Abstract
Aim
Febrile neutropenia (FN) is treated by a broad‐spectrum antimicrobial. Subsequent antimicrobial therapy depends on identifying the source of the infection. Although urinary tract infections (UTIs) are common and urine culture (UC) is a valuable diagnostic tool, uncertainties remain about the specific indications for conducting UC in FN. This study examined whether performing routine UC would affect the subsequent antimicrobial therapy in FN.
Methods
All emergency department patients who received chemotherapy for malignancy and met the definition of FN (neutrophil count <0.5 × 109/L and fever >37.5°C) were included. The patient's demographic data, clinical symptoms, urinalysis results, urine and blood culture results, antibiotic therapy and duration, and patient outcomes were extracted from electronic medical records. UC was defined as positive if >105 colony‐forming units/L were detected.
Results
In total, 115 of the initial 124 cases were included in the analysis. Thirty‐one cases met the Infectious Diseases Society of America guideline definition for recommending UC (recommended group) and 84 cases did not (non‐recommended group). In the recommended group, 16 of 31 cases had a positive UC, and antibiotics were changed for nine, based on UC results. In the non‐recommended group, 15 of 84 cases had a positive UC, and antibiotics were changed for two. The same organism were identified in blood cultures. Seven of 115 cases were detected for the same pathogen in blood and urine cultures.
Conclusion
Performing UC regardless of symptoms could diagnose several asymptomatic UTIs in FN, but seldom impact an antimicrobial treatment strategy.
Keywords: febrile neutropenia, urinary tract infection, urine culture
Urinary tract infection may be missed in febrile neutropenia. We have found that routine urine culture can diagnose asymptomatic urinary tract infections in febrile neutropenic patients, but has little impact on antimicrobial strategy.
INTRODUCTION
Febrile neutropenia (FN), a critical condition characterized by an elevated risk for bacterial and fungal infections caused by an immunocompromised state, is frequently encountered in patients undergoing chemotherapy. Despite advances in care, the mortality rate associated with FN remains significant, ranging from 2.3% to 5.0%. 1 Current guidelines recommend that patients with FN should be treated with broad‐spectrum empiric antibiotics, as FN‐associated bacterial infections progress rapidly and signs of inflammation are often minimal or absent. The primary goal of this approach was to reduce the morbidity and mortality caused by these bacterial infections until they can be properly diagnosed. These measures have been shown to reduce in‐hospital mortality and duration of antimicrobial use. 2
Various culture tests are crucial for identifying pathogens in patients with FN. Blood cultures are particularly essential as bacteremia is present in 10%–25% of FN‐associated infections and carries a high mortality rate. 3 However, other cultures, such as urine cultures (UCs), are recommended only when there is a suspected focal point of infection. For urinary tract infections (UTIs), current FN guidelines suggest urinalysis and evaluation of urinary symptoms as the initial steps, recommending UC only if abnormalities are detected. 4 , 5 This approach may not be fully effective in patients with FN, as their immunocompromised states can lead to diminished urinary symptoms, such as dysuria and suprapubic pain, in addition to reduced sensitivity to urinalysis. 6 , 7 Therefore, patients with FN have minimal findings and may not be adequately identified through guideline‐based examinations.
In this study, we examined whether performing UCs routinely affects treatment following the administration of empiric therapy for FN. Our primary outcome was whether submitting UCs in all cases increased the frequency of antimicrobial changes.
MATERIALS AND METHODS
Study design
This retrospective, single‐center, observational study used data from the Hyogo Prefectural Amagasaki General Medical Center (AGMC) in Japan from January 1, 2015 until June 30, 2023. AGMC, a tertiary care center, serves a population of approximately 1 million, with approximately 35,000 annual emergency department (ED) visits. The study adhered to the Declaration of Helsinki (2013 update) and was approved by the institutional review board of AGMC (approval number: 5–124). Due to the retrospective nature of the study, informed consent was waived.
Study samples
Patients with a neutrophil count of <1.0 × 109/L were selected from all individuals who presented to the AGMC ED and underwent blood tests at the ED. Those who met the criteria for FN were subsequently identified through chart review.
Their UC performances were indicated by clinicians. FN was defined as a single axillary temperature of ≥37.5°C, or a single oral temperature of ≥38.0°C and a neutrophil count of <0.5 × 109/L, or a neutrophil count of <1.0 × 109/L with a predicted decline to <0.5 × 109/L. 8 Recurrent episodes of FN in the same patient were included as separate cases.
Recent chemotherapy was defined as chemotherapy administered within the last 14 days. Patients without urinalyses and UCs or those who had received antimicrobial therapy between the onset of symptoms and their visit to the ED were excluded.
UCs were typically collected before antimicrobial administration. However, in cases of poor general health, treatment was prioritized, and samples were collected as soon as possible. Empiric antibiotic therapy for FN consisted of intravenous cefepime in stable patients. 9 Other antimicrobials were typically selected based on previously detected organisms in the patient and the patient's general condition.
Data collection
Patient data were extracted from the hospital records. These included sex, age, demographic data, underlying malignancy diagnoses, clinical signs and symptoms (e.g., dysuria, frequency, hematuria, acute incontinence, suprapubic pain or tenderness, and flank pain or tenderness), presence of an indwelling urinary catheter, laboratory urinalysis results (leukocytes and nitrites), and urine and blood culture results (microscopy, culture, and sensitivities), Gram staining, antibiotic therapy and duration, and patient outcomes (30‐day mortality and length of stay). Symptomatic patients were defined as those with one or more clinical signs or symptoms. UCs were defined as positive if >105 colony‐forming units (CFU) were detected per liter. 10 Cultures with ≥3 organisms were reported to have “mixed growth” and were considered to be contaminated. 10 Any antibiotic management changes in response to a positive culture were recorded and reviewed by the infectious disease specialist and attending physicians.
Data analysis
Descriptive and bivariate statistics were conducted on the two groups (recommended and non‐recommended) with continuous variables presented as means ± standard deviations (SDs) or medians and their corresponding interquartile ranges (IQRs), and categorical variables were expressed as frequencies and percentages. Descriptive and bivariate statistical analyses were conducted using EZR version 9 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a modified version of the R (The R Foundation for Statistical Computing, Vienna, Austria) commander designed for biostatistics. 11
RESULTS
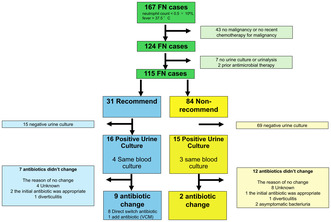
A total of 124 FN cases were identified in 108 patients. Nine cases were excluded: seven that had not undergone urinalyses or UCs and two that had received prior antimicrobial therapy. In total, 115 of the initial 124 cases (93%) were included in our final analysis (Figure 1). The reasons why the excluded cases had not undergone urinalyses or UCs could not be determined by reviewing their charts. Table 1 shows the baseline characteristics of the patients. Their mean age was 72 (±14) years, and females constituted 52%. Thirty‐one cases met the definition of UCs as recommended by the Infectious Diseases Society of America guideline (recommended group), while 84 cases did not (non‐recommended group).
FIGURE 1.

Flowchart of patient recruitment for this study. †FN, febrile neutropenia (defined as a single axillary temperature measurement of ≥37.5°C, or a single oral temperature of ≥38.0°C and a neutrophil count of <0.5 × 10⁹/L, or a neutrophil count of <1.0 × 109/L with a predicted decline to <0.5 × 109/L).
TABLE 1.
Baseline characteristics of patients with urine cultures for febrile neutropenia.
| Variables | All (n = 115) | Recommended (n = 31) | Non‐recommended (n = 84) |
|---|---|---|---|
| Age (years), mean ± SD | 68.8 ± 14.2 | 69.1 ± 14.3 | 68.8 ± 14.2 |
| Female, n (%) | 60 (52%) | 23 (74%) | 37 (43%) |
| Tumor type, n (%) | |||
| Solid | 52 (45%) | 16 (52%) | 36 (44%) |
| Liquid | 63 (55%) | 15 (48%) | 48 (57%) |
| ANC (cell/mm3), median (IQR) | 367.2 (173–704) | 367.2 (181–656) | 367.2 (173–704) |
| Neutropenia, n (%) | |||
| Moderate | 30 (26%) | 12 (39%) | 18 (21%) |
| Severe | 63 (55%) | 13 (42%) | 50 (60%) |
| Profound | 22 (19%) | 6 (19%) | 16 (19%) |
| Previous kidney stones, or genitourinary structural abnormality, n (%) | 6 (5%) | 3 (10%) | 3 (4%) |
| Bacteremia, n (%) | 22 (19%) | 7 (22%) | 15 (18%) |
| Catheterized, n (%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 30 days mortality, n (%) | 5 (4%) | 2 (6%) | 3 (4%) |
| Duration of antibiotics, median (IQR) | 7 (6–12) | 7 (6–12) | 7 (6–12) |
| LOS (days), median (IQR) | 11 (7–16) | 11 (7–16) | 11 (7–15) |
| Gram straining, n (%) | 69 (60%) | 20 (65%) | 49 (58%) |
| Positive symptom, n (%) | 11 (9%) | 11 (35%) | 0 (0%) |
| Positive urinalysis, n (%) | 23 (20%) | 23 (74%) | 0 (0%) |
Note: Neutropenia was divided into moderate (500 cells/mm3 < ANC < 1000 cells/mm3), severe (100 cells/mm3 < ANC < 500 cells/mm3), and profound (<100 cells/mm3).
Abbreviations: ANC, absolute neutrophil count; IQR, interquartile range; LOS, length of stay (in days); SD, standard deviation.
More than two thirds (74%) of the study population exhibited profound or severe neutropenia. Eleven cases presented with urinary symptoms or signs, most commonly flank pain or tenderness. Table 2 shows the bacteria detected in the UCs. Escherichia coli was the most common, followed by E. coli extended‐spectrum β‐lactamase (ESBL) and Enterobacter spp. Table 3 shows the diagnoses and treatment strategies for the patients in both groups. Antibiotics were changed based on UC results in nine cases in the recommended group and two in the non‐recommended group. In the recommended group, eight of nine cases resulted in de‐escalation targeting specific bacteria identified by urine culture. In one case, additional vancomycin was administered because Enterococcus faecium was detected. Five of the nine cases were diagnosed clinically with UTIs at presentation, and three of these had urinary tract symptoms. In four of the nine cases, the diagnosis was changed based on the subsequent UC results. No patients who were diagnosed with UTIs at presentation had their diagnoses changed based on UC results. In these cases, UTIs were ruled out via Gram staining, although urinary tract symptoms were present. In the non‐recommended group, antibiotics were changed in two of the 84 cases. In these cases, the same microorganisms were detected in both blood and urine cultures. None of the patients who had their treatments changed experienced worsening of their general condition after the antibiotic changes. The same pathogen was identified in the blood and UCs in seven of the 115 cases, of which only one of whom had urinary tract symptoms.
TABLE 2.
Organisms identified in urine cultures of patients with bacteriuria.
| Organisms (n = 30) | N (%) |
|---|---|
| Escherichia coli | 11 (36.6) |
| Escherichia coli ESBL | 4 (13.3) |
| Enterococcus sp. | 3 (10.0) |
| Enterobacter cloacae | 2 (6.6) |
| Klebsiella pneumoniae | 2 (6.6) |
| Coryne bacterium sp. | 2 (6.6) |
| Pseudomonas aeruginosa | 2 (6.6) |
| Enterococcus faecalis | 1 (3.3) |
| Enterococcus faecium | 1 (3.3) |
| Klebsiella oxytoca | 1 (3.3) |
| Streptococcus dysgalactiae | 1 (3.3) |
| Proteus mirabilis | 1 (3.3) |
| Staphylococcus aureus | 1 (3.3) |
Abbreviation: ESBL, extended spectrum beta‐lactamases.
TABLE 3.
Urine culture results, impact on management.
| All (n = 115) | Recommended (n = 31) | Non‐recommended (n = 84) | |
|---|---|---|---|
| Contamination | 1 (1%) | 0 | 1 (1%) |
| No growth | 84 (74%) | 15 (49%) | 69 (82%) |
| Positive (organism) | 31 (26%) | 16 (51%) | 15 (18%) |
| Change in antibiotics due to urinary cultures results | 12 (10%) | 9 (29%) | 2 (2%) |
| The same pathogen identified in both blood and urine cultures | 7 (6%) | 5 (16%) | 2 (2%) |
| Change diagnosis due to urinary cultures results | 6 (5%) | 4 (13%) | 2 (2%) |
DISCUSSION
The early administration of empirical therapy represents the most effective curative measure for FN; however, its continuous administration without identifying the specific etiology is not recommended, as this may cause bacterial resistance. 4 Therefore, it is important to identify the source of infection and change antibiotics accordingly during FN treatment. This study aimed to assess the importance of UCs in the management of FN after empirical therapy. Research on UTIs in FN is scarce, with only one small‐scale retrospective study on patients with hematologic tumors currently available in the literature. 12 , 13 , 14 To the best of our knowledge, this is the first study to examine the impact of UC testing on the subsequent treatment of patients with FN and malignancies including solid tumors.
Our results are significant in at least two major aspects. First, UC currently has a limited impact on treatment strategies for FN in patients with malignancies (including solid tumors). Previous reports regarding whether UCs for FN in patients with hematologic malignancies influenced treatment strategies reported that they had minimal impacts on antibiotic treatments and patient outcomes. 12 , 13 Grigg et al. 14 wrote a retrospective review of 317 FN episodes in patients who were undergoing myelosuppressive chemotherapy, reporting that routine UCs in patients with FN but without urinary symptoms rarely affected subsequent antibiotic management. In this study, 29% and 6% of the cases in the recommended and non‐recommended groups, respectively, had the antibiotic regimens changed based on UC results. Additionally, cases in the non‐recommended group, which had the antibiotic regimens changed, had the same organisms detected in both the patient's blood cultures and UCs. Thus, these cases may have had the regimens altered solely based on blood cultures, even without UCs. A possible reason for the small number of cases in the non‐recommended group, which had antibiotic regimen changes, maybe that it is difficult to distinguish between asymptomatic bacteriuria and UTIs in patients with FN. There is no clear definition of UTI in FN, as previous studies have defined UTI differently. 11 , 12 , 13 It is therefore assumed that UCs are unlikely to represent a reason for changing antibiotic regimens, particularly in patients with FN who do not exhibit urinary tract symptoms.
Second, UTIs without urinary tract symptoms are often present in patients with FN. In the present study, six patients (5.2%) were asymptomatic but had positive UCs and blood cultures. When urine and blood cultures are concordant, there is a high probability that the patient has a UTI. 15 These findings are somewhat surprising given the fact that other studies have shown that few asymptomatic patients (0.3%) have concordant pathogens on blood cultures. 14 One hypothesis behind the higher rate of asymptomatic UTIs in our study is due to the sample being taken from an older population. Older individuals often exhibit atypical or impaired responses to severe infections, 16 and age‐related functional and immunological changes can increase the risk of UTIs. 17
Our results suggest that guideline‐guided examinations do not affect antimicrobial treatment strategies, although they may miss some asymptomatic UTIs. However, it is unclear whether the indicative criteria for the tests recommended in the guidelines are the most appropriate. UC is inexpensive and less invasive. Expanding the indications for testing may help prevent the missed detection of rare, asymptomatic UTIs. On the other hand, the expansion of the indications for testing leads to misleading clinical diagnoses due to an increase in false positives, in addition to increased costs. Further studies may allow for more patient severity and background‐specific testing criteria to be established.
This single‐center, retrospective, observational study was constrained by its relatively small sample size, which introduced several limitations. First, this study lacked patients with indwelling urinary catheters, preventing the examination of catheterized patients as defined by FN treatment guidelines. Additionally, the uncertainty surrounding the effectiveness of antimicrobial regimen modifications stems from the observational nature of this study, which is a common limitation for all retrospective studies. Further large‐scale prospective studies are warranted to address this limitation.
CONCLUSION
Performing UC analyses, regardless of the presence of urinary tract symptoms, may be valuable for diagnosing certain asymptomatic UTIs in patients with FN but typically has little impact on antimicrobial treatment strategies.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ETHICS STATEMENT
Approval of the research protocol: All procedures used in this research were approved by the Ethical Committee of Amagasaki General Medical Center.
Informed consent: N/A.
Registry and the registration no. of the study/trial: Approval number: 5–124.
Animal studies: N/A.
Hata N, Ihara T. Impact of urinary culture on diagnosis and treatment strategy after empiric therapy in febrile neutropenic patients. Acute Med Surg. 2024;11:e70012. 10.1002/ams2.70012
DATA AVAILABILITY STATEMENT
Research data are not shared.
REFERENCES
- 1. Leibovici L, Paul M, Cullen M, Bucaneve G, Gafter‐Gvili A, Fraser A, et al. Antibiotic prophylaxis in neutropenic patients: new evidence, practical decisions. Cancer. 2006;107:1743–1751. [DOI] [PubMed] [Google Scholar]
- 2. Schimpff S, Satterlee W, Young VM, Serpick A. Empiric therapy with carbenicillin and gentamicin for febrile patients with cancer and granulocytopenia. N Engl J Med. 1971;284:1061–1065. [DOI] [PubMed] [Google Scholar]
- 3. Klastersky J, Ameye L, Maertens J, Georgala A, Muanza F, Aoun M, et al. Bacteraemia in febrile neutropenic cancer patients. Int J Antimicrob Agents. 2007;30(Suppl 1):S51–S59. [DOI] [PubMed] [Google Scholar]
- 4. Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clin Infect Dis. 2011;52:e56–e93. [DOI] [PubMed] [Google Scholar]
- 5. de Naurois J, Novitzky‐Basso I, Gill MJ, Marti FM, Cullen MH, Roila F, et al. Management of febrile neutropenia: ESMO Clinical Practice Guidelines. Ann Oncol. 2010;21(Suppl 5):v252–v256. [DOI] [PubMed] [Google Scholar]
- 6. Sickles EA, Greene WH, Wiernik PH. Clinical presentation of infection in granulocytopenic patients. Arch Intern Med. 1975;135:715–719. [PubMed] [Google Scholar]
- 7. Zgheib H, El Zakhem AE, Wakil C, Cheaito MA, Cheaito R, Finianos A, et al. Role of urine studies in asymptomatic febrile neutropenic patients presenting to the emergency department. World J Emerg Med. 2021;12:99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Masaoka T. Evidence‐based recommendations for antimicrobial use in febrile neutropenia in Japan: executive summary. Clin Infect Dis. 2004;39(Suppl 1):S49–S52. [DOI] [PubMed] [Google Scholar]
- 9. Furno P, Bucaneve G, Del Favero A. Monotherapy or aminoglycoside‐containing combinations for empirical antibiotic treatment of febrile neutropenic patients: a meta‐analysis. Lancet Infect Dis. 2002;2:231–242. [DOI] [PubMed] [Google Scholar]
- 10. Chan W. Urine cultures. In: Leber A, editor. Clinical microbiology procedures handbook. 4th ed. Washington: ASM Press; 2016. 3.12.6. [Google Scholar]
- 11. Kanda Y. Investigation of the freely available easy‐to‐use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steinrücken J, Pabst T, Zimmerli S, Marschall J. Low impact of urine cultures as a diagnostic tool in patients with neutropenic fever. Infect Dis. 2016;48:872–874. [DOI] [PubMed] [Google Scholar]
- 13. Tran M, Palmer S, Moore DT, Bartelt L, Friedland A, Grgic T, et al. Utility of urine cultures during febrile neutropenia workup in hematopoietic stem cell transplantation recipients without urinary symptoms. Open Forum Infect Dis. 2023;10:ofad236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grigg SE, Date P, Loh Z, Estacio O, Johnson DF, Hawkes EA, et al. Urine cultures at the onset of febrile neutropenia rarely impact antibiotic management in asymptomatic adult cancer patients. Support Care Cancer. 2019;27:1223–1227. [DOI] [PubMed] [Google Scholar]
- 15. Mandell, Douglas, & Bennett's Principles & Practice of Infectious Diseases. Vol 2. 9th ed. 2020. Philadelphia: Elsevier. [Google Scholar]
- 16. Norman DC. Fever in the elderly. Clin Infect Dis. 2000;31:148–151. [DOI] [PubMed] [Google Scholar]
- 17. Castle SC. Clinical relevance of age‐related immune dysfunction. Clin Infect Dis. 2000;31:578–585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.


