Abstract
Background
The standard length of peginterferon plus ribavirin treatment for chronic hepatitis C virus (HCV) genotype 1 infected patients is 48 weeks. However, the number of patients demonstrating a sustained virological response is not high. In order to improve sustained virological response, extending the length of the treatment period has been suggested.
Objectives
To study the benefits and harms of extended 72‐week treatment in comparison with 48‐week treatment with peginterferon plus ribavirin in patients with chronic HCV genotype 1 infection who have shown a slow antiviral response.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, and LILACS until November 2011. We identified further trials by reviewing reference lists and contacting principal authors.
Selection criteria
Trials were eligible for this review if they included patients infected with hepatitis C virus genotype 1 who had a slow antiviral response, and if those patients were randomised to completing 72 weeks versus 48 weeks of treatment with pegylated interferon and ribavirin.
Data collection and analysis
Two authors independently assessed the trials for risk of bias, and extracted the data. The primary outcomes were overall mortality, liver‐related mortality, and liver‐related morbidity. We extracted data separately according to two definitions of slow responders: 1) patients with ≥ 2 log viral reduction but still detectable HCV RNA after 12 weeks of treatment and undetectable HCV RNA after 24 weeks of treatment; 2) patients with detectable HCV RNA after four weeks of treatment. We calculated risk ratios from individual trials as well as in the meta‐analyses of trials.
Main results
We included seven trials with 1369 participants. All trials had high risk of bias. Five trials used our first definition of slow responders, and three other trials (including one that used both definitions) used the second definition. None of the included trials mentioned our primary outcomes. However, regarding the secondary outcomes, extension of the treatment period to 72 weeks increased the sustained virological response according to both definitions (71/217 (32.7%) versus 52/194 (26.8%); risk ratio (RR) 1.43, 95% confidence interval (CI) 1.07 to 1.92, P = 0.02, I2 = 8%; and 265/499 (53.1%) versus 207/496 (41.7%); RR 1.27, 95% CI 1.07 to 1.50, P = 0.006, I2 = 38%), with a risk difference of 0.11 and calculated number needed to treat of nine. The end of treatment response was not significantly different between the two treatment groups. The number of participants who relapsed virologically was found to be lower in the groups that had been treated for 72 weeks using both definitions (27/84 (32.1%) versus 46/91 (50.5%); RR 0.59, 95% CI 0.40 to 0.86, P = 0.007, I2 = 18%, 3 trials; and 85/350 (24.3%) versus 146/353 (41.4%); RR 0.59, 95% CI 0.47, 0.73, P < 0.000001, I2 = 0%, 3 trials). The length of treatment did not significantly affect the adherence (247/279 (88.5%) versus 252/274 (92.0%); RR 0.95, 95% CI 0.84 to 1.07, P = 0.42, I2 = 69%, 3 trials). In the single trial that reported adverse events, no significant difference was seen between the two treatment groups.
Authors' conclusions
This review demonstrates higher a proportion of sustained virological response after extension of treatment from 48 weeks to 72 weeks in HCV genotype 1 infected patients in whom HCV RNA was still detectable but decreased by ≥ 2 log after 12 weeks and became negative after 24 weeks of treatment, and in patients with detectable HCV RNA after four weeks of treatment with peginterferon plus ribavirin. The observed intervention effects can be caused by both systematic error (bias) and random errors (play of chance). There was no reporting on mortality and the reporting of clinical outcomes and adverse events was insufficient. More data are needed in order to recommend or reject the policy of extending the treatment period for slow responders.
Keywords: Adult; Humans; Antiviral Agents; Antiviral Agents/administration & dosage; Drug Administration Schedule; Drug Therapy, Combination; Drug Therapy, Combination/methods; Genotype; Hepacivirus; Hepacivirus/genetics; Hepatitis C, Chronic; Hepatitis C, Chronic/drug therapy; Interferon alpha‐2; Interferon‐alpha; Interferon‐alpha/administration & dosage; Polyethylene Glycols; Polyethylene Glycols/administration & dosage; Randomized Controlled Trials as Topic; Recombinant Proteins; Recombinant Proteins/administration & dosage; Ribavirin; Ribavirin/administration & dosage; Time Factors; Viral Load
Plain language summary
Extended treatment for 72 weeks versus standard treatment for 48 weeks in chronic hepatitis C genotype 1 infected slow responders
Chronic hepatitis C is a leading cause of liver‐related morbidity and mortality. The standard length of treatment with peginterferon plus ribavirin for hepatitis C virus genotype 1 infected patients is 48 weeks, but the number of patients who are treated successfully with regard to disappearance of the virus from the blood (sustained virological response) is limited. In order to improve it, extending the length of the treatment period has been suggested. We attempted to identify whether extending treatment duration to 72 weeks is better than the standard 48 weeks in a subgroup of patients who have shown a slow viral response.
We found seven randomised clinical trials that compared a treatment duration of 72 weeks with 48 weeks in 1369 participants. The quality of all trials was low. Mortality and liver‐related morbidity were not reported in any of the included trials. Sustained virological response (that is, undetectable hepatitis C virus RNA after six months from the end of an entire course of treatment) was increased when the decision to prolong treatment was taken based on viral load after 12 weeks of treatment (RR 1.43, 95% CI 1.07 to 1.92) as well as when the decision to prolong treatment was taken based on the results of the viral load after four weeks of treatment (RR 1.27, 95% CI 1.07 to 1.50). The calculated number needed to treat to achieve an increase in sustained virological response proportions was nine (meaning that among nine participants treated for 72 weeks instead of 48 weeks, only one more will achieve a sustained virological response compared to the participants treated for 48 weeks). The higher sustained virological response after 72 weeks of treatment was caused by a reduction in the number of patients in this group who experienced a virological relapse after treatment. Adherence to treatment was not different between the two groups. Serious adverse events were mentioned in only one trial, and they were not different in the two treatment groups. The findings may be influenced by both risks of systematic errors (bias) and the risk of random errors (play of chance).
Further large‐scale, randomised trials with reporting of patient relevant outcomes are warranted.
Summary of findings
Summary of findings for the main comparison. Extended peginterferon plus ribavirin treatment for 72 weeks versus standard peginterferon plus ribavirin treatment for 48 weeks in chronic hepatitis C genotype 1 infected slow responders.
| 72 weeks of treatment with peginterferon plus ribavirin compared with 48 weeks of this treatment for patients with chronic hepatitis C virus (HCV) genotype 1 infection having shown slow antiviral response according to one of two definitions | ||||||
|
Patient or population: participants with detectable HCV RNA after 12 weeks of treatment with pegylated interferon and ribavirin, but with >= 2 log viral reduction, and undetectable HCV RNA after 24 weeks of treatment. Settings: community. Intervention: 72 weeks of treatment. Comparison: 48 weeks of treatment. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE)2 | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| 48 weeks | 72 weeks | |||||
| Sustained virological response (SVR) | Lower SVR | RR 1.43 [1.07 to 1.92] | 411 (5 trials) | + very low | ||
| 0 per 100 | 0 per 100 | |||||
| Medium SVR | ||||||
| 15 per 100 | 1 per 100 (16 to 29) | |||||
| Higher SVR | ||||||
| 43 per 100 | 61 per 100 (46 to 83) | |||||
| Relapse | Lower relapse | RR 0.59 [0.40 to 0.86] | 175 (3 trials) | + very low | ||
| 47 per 100 | 28 per 100 (19 to 40) | |||||
| Medium relapse | ||||||
| 59 per 100 | 35 per 100 (24 to 51) | |||||
| Higher relapse | ||||||
| 100 per 100 | 59 per 100 (40 to 86) | |||||
| End of treatment response (ETR) |
Lower ETR | RR 0.96 [0.79 to 1.16] |
334 (3 trials) | + very low | ||
| 5 per 100 | 5 per 100 (4 to 6) | |||||
| Medium ETR | ||||||
| 45 per 100 | 43 per 100 (35 to 53) | |||||
| Higher ETR | ||||||
| 79 per 100 | 75 per 1000 (62 to 92) | |||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval. RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Assumed risks were obtained from the three included trials with higher, median, and lower SVR values corresponding to the definition of patients.
2 We decided to reduce three levels of the GRADE quality because of: 1. risk of bias ‐ all trials were unblinded and some of them had other methodological pitfalls such as attrition bias. 2. Imprecision ‐ as shown in Figure 1 the number of patients included in the meta‐analysis was less than the optimum information size. 3. SVR is a surrogate outcome and we do not yet know if reduction in SVR results in reduced mortality and morbidity. The same is true for relapse rate and ETR (of which we know that does not have any correlation to clinical outcomes).
¤ Patients with detectable HCV RNA after 12 weeks of treatment with pegylated interferon and ribavirin, but with ≥ 2 log viral reduction and undetectable HCV RNA after 24 weeks of treatment.
Summary of findings 2. Extended peginterferon plus ribavirin treatment for 72 weeks versus standard peginterferon plus ribavirin treatment for 48 weeks in chronic hepatitis C genotype 1 infected slow responders.
| 72 weeks of treatment with peginterferon plus ribavirin compared with 48 weeks of this treatment for patients with chronic hepatitis C virus (HCV) genotype 1 infection having shown slow antiviral response according to another¤ definition | ||||||
|
Patient or population: participants with detectable HCV RNA after four weeks of treatment with pegylated interferon and ribavirin Settings: community. Intervention: 72 weeks of treatment. Comparison: 48 weeks of treatment. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| 48 weeks | 72 weeks | |||||
| Sustained virological response (SVR) | Low risk population | RR 1.27 [1.07 to 1.50] | 995 (3 trials) | ++ Low | ||
| 28 per 100 | 35 per 100 (29 to 41) | |||||
| Medium risk population | ||||||
| 44 per 100 | 56 per 100 (47 to 66) | |||||
| High risk population | ||||||
| 52 per 100 | 66 per 100 (56 to 78) |
|||||
| Relapse rate | Low risk population | RR 0.59 [0.47 to 0.73] | 703 (3 trials) | ++ Low | ||
| 37 per 100 | 22 per 100 (17 to 27) | |||||
| Medium risk population | ||||||
| 38 per 100 | 22 per 100 (17 to 28) | |||||
| High risk population | ||||||
| 53 per 100 | 31 per 100 (25 to 39) | |||||
| End of treatment response (ETR) |
Low risk population | RR 0.99 [0.91 to 1.07] | 995 (3 trials) | ++ Low | ||
| 58 per 100 | 58 per 100 (53 to 62) | |||||
| Medium risk population | ||||||
| 69 per 100 | 69 per 100 (63 to 74) | |||||
| High risk population | ||||||
| 85 per 100 | 84 per 100 (76 to 90) | |||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
CI = confidence interval. RR = risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1 Assumed risks were obtained from the three included trials corresponding to the definition of patients.
2 We decided to reduce two levels of the GRADE quality because of: 1. risk of bias ‐ all trials were unblinded and some of them had other methodological pitfalls such as attrition bias. 2. SVR is a surrogate outcome and we do not yet know if reduction in SVR results in reduced mortality and morbidity. The same is true for relapse rate and ETR (of which we know that does not have any correlation to clinical outcomes).
¤ GIVE THE DEFINITION! participants with detectable HCV RNA after four weeks of treatment with pegylated interferon and ribavirin.
Summary of findings 3. Extended peginterferon plus ribavirin treatment for 72 weeks versus standard peginterferon plus ribavirin treatment for 48 weeks in chronic hepatitis C genotype 1 infected slow responders.
| 72 weeks of treatment with peginterferon plus ribavirin compared with 48 weeks of this treatment for patients with chronic hepatitis C virus (HCV) genotype 1 infection having shown slow antiviral response (according to both definitions) | ||||||
|
Patient or population: participants with detectable HCV RNA after four weeks of treatment with pegylated interferon and ribavirin or participants with detectable HCV RNA after 12 weeks of treatment, but with >= 2 log viral reduction, and undetectable HCV RNA after 24 weeks of treatment. Settings: community. Intervention: 72 weeks of treatment. Comparison: 48 weeks of treatment. | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE)@ | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| 48 weeks | 72 weeks | |||||
| Adherance to treatment | Low risk population | RR 0.95 [0.84 to 1.07] | 260 (2 trials) | +++ moderate | ||
| 86 per 100 | 81 per 100 (72 to 91) | |||||
| Medium risk population | ||||||
| 91 per 100 | 86 per 100 (76 to 97) |
|||||
| 95 per 100 | 90 per 100 (80 to 100) |
|||||
| *The basis for the assumed risk is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI = confidence interval. RR = risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1Assumed risks were obtained from the three included trials corresponding to the definition of patients.
2 We decided to reduce two levels of the GRADE quality because of all trials were unblinded and some of them had other methodological pitfalls such as attrition bias.
Background
Description of the condition
Hepatitis C virus (HCV) infection is a leading cause of liver‐related morbidity and mortality, with hepatic fibrosis, end‐stage liver cirrhosis, and hepatocellular carcinoma (HCC) as the dominant clinical sequelae. It affects 3% of the population, which equals 170 million people worldwide (Theodore 2006).
Chronic hepatitis C progresses slowly over a time frame of 15 years to 50 years. Approximately 20% to 30% of individuals develop cirrhosis two to three decades after being infected (Thomas 2005). Hepatitis C is responsible for one‐third of HCC patients in the USA (El‐Serag 2003), the annual incidence of HCC in cirrhotic HCV patients being 1% to 4% (Lauer 2001).
HCV infection is the most common indication for orthotopic liver transplantation (Kim 2009). Successful antiviral therapy, defined as a sustained virological response (SVR) (that is, undetectable hepatitis C virus RNA six months from the end of treatment), seems to prevent disease progression and to reduce the risk of HCC (Ueno 2009). However, although a systematic review demonstrates significantly higher proportions of patients with SVR after combination treatment with interferon plus ribavirin than with interferon alone we do not yet know if this results in reduced mortality and morbidity (Brok 2010).
Description of the intervention
The standard treatment for HCV infection is a combination of pegylated interferon (PEG‐IFN) and ribavirin. Recently, protease inhibitors were added to this regimen. The regimen can include either peginterferon alfa‐2b (Peg‐Intron, Schering Plough Corp, Kenilworth, NJ) or peginterferon alfa‐2a (Pegasys, Hoffmann‐La Roche, Nutley, NJ) (Awad 2010). The optimal dose of peginterferon alfa‐2b is 1.5 µg/kg/week (Ghany 2009). Peginterferon alfa‐2a is administered at a fixed dose of 180 µg/week.
Ribavirin dosing depends on the viral genotype, the type of peginterferon used, and the patient's weight. For patients receiving peginterferon alfa‐2a who are infected by genotype 1 or 4 HCV, the ribavirin dose is 1000 to 1200 mg/d (1000 mg if weight < 75 kg, 1200 mg if weight > 75 kg). For patients receiving peginterferon alfa‐2b, the ribavirin dose is as follows: 800 mg for patients weighing < 65 kg; 1000 mg for patients weighing 65 kg to 85 kg; 1200 mg for patients weighing 85 kg to 105 kg; and 1400 mg for patients weighing 105 kg and above. For patients with genotypes 2 and 3, the recommended dose of ribavirin is 800 mg/d independent of type of interferon or patients' weight (Ghany 2009).
The optimal duration of treatment is related to the viral genotype. It has been established that patients with genotype 1 should be treated for 48 weeks, whereas patients with genotypes 2 and 3 should be treated for 24 weeks (Hadziyannis 2004). According to a meta‐analysis of six randomised trials for patients with HCV genotype 4 infection, treatment should be planned for 48 weeks (Khuroo 2004).
The primary outcome in most of the studies dealing with treatment of HCV infection is sustained virological response (SVR), defined as undetectable serum HCV RNA 24 weeks after cessation of therapy. Response to therapy and clearance of the virus as measured by SVR seems a rational surrogate marker. However, SVR cannot be a primary outcome since it has never been validated as a predictor of clinical outcomes (Brok 2010). The primary aim of therapy is to prevent the subsequent development of end‐stage liver disease, HCC, and mortality in the fraction of infected patients who would otherwise do so. Therefore, only clinical outcomes can serve as primary outcomes.
The kinetics of HCV RNA load in response to antiviral treatment is a crucial factor in determining the therapeutic outcome and the possible achievement of SVR. Patients who have undetectable HCV RNA in serum after four weeks of treatment (rapid virological response (RVR)) have around a 90% chance of SVR (Jensen 2006). Patients with less than a 2 log decrease from baseline HCV RNA load after 12 weeks of treatment have a very low chance of developing SVR (Fried 2002).
Recently, attention has focused on tailoring treatment duration according to response to treatment (Berg 2009). Usually, slow virological response is defined as HCV RNA levels > 50 IU/mL at week 12, but more than 2 log decrease from baseline levels, and < 50 IU/ml at week 24.
The duration of treatment once HCV RNA has been cleared from the serum might influence the SVR (Drusano 2004). Based on this hypothesis, some investigators support the extension of treatment to all HCV genotype 1 infected patients who have not cleared their HCV RNA after four weeks of treatment (Sanchez‐Tapias 2006).
In this systematic review with meta‐analyses we tried to establish the benefits and harms of treating genotype 1 hepatitis C patients who are slow virological responders to peginterferon and ribavirin treatment, for 72 weeks rather than with the standard treatment for 48 weeks.
Why it is important to do this review
Several trials have been published regarding the effect of refining the duration of treatment. Our systematic review was aimed at investigating whether extended treatment for 72 weeks for genotype 1 infected patients who are slow responders has a clinical effect as well as increases the SVR. If a 72‐week regimen is found to be more effective than the 48‐week regimen, our systematic review will have practical implications for the way these patients should be treated. As genotype 1 is the most common genotype of HCV in the western world, the possible implications of this systematic review may be important. The clinical, social, and economic implications could be significant.
Recently, five other meta‐analyses have been published demonstrating a higher SVR proportion for the patients who were treated for 72 weeks (Farnik 2010; Parikh 2010; Alavian 2011; Di Martino 2011; Gevers 2011). However, these meta‐analyses used only the common definition for slow responders in their inclusion criteria and did not include two recently published randomised clinical trials (Liu 2011; Lee 2012), so we decided to conduct another, more comprehensive and updated systematic review with meta‐analyses (see Methods, inclusion criteria).
Objectives
To study the benefits and harms of extended treatment for 72 weeks with peginterferon plus ribavirin in patients with chronic hepatitis C virus genotype 1 infection who have shown a slow antiviral response after four or 12 weeks of treatment compared with the 48‐week standard treatment.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials. We have included randomised trials irrespective of publication status, language, or blinding.
Types of participants
Inclusion criteria
People of both sexes and all ethnic origins that are chronic HCV genotype 1 infected naive patients and slow responders, according to the definitions below.
Chronic HCV infected patients: patients who at baseline had detectable serum HCV RNA via nucleic acid testing (irrespective of their serum alanine amino transferase levels) and histologically proven chronic hepatitis C.
Naive patients: patients who did not receive any antiviral treatment for the HCV infection before the trial.
-
Slow responders, with one of two definitions:
patients with detectable HCV RNA after 12 weeks of treatment with pegylated interferon and ribavirin but with ≥ 2 log viral reduction and undetectable HCV RNA after 24 weeks of treatment;
patients with detectable HCV RNA after four weeks of treatment with pegylated interferon and ribavirin.
Exclusion criteria
Age less than 18 years.
Patients with other genotypes than genotype 1 infection.
Patients with other causes of liver disease.
Patients who were co‐infected with hepatitis B virus (HBV) or human immunodeficiency virus (HIV), or both.
Patients with decompensated liver disease.
Patients with clinically significant cardiac or cardiovascular abnormalities, systemic infection, an organ graft, clinically significant bleeding disorders, evidence of malignant diseases, or concomitant immunosuppressive medication.
Patients who were known to have or reported excessive alcohol intake or concomitant drug abuse.
Pregnant or lactating women or male partners of pregnant women.
Types of interventions
Peginterferon (alfa‐2a or alfa‐2b) and ribavirin for 72 weeks versus peginterferon (alfa‐2a or alfa‐2b) and ribavirin for 48 weeks.
Types of outcome measures
Primary outcomes
Overall mortality, defined as the number of participants who died during the follow‐up period of the trials
HCV‐related mortality, defined as the number of participants who died during the studies' follow‐up periods because of their HCV‐related liver disease
Liver‐related morbidity
Secondary outcomes
Number of participants with sustained virological response (SVR), defined as undetectable serum HCV RNA 24 weeks after the end of treatment.
Number of participants with end of treatment response (EOR), defined as undetectable serum HCV RNA at the end of treatment.
Number of participants who relapsed, defined as reappearance of HCV RNA in serum after therapy is discontinued.
Adherance to treatment, defined as the number of participants who adhered to the protocol of treatment.
Reduction of treatment dose, defined as the number of participants in whom the medication doses were reduced.
Occurrence of adverse events: a) any clinical adverse event; b) serious adverse events defined as any untoward medical occurrence in a patient, in either of the two described regimens, which did not necessarily have a causal relationship with the treatment but did, however, result in a dose reduction or discontinuation of treatment. Serious adverse events are defined according to the International Conference on Harmonisation Guidelines (ICH‐GCP 1997) as any event that led to death, was life‐threatening, required inpatient hospitalisation or prolongation of existing hospitalisation, resulted in persistent or significant disability, congenital anomaly or birth defect, or led to any important medical event which might have jeopardised the patient or required intervention to prevent it.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2012), Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded (SCI Expanded), and LILACS (Royle 2003). The search strategies with the time spans of the searches are described in Appendix 1.
Searching other resources
We tried to identify further trials by reviewing the reference lists and contacting the principal authors of the identified trials.
Data collection and analysis
We performed the review following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (Gluud 2012). We performed the analyses using Review Manager 5 (RevMan 2011).
Selection of studies
Two authors (LK, HG) independently inspected each reference identified by the searches and applied the inclusion criteria. For possibly relevant publications, or in cases of disagreement between the two authors, we obtained the full publication and LK and HG inspected it independently. If disagreement persisted, we consulted a third author (RTK).
Data extraction and management
Two authors (LK, HG) independently extracted data. In the case of disagreement between the two authors, a third author (RTK) extracted the data. We discussed the data extraction, documented decisions and, where necessary, contacted authors of trials for clarification. We identified trials by the name of the first author and year in which the trial was published in full; and we then ordered them chronologically.
We extracted, checked, and recorded the following data.
Characteristics of trials: date, location and setting; publication status; sponsor (specified, known, or unknown); duration of follow‐up.
Characteristics of participants: number of participants in each group; age; sex; ethnicity; weight or body mass index (BMI); viral load at the beginning of treatment and after four weeks and 12 weeks; degree of fibrosis at the beginning of treatment.
Characteristics of interventions: type and dose of peginterferon; dose of ribavirin; schedule.
Characteristics of outcome measures: whenever possible, we recorded the number of events previously listed under 'outcome measures' in each of the groups of the trials. We applied outcome measures separately to each of the mentioned definitions of slow responders.
Assessment of risk of bias in included studies
Methodological quality is defined as confidence that the design and the report of the randomised clinical trial would restrict bias in the comparison of the intervention (Moher 1998). According to the empirical evidence (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008), the methodological quality of the trials was assessed based on sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessors; incomplete outcome data; selective outcome reporting; and other sources of bias. The domains for assessment of risk of bias that we have chosen to apply to the studies are as follows.
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice are adequate if performed by an independent adjudicator.
Uncertain risk of bias: the trial is described as randomised but the method of sequence generation was not specified.
High risk of bias: the sequence generation method is not, or may not be, random. Quasi‐randomised studies, those using dates, names, or admittance numbers in order to allocate patients are inadequate and were excluded for the assessment of benefits but not for assessing harms.
Allocation concealment
Low risk of bias: allocation was controlled by a central and independent randomisation unit, sequentially numbered, opaque and sealed envelopes or similar so that intervention allocations could not have been foreseen in advance of, or during, enrolment.
Uncertain risk of bias: the trial was described as randomised but the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: if the allocation sequence was known to the investigators who assigned participants, or if the study was quasi‐randomised. Quasi‐randomised studies were excluded for the assessment of benefits but not for assessing harms.
Blinding of participants, personnel, and outcome assessors
Low risk of bias: blinding was performed adequately, or the outcome measurement is not likely to be influenced by lack of blinding.
Uncertain risk of bias: there is insufficient information to assess whether the type of blinding used is likely to induce bias on the estimate of effect.
High risk of bias: no blinding or incomplete blinding, and the outcome or the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: the underlying reasons for missing data are unlikely to make treatment effects depart from plausible values, or proper methods have been employed to handle missing data.
Uncertain risk of bias: there is insufficient information to assess whether the missing data mechanism in combination with the method used to handle missing data are likely to induce bias on the estimate of effect.
High risk of bias: the crude estimate of effects (for example, complete case estimates) will clearly be biased due to the underlying reasons for missing data, and the methods used to handle missing data are unsatisfactory.
Selective outcome reporting
Low risk of bias: pre‐defined, or clinically relevant and reasonably expected outcomes are reported on.
Uncertain risk of bias: not all pre‐defined or clinically relevant and reasonably expected outcomes are reported on, or are not reported fully, or it is unclear whether data on these outcomes were recorded or not.
High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported on; data on these outcomes were likely to have been recorded.
Other sources of bias
Such bias sources could be academic bias or other conflicts of interest (for example, industry bias).
Low risk of bias (the trial appears to be free of other sources of bias).
Uncertain risk of bias (there is insufficient information to assess whether other sources of bias are present).
High risk of bias (it is likely that potential sources of bias related to the specific design used, early termination due to some data‐dependent process, lack of sample size or power calculation, or other bias risks are present).
We judged trials as trials with low risk of bias if assessed as having low risk of bias in all of the specified individual domains. One or more domains with 'uncertain risk of bias' or 'high risk of bias' assessments defined the trial as a trial with 'high risk of bias'.
Measures of treatment effect
The treatment effects in this meta‐analysis were all dichotomous and were expressed as risk ratio (RR) with 95% confidence interval (CI). The number needed to treat (NNT) was derived from the risk difference (RD).
Dealing with missing data
For trials with missing data, assessment would have been made in order to decide whether the missing data were 'missing at random' or not. For 'missing at random data', only analyses based on the available data would have been undertaken. For 'not missing at random data', we would have tried to contact the primary investigators in order to request the missing data. If the information had not been available, we would have assessed the adequacy of the methods used to deal with 'missingness'. In the Discussion section, we would have addressed the potential impact of missing data on the findings of the review.
If patients had been lost to follow‐up and missing data methods not applied, data would have been analysed according to the intention‐to‐treat (ITT) principle and an available case analysis using as the denominator the total number of people who had data recorded for the particular outcome in question. ITT would have been performed based on consideration of 'best‐case' and 'worst‐case' scenarios (Gamble 2005). However, after completing the data extraction we did not identify any trials with missing data.
Assessment of heterogeneity
We assessed heterogeneity using the Chi2 test of heterogeneity and determined the quantity of heterogeneity by the I2 statistic as a measure of inconsistency (Higgins 2002). Significant heterogeneity was defined as a Chi2 test P value less than 0.1 or an I2 greater than 50%.
Assessment of reporting biases
We handled reporting biases following the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used funnel plot asymmetry (Higgins 2011) even though asymmetric funnel plots are not necessarily caused by publication bias, and publication bias does not necessarily cause asymmetry in a funnel plot (Egger 1997).
Data synthesis
For all analyses, we used the fixed‐effect model meta‐analysis (DeMets 1987). In case of significant heterogeneity, as described earlier, we also conducted random‐effects model meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analyses to compare findings with:
type of patients, according to the above mentioned two definitions of 'slow responders';
intervention, according to the dose of ribavirin and the type of peginterferon;
data analysis in included trials according to the ITT principle (main analysis) as well as 'as treated' (per protocol) analysis;
trials with low risk of bias compared to trials with high risk of bias.
Sensitivity analysis
Trial sequential analysis
In order to asses the reliability of the results in our meta‐analysis regarding SVR, we calculated the required meta‐analysis sample (the required information size) based upon the proportion of patients in the control group with SVR; a relative risk reduction as suggested by the meta‐analysis; and an alpha = 5% and a beta = 10% (90% power). We substituted the conventional 5% threshold for statistical significance with those of the Lan‐DeMets alpha spending monitoring boundaries. We used the TSA software (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009, Wetterslev 2009; Thorlund 2010; CTU 2011; Thorlund 2011).
Results
Description of studies
Results of the search
Our initial search identified 845 references: we obtained 46 references from the Cochrane Hepato‐Biliary Group Controlled Trials Register (November 2011); 257 from CENTRAL (Issue 4 of 4, 2011); 126 from MEDLINE (Ovid SP) (1950 to November 2011); 213 from EMBASE (Ovid SP) (1980 to February 2011); 190 from Science Citation Index Expanded (http://apps.isiknowledge.com) (1900 to November 2011); and 13 from LILACS (http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online) (1982 to November 2011).
After reading the titles and abstracts, we excluded 381 references because they were duplicates; 338 because they had objectives different from this review; 74 because they were reviews of the literature; nine because they were meta‐analyses; eight because they dealt with extension of interferon treatment for chronic hepatitis C patients and not with the extension of peginterferon treatment; seven because they dealt with treatment of non‐naive chronic HCV patients; seven because they were relevant studies but were not randomised trials; four because they were editorials; two were trials that included patients with HCV‐HIV co‐infection; one was an article which published guidelines for treatment for chronic HCV; one trial did not deal with peginterferon treatment; and one publication dealt with patients who were non‐responders to previous antiviral therapy.
We retrieved a total of 12 studies for further evaluation.
Included studies
We have included seven trials in our review (Berg 2006; Sanchez‐Tapias 2006; Pearlman 2007; Mangia 2008; Buti 2010; Liu 2011; Lee 2012). We have described these trials in the 'Characteristics of included studies' table.
One trial was a multi‐centre trial performed in Canada, Europe, Israel, and Puerto Rico. The other six trials took place in Germany, Italy, Spain, Taiwan, Canada, and the United States of America. All trial reports were published in English. We wrote to the corresponding authors of all trials in order to obtain more information and received answers from four of them (Sanchez‐Tapias 2006; Buti 2010; Liu 2011; Lee 2012). We have included detailed information in the Characteristics of included studies table.
Altogether, the seven trials included 2268 participants (range from 101 to 696 for each trial); we could include 1369 of them in our meta‐analysis. We have excluded the remaining participants either because they were not slow responders or because they had genotypes other than genotype 1.
In four trials (Pearlman 2007; Mangia 2008; Buti 2010; Lee 2012) the definition of slow responders was detectable HCV‐RNA with ≥ 2 log viral reduction at week 12 and undetectable HCV RNA at week 24 (matching our review's first definition of slow responders). The Mangia 2008 trial also reported the data of participants with positive HCV RNA in week 8 and negative in week 24; these data are not a part of our meta‐analysis. The Lee 2012 trial evaluated a treatment duration of 24, 36, 48, and 72 weeks, based on the HCV RNA status. We included only patients who were able to match our first definition of slow responders. One trial's definition of slow responders was positive HCV RNA at week four, matching our review's second definition of detectable HCV RNA at week four (Sanchez‐Tapias 2006). In Berg 2006, two definitions were given for slow responders: 1) participants with detectable HCV RNA after four weeks of treatment, that were included in our meta‐analysis; and 2) participants with no early virological response based on HCV RNA status after 12 weeks of treatment. However, neither their HCV RNA status at week 24 nor the amount of reduction of viral load at week 12 were mentioned, so subsequently we could not include this second subgroup in our meta‐analysis. In the last trial (Liu 2011), patients were recruited and randomised according to our second definition (that is, positive HCV RNA after four weeks of treatment). However, a subgroup analysis of this trial also included a comparison according to our first definition of slow responders (detectable HCV‐RNA with ≥ 2 log viral reduction at week 12 and undetectable HCV RNA at week 24), which made it possible to include this subgroup of patients in the analyses of both definitions.
The mean age of the participants ranged from 42.7 to 56 years; 54.2% to 69% were males. There were no noteworthy baseline differences between the participants in the different arms of each of the trials.
In all trials, naive chronic hepatitis C participants were treated with combination of subcutaneous peginterferon alfa‐2a or subcutaneous peginterferon alfa‐2b and daily ribavirin (doses and regimens are specified in Characteristics of included studies). Two trials used low dose of ribavirin (Berg 2006; Sanchez‐Tapias 2006) and the others used the accepted weight‐dependent dose. In all trials data were extracted on genotype 1 patients only.
In all trials a comparison of an extended 72‐week treatment with a standard 48‐week treatment was undertaken. In one trial not only the slow responders but all participants were randomised to a 72‐week treatment versus a 48‐week treatment (Berg 2006). However, only data for the slow responders were extracted. In four trials only the slow responders were randomised (Sanchez‐Tapias 2006; Pearlman 2007; Buti 2010; Liu 2011). In one trial (Mangia 2008) the standard group of a 48‐week treatment was compared with a variable group of 24, 48, and 72‐week treatment if the HCV‐RNA was negative at weeks 4, 8, or 12, respectively. In the variable group, participants who were slow responders were also treated for 72 weeks and only data for the participants matching our first definition of slow responders were extracted. The remaining trial, the IMPROVE trial (Lee 2012), evaluated treatment duration according to virological responses on weeks 4, 8, and 12; so patients with negative HCV RNA at these points of time were randomised to be treated for 24 or 48 weeks, 36 or 48 weeks, and 72 or 48 weeks, respectively. Only part of the last group (those with partial early virological response (pEVR) (see Characteristics of included studies table) was included in our meta‐analysis.
The primary outcome of all trials was SVR. For the secondary outcomes, see the Characteristics of included studies table.
Excluded studies
Of the 12 potentially eligible trials, we excluded five. The reasons for exclusion are listed in the Characteristics of excluded studies table.
Briefly, in Ide 2009 the treatment duration was less than 72 weeks: treatment was administered for 44 weeks after participants became negative for HCV RNA (total duration 48 to 68 weeks). Miyase 2010 compared 48 with 72 weeks of treatment but the participants in this trial had positive HCV RNA at week eight and negative HCV RNA at week 12. So, theoretically they could fit into our second definition of slow responders. However, several participants in this trial were non‐naive patients, so we had to exclude the trial. In the Ferenci 2010 trial, we could not segregate data about genotype 1 slow responders. Data were extracted from this trial on slow responders with genotypes 1 and 4, or on genotype 1 participants with partial and complete early response. We contacted Dr Ferenci to obtain clarifications and we received a reply. However, even after the reply we still had some missing data and could not include this trial. We excluded the trial by Nagaki 2009 because treatment was extended for 96 weeks and some of the participants were non‐naive. So far, Sarrazin et al has published only an abstract (INDIV‐2 study) (Sarrazin 2010). From their data we could not extract enough relevant information for the group treated for 72 weeks and their controls. We therefore consider this trial as an 'ongoing study'.
Risk of bias in included studies
Five trials reported that the allocation sequence was adequately generated. However, in one trial (Pearlman 2007) there was insufficient information about the sequence generation process. Allocation was adequately concealed in six trials. In one trial (Mangia 2008), participants were allocated in blocks of five and a randomisation list was sent to each participating centre. Therefore, investigators could have predicted allocation for some of the participants. Lack of blinding in all included trials might have influenced most of the outcomes, like SVR and especially adverse events. Following the definitions in the domains for bias risk, all trials were judged to have high risk of bias. Since we found no clinical data on mortality or liver‐related morbidity in any of the trials, these trials ought to be viewed as having high risk of bias when assessing the domain for selective outcome reporting. Regarding the remaining outcomes, data for all included and randomised participants were given in two trials (Buti 2010; Liu 2011). In another trial (Pearlman 2007) incomplete outcome data were adequately addressed. In the four other trials not all the participants were truly slow responders; therefore, data on some of the secondary outcomes were general and not specified for the slow‐responder population, or were not mentioned. However, reasons for missing outcome data were unlikely to be related to the true outcome. Trial protocols were not available for all trials, and it is unclear if all trials were free from selective reporting. We have provided individual detailed descriptions in the 'Risk of bias' tables and in Figure 2 and Figure 3.
2.
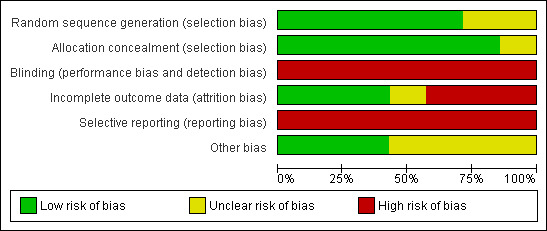
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
3.
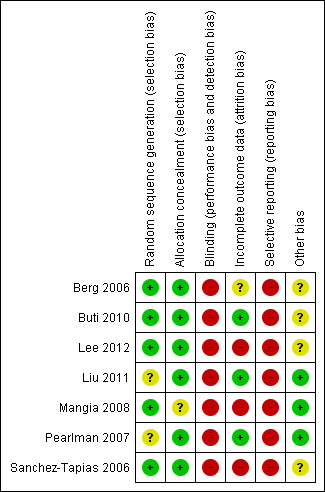
Risk of bias summary: review authors' judgements about each risk of bias item for each included trials.
Effects of interventions
See: Table 1; Table 2; Table 3
We included seven trials in our systematic review and meta‐analysis (see Characteristics of included studies). We performed two separate meta‐analyses according to the two definitions of slow responders in our protocol (see inclusion criteria). Five trials defined slow responders according to our first definition, that is, participants with detectable HCV RNA after 12 weeks of treatment but with ≥ 2 log viral reduction and undetectable HCV RNA after 24 weeks of treatment (Pearlman 2007; Mangia 2008; Buti 2010; Liu 2011; Lee 2012). In all these trials, the ribavirin dose was the accepted dose ('high dose'), that is, 800 mg/d to 1400 mg/d (Pearlman 2007; Buti 2010), 1000 mg/d to 1400 mg/d (Lee 2012), or 1000 mg/d to 1200 mg/d (Mangia 2008; Liu 2011) according to the participant's weight. Two trials used peginterferon alfa 2b (Pearlman 2007; Buti 2010), two trials used peginterferon alfa‐2a (Liu 2011; Lee 2012), and in the fifth trial peginterferon alfa‐2a or ‐2b was given (Mangia 2008). Three trials defined slow responders according to our second definition, tat is, participants with detectable HCV RNA after four weeks of treatment (participants without a rapid virological response (RVR)) (Berg 2006; Sanchez‐Tapias 2006; Liu 2011). The first two trials were conducted before the era of high ribavirin dose and used lower doses of ribavirin (800 mg/d) and the third trial used 1000 mg/d to 1200 mg/d ribavirin, as mentioned. In all three trials, peginterferon alpha 2a was given to the participants.
We present the effect of the interventions for each outcome separately and for each of the definitions of slow responders. In addition, we have created 'Summary of findings' tables (Table 1; Table 2; Table 3) as these tables present the collected evidence in a succinct, transparent, and informative way (GRADEpro).
Primary outcomes
Overall mortality, HCV‐related mortality, and liver‐related morbidity were not reported by any of the included trials.
Secondary outcomes
Sustained virological response
When pooling the results of the five trials which defined slow responders according to our first definition (Pearlman 2007; Mangia 2008; Buti 2010; Liu 2011; Lee 2012), a small but significant increase in the SVR proportion was seen after extending treatment to 72 weeks (RR 1.43, 95% CI 1.07 to 1.92, P = 0.02, I2 = 8%). The risk difference between the two groups was 0.11 (95% CI 0.02 to 0.19) so the calculated number needed to treat to achieve a higher proportion of SVR (NNT) was nine, meaning that among nine participants treated for 72 weeks instead of 48 weeks, only one more will achieve SVR compared to participants treated for 48 weeks.
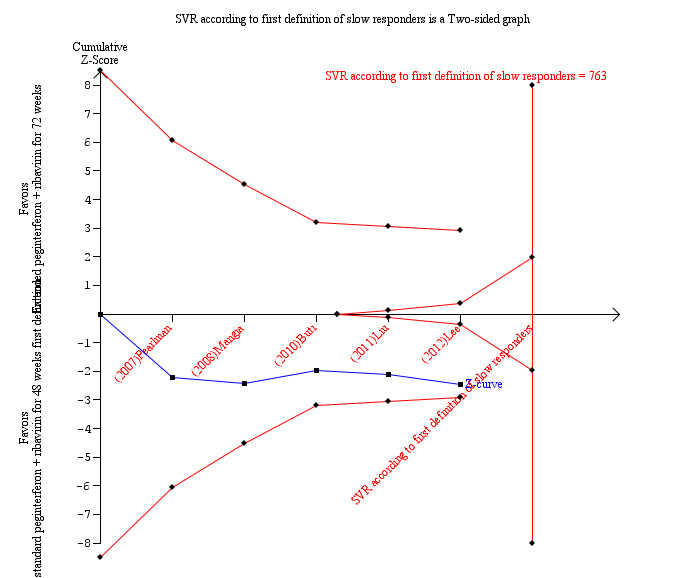
In order to assess the reliability of the conclusion drawn from the pooled results for SVR, we calculated the information size required to detect a 44% relative risk reduction in SVR (38% SVR after 72 weeks versus 27% after 48 weeks) to be 763 patients (Figure 1). Using Lan‐DeMets alpha‐spending monitoring boundaries and futility boundaries, we showed that the number of patients included in the meta‐analysis (411) is not enough for drawing any conclusion regarding SVR based on our first definition of slow responders. Neither the Lan‐Demets alpha‐spending monitoring boundaries nor the beta‐spending futility boundaries were crossed.
1.
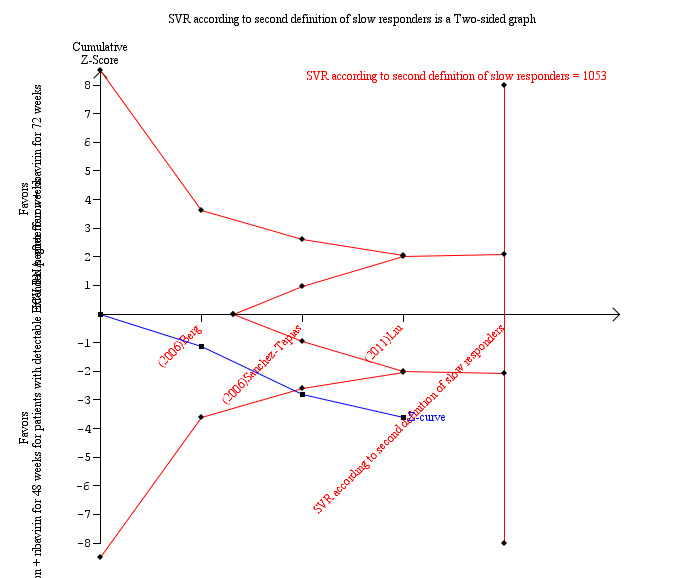
In a meta‐analysis of the three trials which defined the slow responders as patients without RVR (Berg 2006; Sanchez‐Tapias 2006; Liu 2011), we also found a statistically significant difference between the two groups (RR 1.27, 95% CI 1.07 to 1.50, P = 0.006, I2 = 38%) with a risk difference of 0.11 (95% CI 0.05 to 0.18) and calculated NNT of nine. When we calculated the required information size according to this definition in order to detect 26% relative risk reduction we found it to be 1053 participants (as appeared in the meta‐analysis: 53% SVR after 72 weeks of treatment versus 42% after 48 weeks) (Figure 4). Our meta‐analysis included 995 patients, and this number was sufficient to show a statistically significant advantage in treating slow responders based on our second definition.
4.
End of treatment response and number of participants who experienced virological relapse after treatment
The end of treatment response was not significantly different between slow responders who were treated for 48 weeks and those treated for 72 weeks. We identified this lack of difference when we performed meta‐analysis according to both definitions of slow responders (first definition: RR 0.95, 95% CI 0.80 to 1.14, P = 0.60, I2 = 49%, 3 trials; second definition: RR 0.99, 95% CI 0.91 to 1.07, P = 0.71, I2 = 0%, 3 trials). The number of participants who experienced virological relapse after treatment was found to be significantly lower in the groups of participants who had been treated for 72 weeks, in both the first definition (RR 0.59, 95% CI 0.40 to 0.86, P = 0.007, I2 = 18%) and the second definition (RR 0.59, 95% CI 0.47 to 0.73, P < 0.00001, I2 = 0%). It seems that the increased SVR in participants who were treated for 72 weeks was caused by a reduction in the number of participants who experienced relapse after treatment without improvement in the end of treatment response.
Adherence to treatment, reduction of treatment dose, and adverse events
In the meta‐analyses of these three outcomes we did not divide the trials according to the definition of slow responders since the length of treatment per se was the only variable which might influence these outcomes, and the time point in which the length of treatment was set could not have any relation to these outcomes. And yet we omitted Berg 2006 and Lee 2012 from these outcomes since the relevant outcome data in these trials were taken from the total number of included patients or patients with EVR, respectively, and not only for patients with a slow response or with pEVR. Since in Mangia 2008 there was no subgrouping of their 'variable group' according to one of our definitions of slow responders, we could not include this trial in the assessment of the three outcomes. We also excluded Sanchez‐Tapias 2006 from the meta‐analyses since in the Sanchez‐Tapias 2006 trial all HCV genotypes were grouped together without mentioning the outcomes for genotype 1 alone. The length of treatment did not affect the adherence proportion (RR 0.95, 95% CI 0.84 to 1.07, P = 0.42, I2 = 69%, 3 trials). Reduction of treatment dose and serious adverse events in the included participants were mentioned in one trial each (Pearlman 2007; Buti 2010), respectively, so we could not perform a meta‐analysis. Dose reduction was not statistically different between patients who were treated for 72 weeks and for 48 weeks (35% versus 37%, respectively) (Pearlman 2007). Serious adverse events were also not significantly different between the two groups (8.2% versus 7.0%) (Buti 2010).
Subgroup analysis
Subgroup analysis was undertaken according to the dose of ribavirin given to patients. The earliest two trials included in our meta‐analysis (Berg 2006; Sanchez‐Tapias 2006) used low‐dose ribavirin. As well as these two trials, only one trial used our second definition of slow responders (Liu 2011); we compared the results of these two earlier trials with the results of the newer one. We found that when a low dose of ribavirin was used, extension of treatment to 72 weeks did not increase the SVR (RR 1.33, 95% CI 0.94 to 1.87, P = 0.11, I2 = 69%). When the ribavirin dose was determined according to body weight, prolongation of treatment to 72 weeks increased the SVR significantly (RR 1.23, 95% CI 1.03 to 1.48, P = 0.02).
Subgroup analysis according to the type of pegylated interferon was performed for four trials which included patients according to our first definition of slow responders. In Liu 2011 and Lee 2012 patients were treated with pegylated interferon alfa‐2a, and in Buti 2010 and Pearlman 2007 patients were treated with pegylated interferon alfa‐2b. The Mangia 2008 trial included patients treated with both drugs. Since the number of patients in each subgroup was low, no significant difference in SVR was observed between patients treated for 72 weeks and 48 weeks in each group. All the trials that used the second definition of slow responders used pegylated interferon alfa‐2a.
All included trials published their results according to an intention‐to‐treat (ITT) analysis. However, following the methods sections, two of the trials (Buti 2010; Liu 2011) also performed a 'per‐protocol' analysis. Only Liu 2011 mentioned the SVR according to that analysis (in Buti 2010 the results of the 'per protocol' analysis were mentioned only for the subgroup of adhering patients). A statistically significant difference was found in the 'per protocol' analysis by Liu 2011; with the same magnitude as with the ITT analysis (P = 0.03). Another trial (Sanchez‐Tapias 2006) mentioned a 'per‐protocol' analysis of participants with SVR without mentioning the actual numbers. Extending the treatment period to 72 weeks was associated with an increased SVR in the ITT analysis. Since the trials' definitions of slow responders varied, we could not perform a meta‐analysis. We have asked the contact authors of the remaining trials about per‐protocol analysis; unfortunately we have not received any additional information.
We intended to perform a subgroup analysis according to the quality of the methodology of the included trials. Since all our included trials were defined as having a high risk of bias, we could not undertake this subgroup analysis.
Discussion
The standard of care for chronic HCV genotype 1 infected patients is 48 weeks of treatment with peginterferon plus ribavirin (Ghany 2009). With this regimen, only 40% to 52% of patients will attain SVR (Manns 2001; Fried 2002; Hadziyannis 2004; McHutchison 2009; Awad 2010). One of the strategies to improve the SVR is to refine or individualise the length of treatment according to the virological response after the first four or 12 weeks. Patients who after 12 weeks of treatment with peginterferon and ribavirin have not achieved at least a 2 log reduction in their HCV RNA level have negligible chance of achieving a SVR (Davis 2003; Ferenci 2005). Patients with detectable viraemia after four weeks of treatment, as well as patients with ≥ 2 log reduction in their HCV RNA level after 12 weeks of treatment and who still have detectable viral load have a higher probability of relapse after the standard 48 weeks of treatment, suggesting that this treatment duration may not be sufficient (Sanchez‐Tapias 2006).
Several randomised trials have been performed in order to establish whether prolongation of treatment to 72 weeks in slow responders improves the SVR. However, these trials used different definitions for slow responders. In our meta‐analysis we extracted the results of these trials after choosing the two common definitions and we meta‐analysed the results separately for each definition. Our first definition required detectable HCV RNA after 12 weeks of treatment with ≥ 2 log viral reduction and undetectable HCV RNA after 24 weeks of treatment. The second definition was based on detectable HCV RNA after four weeks of treatment without mentioning HCV RNA status after 12 or 24 weeks. We found four trials compatible with our first definition, two compatible with the second definition, and one trial which defined the patients according to both definitions (Liu 2011). Extension of the treatment period to 72 weeks seemed to increase the SVR according to the two definitions (RR 1.43, 95% CI 1.07 to 1.92, P = 0.02, I2 = 8%; and RR 1.27, 95% CI 1.07 to 1.50, P = 0.006, I2 = 38%). This effect is susceptible to both risks of systematic errors ('bias') and risks of random errors ('play of chance'). The end of treatment response was not different for the two treatment groups, but the number of patients who relapsed was higher in the 48‐week group than in the 72‐week group. There was no significant difference in the occurrence of severe adverse events in the two intervention groups. Reduction of treatment dose episodes was not increased in the 72‐week group, and adherence proportions were the same (RR 0.95, 95% CI 0.84 to 1.07, P = 0.42, I2 = 69%).
Drusano 2004 hypothesised in a post hoc analysis of data from a phase III trial of peginterferon‐alfa‐2a and ribavirin that the longer the serum HCV RNA remained undetectable during treatment, the higher the probability of a SVR. They conclude that continuous absence of detectable HCV RNA in the serum for 36 weeks is needed in HCV genotype 1 infected patients in order to achieve a SVR of 90%. This claim motivated investigators to offer patients who were defined as slow responders a prolonged course of treatment, for 72 weeks. This idea was successfully tried before in the interferon era, in a randomised trial that compared interferon monotherapy of 3,000,000 IU three times weekly for 18 months with other regimens lasting a shorter time or containing lower doses (Poynard 1995). The SVR was increased and the number of patients who experienced relapse after treatment decreased in the group of patients who received 18‐month interferon and ribavirin treatment compared to six‐month treatment with both drugs. Buti 2003 showed, for the first time, in a group of nine slow responders that administration of peginterferon‐alfa‐2b (1 µg/kg/week) and ribavirin (800 mg/d) attained a SVR in seven patients (77.7%) after 72 weeks of treatment. However, when treatment was extended to 72 weeks in a group of chronic HCV genotype 1 infected patients who were not only slow responders, no significant difference was found in the SVR compared with participants who were treated for 48 weeks (Berg 2006). The 72‐week treatment was demonstrated to be significantly more effective (29% SVR versus 17%, OR of 2.02) in a subgroup of participants with no early virological response based on HCV RNA status after 12 weeks of treatment. However, since their HCV RNA status at week 24 and the extent of the reduction in viral load at week 12 were not mentioned, we could not include this subgroup in our meta‐analysis. We did include in the meta‐analysis a subgroup of participants who fitted our second definition (absence of RVR at week four). However, in this subgroup we found no statistically significant difference between the 72‐week treatment groups and the 48‐week treatment groups (49% SVR in the 72‐week group versus 44% in the 48‐week group, P = 0.26).
Two other randomised trials comparing treatment with peginterferon and ribavirin for 72 weeks with treatment for 48 weeks were published between 2006 and 2007 (Sanchez‐Tapias 2006;Pearlman 2007). Only slow responders were included in both trials. A higher SVR was reported in participants who were treated for 72 weeks (see Data and analyses). Unfortunately, the results of Mangia 2008 and Buti 2010 challenged these results and showed only non‐significant increases in SVR in the 72‐week groups (see Data and analyses).
In order to meta‐analyse the results of the aforementioned randomised trials, we decided to group the trials according to their definitions of slow responders. The results of our meta‐analysis show that extension of the treatment period to 72 weeks seemed to increase the SVR according to both definitions of slow responders (RR 1.43, 95% CI 1.07 to 1.92, P = 0.02, I2 = 8%; RR 1.27, 95% CI 1.07 to 1.50, P = 0.006, I2 = 38%), respectively. Accordingly, the time point for a decision on the extension of treatment can be at four or 12 weeks of treatment, but at these time points the strategies improve the SVR minimally, with a NNT of nine.
Unfortunately, none of our included trials have reported on mortality and liver‐related morbidity, and in all of them SVR proportion was chosen as the primary outcome. The practical reason for this decision is probably the time limit of the primary trials. While SVR occurs exactly 24 weeks after the end of treatment, clinical outcomes such as mortality and liver‐related morbidity may not be seen for many years. Although there are data to support a positive relationship between SVR and clinical parameters (Abergel 2004; Morgan 2010; Innes 2011), other studies did not succeed in validating SVR as a prognostic surrogate marker for a long‐term clinical outcome (Brok 2010). Indeed, a recent study (DiBisceglie 2011) showed that the all‐cause mortality of the patients in the treatment arm in the HALT‐C cohort was higher than that in the control arm in spite of a high proportion of SVR in the treatment arm (albeit a very low 3.5%). This uncertainty regarding the clinical and prognostic meaning of SVR precluded us from defining it as a primary outcome. By deciding this we followed the Cochrane policy demanding that surrogate outcomes can no longer be employed as primary outcomes. This change in the Cochrane policy necessitated us to make our change from the approved protocol.
In order to refine our results, we tried to undertake subgroup analyses. We compared two trials that used low dose ribavirin (Berg 2006; Sanchez‐Tapias 2006) to the one trial which defined slow responders in the same way as those two trials and used a weight‐based ribavirin dose (Liu 2011). We found that when a low dose of ribavirin was used, extension of treatment to 72 weeks did not increase the SVR (RR 1.33 CI, 95% 0.94 to 1.87, P = 0.11, I2 = 69%). However, when the ribavirin dose was determined according to body weight, prolongation of treatment to 72 weeks increased the SVR (RR 1.23, CI 95% 1.03 to 1.48, P = 0.02, I2 = 38%). This result shows that extension of treatment to 72 weeks would be successful in increasing the SVR proportions, that appropriate dose of ribavirin should be used, and that the importance of adequate ribavirin treatment should be emphasised. Subgroup analysis according to the type of peginterferon did not demonstrate a significant difference in SVR between patients treated for 72 weeks and 48 weeks in each subgroup.
Trial sequential analysis according to the first definition of slow responders showed that our meta‐analysis did not include enough patients in order to draw the conclusion that 72 weeks of treatment is significantly better in terms of a 44% relative risk reduction. However, enough patients were recruited in trials that compared 72 weeks of treatment to the standard 48 weeks in non‐RVR patients, so we can conclude that according to that definition of slow responders extension of treatment to 72 weeks seems to cause a relative risk reduction of 22%.
Low adherence might be a major limitation of the 72‐week protocol. However, we showed that adherence proportions were similar in both the 72 and 48 week groups (RR 0.95, 95% CI 0.84 to 1.07, P = 0.42, I2 = 69%). We believe that complete adherence to the entire treatment duration may improve the SVR (unfortunately we could not show this in a per‐protocol analysis) so patient education regarding the importance of taking medications for 72 weeks and motivating them to do so are crucial steps in order to achieve better results.
Our results demonstrate that week four as well as week 12 of treatment may be used as time points for a decision regarding treatment prolongation. A recent trial challenged week 12 as the optimal decision time point and recommended week eight for decision making (Ferenci 2010). In this trial a reduction in the number of participants who experienced relapse after treatment occurred in all participants with an early virological response (EVR), including those with a complete EVR, that is, negative HCV RNA after 12 weeks of treatment. A preliminary analysis from this trial showed the advantage of week eight as a decision time point (Scherzer 2009). Mangia 2008, who compared standard treatment to individualised treatment, also showed that participants with undetectable HCV RNA at week 12 had a higher SVR when treated for 72 weeks, suggesting that decisions should be made before week 12. In non‐RVR patients, Liu 2011 also suggests week eight as a point for decisions on treatment extension.
Our systematic review has several limitations. The main shortcoming is the paucity of data. Since properly defining the term 'slow responders' was a prerequisite for obtaining methodologically accurate meta‐analyses, we had to divide the included trials into two groups according to the two definitions of slow responders. Only seven trials followed these definitions and were included in our meta‐analysis; five for the first definition and three for the second. As mentioned earlier, one trial used both definitions and was included twice. However, the subgroups included only 411 and 995 participants, respectively. This was insufficient for drawing conclusions. Our meta‐analysis included more patients than all other meta‐analyses published on this topic. Some of our meta‐analyses were based on two trials. A meta‐analysis of two trials may be limited due to a small sample size; this, of course, may limit the statistical power. Yet, if feasible, it is still better to meta‐analyse the results of two trials than to give only descriptions of the trials. Thus, whenever possible, we did conduct a meta‐analysis, even with two trials. Another drawback is the low quality of the data, as implicated in the low GRADE score that was given to most of the outcomes. The three main reasons for lowering the score are first of all the risk of bias. All included trials were unblinded and suffered from reporting bias since none of them included clinical outcomes such as overall mortality and HCV‐related morbidity or mortality. Some trials had attrition bias as well. The second is imprecision as all trials that used our first definition did not reach the required information size, so conclusions regarding SVR in this group could not be drawn. The third is indirectness, as all trials used the SVR as their primary outcome. This is a surrogate outcome that is not correlated directly to a reduction in mortality or morbidity. Another limitation is that only three trials included exclusively slow responders, fitting our inclusion criteria (Pearlman 2007; Buti 2010; Liu 2011). All of the other trials had other different objectives and also included participants who were not suitable for our meta‐analysis. Two other facts limit the meta‐analysis results for the non‐RVR group (second definition): the lower dose of ribavirin used, as discussed previously, and the absence in this group of a requirement for negative HCV RNA after 24 weeks in the definition of slow responders. According to the literature, patients who are still HCV RNA positive after 24 weeks of treatment have a very low chance of achieving a SVR, and most authorities recommend stopping treatment after 24 weeks if HCV RNA is positive (Ghany 2009). We can hypothesise that for participants with positive HCV RNA after 24 weeks of treatment the yield of extending the treatment period is much lower since the total period of absence of detectable HCV RNA in the serum will still remain short.
The inclusion of the SUCCESS (Buti 2010) and the IMPROVE (Lee 2012) trials and the analysis according to different definitions of slow responders give our results greater reliability and allow us to present the up‐to‐date literature in the most accurate way. Although all included trials were unblinded, we think that SVR, which is an objective virologic parameter, is not likely to be influenced by the lack of blinding. However, some other secondary outcomes such as adherence to treatment, reduction of treatment dose, and adverse events might have been influenced by the absence of blinding. Data in the included trials were reported fully, and all trials were probably free of selective reporting.
Newer drugs for treating chronic hepatitis C have emerged in the last couple of years (Michaels 2010). Some of these, such as telaprevir and bocepravir, show an increase in SVR when given with peginterferon and ribavirin (Hezode 2009; McHutchison 2009b). This kind of triple therapy has not been tried yet on slow responders in order to evaluate the most appropriate duration of treatment. We think that the safety, efficacy, tolerability, and cost of newer treatment combinations should be determined in this setting as well.
Authors' conclusions
Implications for practice.
This review suggests that the small extension from 48 weeks of treatment to 72 weeks in HCV genotype 1 infected slow responders, according to either of the definitions below, may increase the chance of SVR. The first definition is patients in whom HCV RNA was still detectable but decreased by ≥ 2 log after 12 weeks of treatment with peginterferon and ribavirin and became negative after 24 weeks of treatment; and the second is patients with a positive HCV RNA after four weeks of treatment. We were unable to find any data on all‐cause mortality, liver‐related mortality, and morbidity.
Taking into account the small benefit of the extended treatment in terms of SVR (NNT = 9) as opposed to the increase in the cost of treatment, inconvenience for patients, low GRADE score, and the lack of data regarding long‐term clinical outcomes, we cannot recommend routine prolongation of treatment. The fact that trial sequential analysis has shown that according to our first definition of slow responders we did not reach the required information size and we did not any breaking of the alpha spending monitoring boundaries strengthens our opinion.
Implications for research.
Among the limitations of our review are the low number of included trials as well as their high risk of bias, particularly those trials with the primary objective of extension of treatment in slow responders. More data are needed in order to recommend or reject the policy of extending the treatment period for slow responders. We suggest that further large scale randomised trials are warranted. These trials should have adequate sample sizes to try and show superiority of extended treatment and be performed following the CONSORT statement (www.consort‐statement.org). We recommend including long‐term clinical outcomes such as overall mortality and HCV‐related morbidity and mortality (in particular cirrhosis and its complications and development of HCC) in these trials besides the usually accepted surrogate outcome (that is, SVR). An acceptable dose of ribavirin should be given to the participants, and the results should be determined using either an intention‐to‐treat analysis or a 'per‐protocol' analysis. A subgroup analysis according to HCV RNA level at the beginning of the treatment or after 12 weeks might help to elucidate who the most appropriate patients for treatment prolongation are. Further research can take place using a definition based on HCV RNA status after four or eight weeks of treatment, but only for patients who become HCV RNA negative after 24 weeks, and obviously using the correct and common dosage of ribavirin. Combined triple therapy (peginterferon + ribavirin + protease inhibitor) and extension of treatment for slow responders should also be tested.
Acknowledgements
Protocol Peer reviewers: Salvo Madonia, Italy; S Petta, Italy. Contact editor: Gennaro D'Amico, Italy.
Review Peer reviewers: Salvo Madonia, Italy; S Petta, Italy. Contact editors: Gennaro D'Amico, Italy; Ronald Koretz, USA.
Appendices
Appendix 1. Search strategy for identification of studies
| Database | Time span | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | November 2011. | ('hepatitis C' OR HCV OR hepacivirus) AND ('pegylated interferon' OR peginterferon) AND (extension OR extended OR '72 weeks' OR prolonged OR prolongation) |
| The Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library | Issue 4 of 4, 2011. | #1 MeSH descriptor Hepatitis C explode all trees #2 MeSH descriptor Hepacivirus explode all trees #3 hepatitis C OR HCV OR hepacivirus #4 (#1 OR #2 OR #3) #5 MeSH descriptor Interferons explode all trees #6 pegylated interferon OR peginterferon #7 (#5 OR #6) #8 extension OR extended OR 72 weeks OR prolonged OR prolongation #9 (#4 AND #7 AND #8) |
| MEDLINE (Ovid SP) | 1948 to November 2011. | 1. exp Hepatitis C/ 2. exp Hepacivirus/ 3. (hepatitis c or HCV or hepacivirus).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 4. 1 or 2 or 3 5. exp Interferons/ 6. (pegylated interferon or peginterferon).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 7. 5 or 6 8. (extension or extended or 72 weeks or prolonged or prolongation).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 9. 4 and 7 and 8 10. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 11. 9 and 10 |
| EMBASE (Ovid SP) | 1980 to November 2011. | 1. exp hepatitis C/ 2. exp Hepatitis C virus/ 3. (hepatitis c or HCV or hepacivirus).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 4. 1 or 2 or 3 5. exp interferon/ 6. (pegylated interferon or peginterferon).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 7. 5 or 6 8. (extension or extended or 72 weeks or prolonged or prolongation).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 9. 4 and 7 and 8 10. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 11. 9 and 10 |
| Science Citation Index Expanded (http://pcs.isiknowledge.com) | 1900 to November 2011. | #6 #5 AND #4 #5 TS=(random* or blind* or placebo* or meta‐analysis) #4 #3 AND #2 AND #1 #3 TS=(extension OR extended OR '72 weeks' OR prolonged OR prolongation) #2 TS=(pegylated interferon OR peginterferon) #1 TS=(hepatitis C OR HCV OR hepacivirus) |
| LILACS | 1982 to November 2011. | (hepatitis C OR HCV OR hepacivirus) [Words] and (pegylated interferon OR peginterferon) [Words] |
Data and analyses
Comparison 1. Extended peginterferon + ribavirin for 72 weeks versus standard peginterferon + ribavirin for 48 weeks for patients with more than 2log drop after week 12 and negative HCV RNA at week 24.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sustained virological response | 5 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.07, 1.92] |
| 2 End of treatment response | 3 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.14] |
| 3 Number of patients with relapse | 3 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.40, 0.86] |
1.1. Analysis.
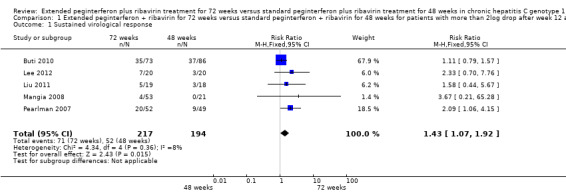
Comparison 1 Extended peginterferon + ribavirin for 72 weeks versus standard peginterferon + ribavirin for 48 weeks for patients with more than 2log drop after week 12 and negative HCV RNA at week 24, Outcome 1 Sustained virological response.
1.2. Analysis.
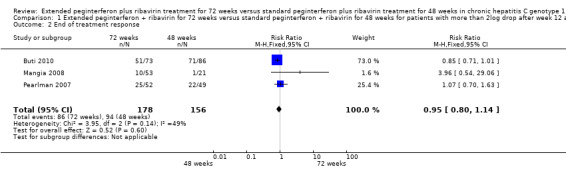
Comparison 1 Extended peginterferon + ribavirin for 72 weeks versus standard peginterferon + ribavirin for 48 weeks for patients with more than 2log drop after week 12 and negative HCV RNA at week 24, Outcome 2 End of treatment response.
1.3. Analysis.
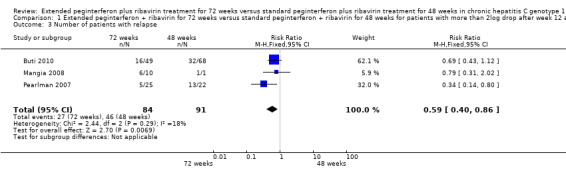
Comparison 1 Extended peginterferon + ribavirin for 72 weeks versus standard peginterferon + ribavirin for 48 weeks for patients with more than 2log drop after week 12 and negative HCV RNA at week 24, Outcome 3 Number of patients with relapse.
Comparison 2. Extended peginterferon + ribavirin for 72 weeks versus standard peginterferon + ribavirin for 48 weeks for patients with detectable HCV RNA after four weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sustained virological response | 3 | 995 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.07, 1.50] |
| 2 End of treatment response | 3 | 995 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.91, 1.07] |
| 3 Number of patients with relapse | 3 | 703 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.47, 0.73] |
2.1. Analysis.
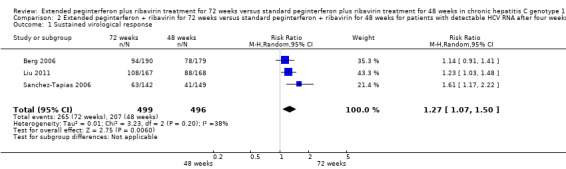
Comparison 2 Extended peginterferon + ribavirin for 72 weeks versus standard peginterferon + ribavirin for 48 weeks for patients with detectable HCV RNA after four weeks, Outcome 1 Sustained virological response.
2.2. Analysis.
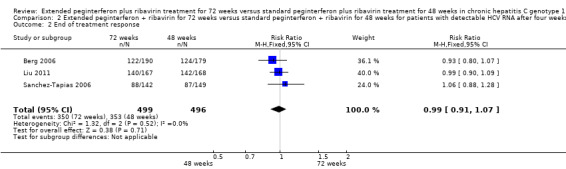
Comparison 2 Extended peginterferon + ribavirin for 72 weeks versus standard peginterferon + ribavirin for 48 weeks for patients with detectable HCV RNA after four weeks, Outcome 2 End of treatment response.
2.3. Analysis.
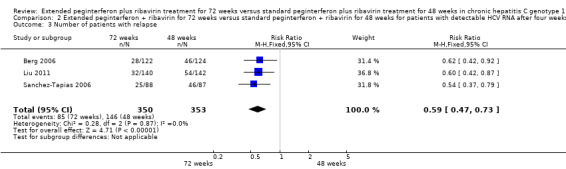
Comparison 2 Extended peginterferon + ribavirin for 72 weeks versus standard peginterferon + ribavirin for 48 weeks for patients with detectable HCV RNA after four weeks, Outcome 3 Number of patients with relapse.
Comparison 3. Slow responders according to the two definitions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adherence to treatment | 3 | 553 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.84, 1.07] |
3.1. Analysis.
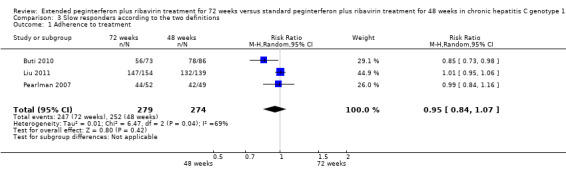
Comparison 3 Slow responders according to the two definitions, Outcome 1 Adherence to treatment.
Comparison 4. Subgroup analysis according to the ribavirin dose for patients with detectable HCV RNA after four weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sustained virological response | 3 | 995 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.07, 1.50] |
| 1.1 Low dose ribavirin | 2 | 660 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.94, 1.87] |
| 1.2 Weight based ribavirin dose | 1 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [1.03, 1.48] |
4.1. Analysis.
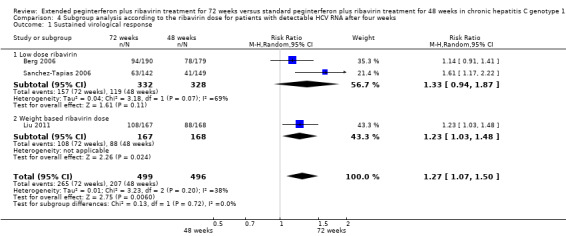
Comparison 4 Subgroup analysis according to the ribavirin dose for patients with detectable HCV RNA after four weeks, Outcome 1 Sustained virological response.
Comparison 5. Subgroup analysis according to the type of peginterferon used for patients with ≥ 2log drop after week 12 and negative HCV RNA on week 24.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sustained virological response | 4 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.05, 1.87] |
| 1.1 PEG‐IFN alfa 2a | 2 | 77 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.82, 4.67] |
| 1.2 PEG‐IFN alfa 2b | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.97, 1.80] |
5.1. Analysis.
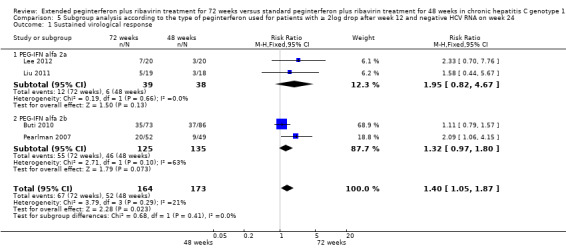
Comparison 5 Subgroup analysis according to the type of peginterferon used for patients with ≥ 2log drop after week 12 and negative HCV RNA on week 24, Outcome 1 Sustained virological response.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Berg 2006.
| Methods | Randomised clinical trial. Study design: Genotype 1 compensated chronic HCV infected naive patients were randomised to 48 weeks or 72 weeks of treatment. Follow‐up period: 24 weeks after the end of treatment. |
|
| Participants | Location: 18 centres in Germany. Time of participants selection: 12/2000 to 7/2001. Included participants in our meta‐analysis: chronic hepatitis C genotype 1 naive patients with positive HCV RNA at week 4 of treatment. No. participants: 179 in the 48‐week treatment group and 190 in the 72‐week treatment group. Mean age: no data. Males: no data. Mean BMI: no data. Liver biopsy: preformed within the preceding 24 months of trial, enrolment conforming chronic hepatitis. |
|
| Interventions | Experimental: s.c. peginterferon‐alfa‐2a 180 µg/week and p.o. ribavirin 800 mg*1/d for 72 weeks. Control: s.c. peginterferon‐alfa‐2a 180 µg/week and p.o. ribavirin 800 mg*1/d for 48 weeks. |
|
| Outcomes | Outcomes and time points considered in the review: Primary outcome: SVR. Secondary outcomes: relapse; serious adverse events; dose reduction and therapy discontinuation owing to adverse events;completed therapy (adherence). Outcomes and time points not considered in the review: breakthrough (a reappearance of hepatitis C viraemia during the treatment phase); non‐responders (patients who failed to test HCV‐RNA negative at any point during the trial); patients for whom not enough data were available for categorisation; sustained biochemical response; on‐treatment virologic response rates; evaluation of predictive parameters associated with SVR: patient’s age, ethnicity, sex, body weight, BMI, GGT, ALT, glucose, platelet count, HCV‐RNA serum concentrations, HCV subtype (1a vs 1b), presence of cirrhosis, and the relevance of early virologic response (EVR) at weeks 4 and 12. |
|
| Notes | Total of 445 participants included, data extracted for the meta‐analysis only about slow responders (according to our second definition). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomisation was performed centrally... in fixed blocks of 4". |
| Allocation concealment (selection bias) | Low risk | "randomisation was performed centrally...". |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were not available data about number of slow responders that completed therapy, number of slow responders in whom treatment reduction was needed or therapy was discontinued due to adverse events, and the rate of serious adverse events in slow responders. |
| Selective reporting (reporting bias) | High risk | No clinical data on mortality were reported. |
| Other bias | Unclear risk | Sponsored by industry. |
Buti 2010.
| Methods | Randomised clinical trial. Study design: Genotype 1 compensated chronic HCV infected naive patients were treated for an initial 12‐week period and further treatment duration was set in accordance with week 12 HCV RNA levels. Patients with detectable HCV RNA and a ≥2 log drop at week 12 continued to receive the same treatment regimen until week 24. At week 24, patients with detectable HCV RNA were withdrawn from treatment. Those patients with undetectable HCV RNA at week 24 were considered slow responders and randomised to treatment for an additional 24 weeks or 48 weeks. Duration of follow‐up: 24 weeks after the end of treatment. |
|
| Participants | Location: 118 centres in Europe, Canada, Puerto Rico, and Israel. Time of participant selection: December 2004 to May 2008. Included participants in our meta‐analysis: chronic hepatitis C genotype 1 naive patients with detectable HCV‐RNA and ≥ 2 log viral reduction at week 12 and undetectable HCV RNA at week 24. No. participants: 86 in the 48‐week treatment group and 73 in the 72‐week treatment group. Mean age: 44.5 (± 9.9) years in the 48‐week treatment group and 46.5 (± 11.6) years in the 72‐week treatment group. Males: 52/86 in the 48‐week treatment group and 46/73 in the 72‐week treatment group. Mean weight (kg): 78.0 (± 15.2) in the 48‐week treatment group and 77.5 (± 16.6) in the 72‐week treatment group. Liver biopsy: preformed within the preceding 18 months of trial enrolment conforming chronic hepatitis. |
|
| Interventions | Experimental: s.c. peginterferon‐alfa‐2b 1.5 mcg/kg/week and p.o. ribavirin 800 to 1400 mg*1/d (weight depended) for 72 weeks. Control: s.c. peginterferon‐alfa‐2b 1.5 mcg/kg/week and p.o. ribavirin 800 to 1400 mg*1/d (weight depended) for 48 weeks. |
|
| Outcomes | Outcomes and time points considered in the review Primary outcome: SVR. Secondary outcomes: relapse; adverse events; completed therapy (adherence); therapy discontinuation due to adverse events. Outcomes and time points not considered in the review: SVR among participants who took at least 80% of the planned dose of each of the trial medications for at least 80% of the assigned treatment duration; viral load of slow responders; variables associated with SVR: age, weight, week 12 HCV RNA levels, week 8 viral load. |
|
| Notes | A total of 1428 naive patients with chronic HCV genotype 1 were enrolled. One did not receive the drugs. 452 (31.7%) were nonresponders or had positive HCV RNA at week 24, and were excluded. 816 (57.5%) had complete EVR, or negative HCV RNA at week 12, and were excluded as well. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed independent of sponsor and investigators via a data fax response system using a computer‐generated randomisation scheme in blocks of 4 (Everest Clinical Research Services, Ontario, Canada). trial groups were stratified by center". |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed independent of sponsor and investigators via a data fax response system using a computer‐generated randomisation scheme in blocks of 4....Study groups were stratified by center". |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were given about all included and randomised patients. |
| Selective reporting (reporting bias) | High risk | No clinical data on mortality were reported. |
| Other bias | Unclear risk | Sponsored by industry. |
Lee 2012.
| Methods | Randomised clinical trial. Study design: Previously untreated patients with HCV genotypes, other than 2 or 3, initiated therapy. HCV‐RNA‐negative patients at week 4 were randomised to 24 or 48 weeks of treatment; those negative at week 8 were randomised to 36 or 48 weeks; and those who were negative or had a ≥2‐log drop at week 12 were randomised to 72 or 48 weeks. Only the last group was included in our meta‐analysis. Follow‐up period: 24 weeks after the end of treatment. |
|
| Participants | Location: 24 centres in Canada. Time of participant selection: June 2007 to July 2008. Included participants in our meta‐analysis: chronic hepatitis C genotype 1 naive patients with detectable HCV‐RNA and ≥ 2 log viral reduction at week 12 and undetectable HCV RNA at week 24. No. participants: 20 in the 48‐week treatment group and 20 in the 72‐week treatment group. Mean age: not reported for these groups. Males: not reported for these groups. Mean weight (kg): not reported for these groups. Liver biopsy: not obligatory. |
|
| Interventions | Experimental: s.c. peginterferon‐alfa‐2a (180 mcg/week) plus ribavirin (1,000‐1,400 mg/day (weight depended) for 72 weeks. Control: s.c. peginterferon‐alfa‐2a (180 mcg/week) plus ribavirin (1,000‐1,400 mg/day (weight depended) for 72 weeks. |
|
| Outcomes | Outcomes and time points considered in the review: Primary outcome: SVR. Secondary outcomes: not reported for our groups. Outcomes and time points not considered in the review: not reported for our groups. |
|
| Notes | 1. The study was terminated in July 2008 after several months of lagging enrolment, most probably due to the use of the newer direct anti viral agents in competing clinical trials. 2. As mentioned earlier, the patients included in our meta‐analysis are only one subgroup of the study. After personal communication with Dr Lee we received the data of the G1 patients with partial early viral response (EVR). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Three independent sets of randomisation numbers were generated by computer, one for each randomisation point by Syreon Corporation, Vancuver". |
| Allocation concealment (selection bias) | Low risk | "The sponsor and investigators did not have access to the randomisation sequence or assignments". |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data were given about all included and randomised patients. However, from the whole trial population, 34% did not complete the treatment and the follow‐up period. More than half of the patients that withdrew consent had been randomised to the 72 weeks group. Data about compliance and AEs were given to the whole group of patients with EVR and not given separately to our included patients whom are a subgroup with partial EVR. |
| Selective reporting (reporting bias) | High risk | No clinical data on mortality were reported. |
| Other bias | Unclear risk | The trial was terminated earlier than expected and during its last months only few patients were enrolled. It might be that those patients could not be enrolled in clinical trials that used direct antivirals. The trial was sponsored by the industry. However, the sponsors did not have veto power over the content of the manuscript or the decision to publish. |
Liu 2011.
| Methods | Randomised clinical trial. Study design: Treatment‐nave HCV genotype 1 patients who failed to achieve rapid virologic response (RVR), defined as undetectable HCV RNA at week 4 of therapy, were randomised to receive either 48 or 72 weeks of peginterferon alfa‐2a plus ribavirin. Serial HCV RNA levels were evaluated at weeks 8, 12, 24, at end‐of‐treatment and at week 24 off therapy. Duration of follow‐up: 24 weeks after the end of treatment. |
|
| Participants | Location: 6 academic centres in Taiwan. Time of participants selection: 02/2007‐06/2010. Included participants in our meta‐analysis: chronic hepatitis C genotype 1 naive patients with positive HCV RNA at week 4 of treatment. No. participants: 168 in the 48‐week treatment group and 167 in the 72‐week treatment group. Age > 65 years: 59 in the 48‐week treatment group and 54 in the 72‐week treatment group. Males: 95 in the 48‐week treatment group and 91 in the 72‐week treatment group. Mean BMI: 25.9 ± 3.8 in the 48‐week treatment group and 25.9 ± 3.9 in the 72‐week treatment group. Liver biopsy: was mandatory (within 3 months before enrolment). |
|
| Interventions | Experimental: s.c. peginterferon alfa‐2a (180 mcg/week) plus ribavirin (1000 to 1200 mg/day (weight depended) for 72 weeks. Control: s.c. peginterferon alfa‐2a (180 µg/week) plus ribavirin (1000 to 1200 mg/day (weight depended) for 72 weeks. |
|
| Outcomes | Outcomes and time points considered in the review: Primary outcome: SVR. Secondary outcomes: relapse. Outcomes and time points not considered in the review: SVR among different IL‐28 status; weeks 8 and 12 HCV RNA levels; Baseline factors predictive of SVR and Wk‐8R. |
|
| Notes | Of the 168 patients in the 48‐week group, 147 completed the treatment period and 166 completed the follow‐up period. 11 patients were non‐response at wk 24, three had viral breakthrough at wk 24 and 7 discontinue treatment due to AEs. Of the 167 patients in the 48‐week group, 132 completed the treatment period and 163 completed the follow‐up period. 12 patients were non‐response at wk 24, another 10 were non‐response at wk 24, six had viral breakthrough at wk 24 and 7 discontinue treatment due to AEs (five before wk 48 and two after). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Central. |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were given about all included and randomised patients. |
| Selective reporting (reporting bias) | High risk | No clinical data on mortality were reported. |
| Other bias | Low risk | The trial appears to be free of other sources of bias. |
Mangia 2008.
| Methods | Randomised clinical trial. Study design: Chronic HCV G1 patients were randomised to receive therapy for 48 weeks (standard group) or for an individualized duration, based on the time when HCV RNA first became undetectable (variable group). In the variable group, patients who were first HCV‐RNA negative at week 4 were treated for a total of 24 weeks (not included in this meta‐analysis), whereas those who were first HCV‐RNA negative at weeks 8 and 12 were treated for a total of 48 and 72 weeks, respectively (not included as well). Patients with a 2 log drop in HCV RNA levels at week 12 were treated for 48 and 72 weeks in the standard or variable group, respectively. All patients viraemic at week 24 were considered nonresponders and excluded from further treatment. Duration of follow‐up: 24 weeks after the end of treatment. |
|
| Participants | Location: 11 centres in the south of Italy. Time of participant selection: 6/2004 to 12/2005. Included participants in our meta analysis: chronic hepatitis C genotype 1 naive patients with detectable HCV‐RNA and ≥ 2 log viral reduction at week 12 and undetectable HCV RNA at week 24. No. participants: 21 in the 48‐week treatment group and 53 in the 72‐week treatment group. Mean age: no data. Males: no data. Mean BMI: no data. Liver biopsy: was not mandatory for the patients to be enrolled. |
|
| Interventions | Experimental: s.c. peginterferon alfa‐2a 180 mcg/week or s.c. peginterferon alfa‐2b 1.5 µg/kg/week and p.o. ribavirin 1000/1200 mg*1/d (weight dependent) for individualised duration (variable group). In the trial, the variable group treatment's duration periods were 24, 48, and 72 weeks for HCV‐RNA negative at week 4, 8, and 12 participants, respectively. Slow responders, defined as patients with detectable HCV RNA and ≥ 2 log viral reduction at week 12 and undetectable HCV RNA at week 24 were treated for 72 weeks and were included in our meta‐analysis. Control: s.c. peginterferon alfa‐2a 180 µg/week or s.c. peginterferon alfa‐2b 1.5 µg/kg/week and p.o. ribavirin 1000/1200 mg*1/d (weight depended) for 48 weeks. |
|
| Outcomes | Outcomes and time points considered in the review: Primary outcome: SVR. Secondary outcomes: treatment failures: relapse, nonresponse, discontinuation. Outcomes and time points not considered in the review: ascertaining SVR according to on‐treatment virologic response as determined by qualitative HCV RNA assays at weeks 4, 8, and 12 and comparison of these rates in the standard and variable groups; evaluation of predictors of 12‐week virologic response and SVR‐associated parameters: age, gender, body weight, BMI, serum alanine transferase level, serum HCV RNA level, HCV genotype, stage of fibrosis and grade of inflammation. |
|
| Notes | A total of 696 naive patients with chronic HCV genotype 1 were enrolled. The standard group included 237 participants : 62 had RVR (HCV RNA negative at week 4), 64 were HCV‐RNA negative at week 8, 21 were HCV‐RNA negative at week 12 and 21 were slow responders based on HCV RNA results at week 12 and 24. 69 participants had less than 2 log viral reduction at week 12 and therefore treatment was discontinued. The variable group included 459 patients: 123 were RVR, 128 were HCV‐RNA negative at week 8, 52 were HCV‐RNA negative at week 12 and 53 were slow responders. 103 participants had less than 2 log viral reduction at week 12 and with discontinuation of treatment . | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated list of randomisation was sent to each participating center, where, at the study entry, patients were allocated 1:2 to the standard or variable groups in blocks of five." |
| Allocation concealment (selection bias) | Unclear risk | Participants were allocated in blocks of five, therefore investigators could predict allocation for some of the participants. |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were not available data about number of slow responders that completed therapy, about the number of slow responders that treatment reduction was needed or therapy was discontinued due to adverse events, and about the rate of serious adverse events in slow responders. |
| Selective reporting (reporting bias) | High risk | No clinical data on mortality were reported. |
| Other bias | Low risk | The trial appears to be free of other sources of bias. |
Pearlman 2007.
| Methods | Randomised clinical trial. Study design: Chronic HCV G1 patients were defined as slow responders if they had at least 2‐lo decrement in baseline serum HCV RNA, albeit detectable viraemia at 12 weeks and undetectable serum HCV RNA at 24 weeks. After 24 weeks they were randomised to continue therapy for an additional 24 weeks or 48 weeks. Duration of follow‐up: 24 weeks after the end of treatment. |
|
| Participants | Location: Atlanta Medical Center for Hepatitis C and Sheffield Health Center ‐ ambulatory clinics in downtown Atlanta, GA, USA. Time of participant selection: 6/2003 ‐ 9/2005 Included participants in our meta‐analysis: chronic hepatitis C genotype 1 naive patients with detectable HCV RNA and ≥ 2 log viral reduction at week 12 and undetectable HCV RNA at week 24. No. participants: 49 in the 48‐week treatment group and 52 in the 72‐week treatment group. Mean age: 56 years in the 48‐week treatment group and 54 years in the 72‐week treatment group. Males: 33/49 in the 48‐week treatment group and 34/52 in the 72‐week treatment group. Mean BMI: 28.9 in the 48‐week treatment group and 28.8 in the 72‐week treatment group. Liver biopsy: preformed within the past two years, consistent with chronic hepatitis. |
|
| Interventions | Experimental: s.c. peginterferon alfa‐2b 1.5 µg/kg/week and p.o. ribavirin 800 to 1400 (weight depended) mg*1/d for 72 weeks. Control: s.c. peginterferon alfa‐2b 1.5 µg/kg/week and p.o. ribavirin 800 to 1400 (weight depended) mg*1/d for 48 weeks. |
|
| Outcomes | Outcomes and time points considered in the review Primary outcome: SVR. Secondary outcomes: relapse rate. Additional data that were extracted: dose reduction or therapy discontinuation due to adverse events; serious adverse events; adherence. Outcomes and time points not considered in the review: none. |
|
| Notes | A total of 361 naive participants with chronic HCV genotype 1 were treated, 112 of them matched the definition of slow responders and were included in this trial. 'Eleven slow responders either declined to participate in the study or were ineligible'. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated, but there is Insufficient information about the sequence generation process. |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised to 1 of 2 study treatment durations at a ratio of 1:1 without stratification, and randomisation groups were concealed until after patients consented to participate and interventions were assigned." |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups: Seven participants in the standard duration arm (14%) and eight participants in the extended duration arm (15%) discontinued treatment, but all the discontinuations occurred before week 48 in both groups. Data about major adverse events were given. |
| Selective reporting (reporting bias) | High risk | No clinical data on mortality were reported. |
| Other bias | Low risk | The trial appears to be free of other sources of bias. |
Sanchez‐Tapias 2006.
| Methods | Randomised clinical trial. Study design: patients with chronic HCV infection without RVR (after 4 weeks of treatment) were randomised to continue therapy for 44 additional weeks or for 68 additional weeks. Duration of follow‐up: 24 weeks after the end of treatment. |
|
| Participants | Location: 28 specialist hepatology centres in Spain. Time of participants selection: 4‐9/2001. Included participants in our meta‐analysis: chronic hepatitis C genotype 1 naive patients with detectable HCV RNA at week 4. No. participants: 149 in the 48‐week treatment group and 142 in the 72‐week treatment group. Mean age: no data. Males: no data. Mean BMI: no data Liver biopsy: preformed within the past two years consistent with chronic hepatitis. |
|
| Interventions | Experimental: s.c. peginterferon alfa‐2a 180 µg/week and p.o. ribavirin 800 mg*1/d for 72 weeks. Control: s.c. peginterferon alfa‐2a 180 µg/week and p.o. ribavirin 800 mg*1/d for 48 weeks. |
|
| Outcomes | Outcomes and time points considered in the review Primary outcome: SVR. Additional data that were extracted: end of treatment response; relapse rate. Outcomes and time points not considered in the review: the influence of baseline viraemia less than 800,000 IU/mL on SVR; predictive value of the on‐treatment virologic response (defined as an undetectable serum HCV‐RNA level or a 2 log10 or more decrease from baseline in serum HCV RNA levels at weeks 12 or 24 of treatment). Safety and adherence data about participants from all four genotypes. |
|
| Notes | A total of 510 chronic HCV naive patients (genotype 1, 2, 3, 4) underwent evaluation at week 4 of treatment: 326 were slow responders/non RVR and were randomised to 48 or 72 weeks' treatment, 184 were RVR and were randomised to 24 or 48 weeks treatment. Among the slow responders 291 were genotype 1 patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | participants were randomly allocated. "The computerized randomisation list (by blocks of 4 patients, without stratification) was generated automatically by SAS software". |
| Allocation concealment (selection bias) | Low risk | "PRA‐Staticon International S.L. (Madrid, Spain) managed the centralized randomisation procedure, maintained the randomisation list, and communicated with study centers by phone and confirmed by fax". |
| Blinding (performance bias and detection bias) All outcomes | High risk | There was no blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was not isolated available data about genotype 1 participants regarding number of slow responders that completed therapy, number of slow responders in which treatment reduction was needed or therapy was discontinued due to adverse events, and about the rate of serious adverse events in slow responders. |
| Selective reporting (reporting bias) | High risk | No clinical data on mortality were reported. |
| Other bias | Unclear risk | The authors used fixed blocked randomisation in an unblinded trial, so it might be possible to predict future assignments. Sponsored by the industry. |
EVR = early virological response HCV = hepatitis C virus p.o. = peroral RNA = ribonucleic acid s.c. = subcutaneous SVR = sustained virological response
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ferenci 2010 | Data were extracted about either slow responders with genotype 1 and 4 or about genotype 1 patients with partial and complete early response. Data about genotype 1 slow responders could not be isolated. |
| Ide 2009 | Treatment duration was not 72 weeks; treatment was administered for 44 weeks after participants became negative for HCV RNA (total duration 48 to 68 weeks). |
| Miyase 2010 | The participants had positive HCV RNA on week 8 and negative on week 12, so they could match only to our second definition of slow responders (as a subgroup of non RVR participants). However, 9/40 participants were non‐naive and had received interferon treatment earlier. |
| Nagaki 2009 | Some of the participants that were included were non‐naive chronic HCV patients. Treatment was extended to 96 weeks. |
HCV = hepatitis C virus RNA = ribonucleic acid SVR = sustained virological response
Characteristics of ongoing studies [ordered by study ID]
Sarrazin 2010.
| Trial name or title | Completely individualized treatment durations (24, 30, 36, 42, 48, 60 or 72 weeks) with peginterferon‐alfa‐2b and ribavirin in HCV genotype 1‐infected patients (INDIV‐2 study). Journal of Hepatology 2010;52:S25‐S26. |
| Methods | Multi‐centre controlled prospective open label trial. |
| Participants | 398 treatment‐naive HCV genotype 1 patients in the individualised group and 225 controls. |
| Interventions | Patients received individualised treatment durations for 24, 30, 36, 42, 48, 60, or 72 weeks according to low or high baseline viral load (LVL/HVL, cut‐off 800.000 IU/ml) and undetectable HCV RNA at week 4, 6, 8, 12, 24, and 30. |
| Outcomes | SVR. |
| Starting date | |
| Contact information | |
| Notes | Published as abstract in Journal of Hepatology 2010;52:S25‐S26. |
HCV = hepatitis C virus SVR = sustained virological response
Differences between protocol and review
While in the protocol SVR was chosen to be the primary outcome we had to change the primary outcome in the review and define overall mortality, HCV‐related mortality, and liver‐related morbidity as the primary outcomes, and SVR as a secondary outcome. The reason for this difference stems from a change in the Cochrane Hepato‐Biliary Group policy that demands that unvalidated surrogate markers cannot be primary outcome measures. Therefore, only clinical outcomes can be employed as primary outcome measures.
We did not plan the performance of 'Summary of findings' tables in our protocol as they were introduced in Cochrane reviews after its publication.
Contributions of authors
Lior Katz (LK) ‐ responsible for the reference searches, article retrieval, study inclusion and exclusion, data extraction, analysis, interpretation of results, and writing of the review. Hadar Goldvaser (HG) ‐ responsible for the reference searches, study inclusion and exclusion, data extraction, and writing of the review. Anat Gafter‐Gvili (AG) ‐ responsible for analysis, interpretation of results, and writing of the review. Ran Tur‐Kaspa (RTK) ‐ responsible for interpretation of results and writing of the review.
Declarations of interest
None known
New
References
References to studies included in this review
Berg 2006 {published data only}
- Berg T, Wagner M, Nasser S, Sarrazin C, Heintges T, Gerlach T, et al. Extended treatment duration for hepatitis C virus type 1: comparing 48 versus 72 weeks of peginterferon‐alfa‐2a plus ribavirin. Gastroenterology 2006;130:1086–97. [DOI] [PubMed] [Google Scholar]
Buti 2010 {published data only}
- Buti M, Lurie Y, Zakharova N, Blokhina N, Horban A, Teuber G, et al. Randomized trial of peginterferon alfa‐2b and ribavirin for 48 or 72 weeks in patients with HCV genotype 1 and slow virologic response. Hepatology 2010;52(4):1201‐7. [DOI] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee SS, Sherman M, Ramji A, Greenbloom S, Elkashab M, Pluta H, et al. Randomised clinical trial: the efficacy of treatment, guided by a shorter duration of response, using peginterferon alfa‐2a plus ribavirin for hepatitis C virus other than genotypes 2 or 3. Alimentary Pharmacolology and Therapuetics 2012;35(1):37‐47. [DOI] [PubMed] [Google Scholar]
Liu 2011 {published and unpublished data}
- Liu C‐H, Liang C‐C, Liu C‐J, Lin C‐L, Yang S‐S, Hsu S‐J, et al. Extended peginterferon plus ribavirin treatment for 72 weeks versus standard peginterferon plus ribavirin treatment for 48 weeks for patients with chronic hepatitis C virus genotype 1 infection having shown slow antiviral response. Hepatology International. 2011; Vol. 21st Conference of the Asian Pacific Association for the Study of the Liver, APASL 2011.
Mangia 2008 {published data only}
- Mangia A, Minerva N, Bacca D, Cozzolongo R, Ricci G, Carretta V, et al. Individualized treatment duration for hepatitis C genotype 1 patients: a randomized controlled trial. Hepatology 2008;47:43‐50. [DOI] [PubMed] [Google Scholar]
Pearlman 2007 {published data only}
- Pearlman B, Ehleben C, Saifee S. Treatment extension to 72 weeks of peginterferon and ribavirin in hepatitis C genotype 1–infected slow responders. Hepatology 2007;46:1688‐94. [DOI] [PubMed] [Google Scholar]
Sanchez‐Tapias 2006 {published data only}
- Sánchez‐Tapias J, Diado M, Escartín P, Enríquez J, Romero‐Gómez M, Bárcena R, et al. Peginterferon‐alfa2a plus ribavirin for 48 versus 72 weeks in patients with detectable hepatitis C virus RNA at week 4 of treatment. Gastroenterology 2006;131:451–60. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ferenci 2010 {published data only}
- Ferenci P, Laferl H, Scherzer TM, Maieron A, Hofer H, Stauber R, et al. Peginterferon alfa‐2a/ribavirin for 48 or 72 weeks in hepatitis C types 1and 4 patients with slow virologic response. Gastroenterology 2010;138:503‐12. [DOI] [PubMed] [Google Scholar]
Ide 2009 {published data only}
- Ide T, Hino T, Ogata K, Miyajima I, Kuwahara R, Kuhara K, et al. A randomized study of extended treatment with peginterferon α‐2b plus ribavirin based on time to HCV RNA negative – status in patients with genotype 1b chronic hepatitis C. American Journal of Gastroenterology 2009;104:70‐5. [DOI] [PubMed] [Google Scholar]
Miyase 2010 {published data only}
- Miyase S, Ogata K, Haraoka K, Morishita Y, Fujiyama S. The efficacy of prolonging treatment with peginterferon alfa‐2b and ribavirin to 72 weeks in chronic hepatitis C genotype 1 patients HCV RNA positive at week 8 but negative at week 12. Acta Hepatologica Japonica 2010;51:48‐50. [Google Scholar]
Nagaki 2009 {published data only}
- Nagaki M, Shimizu M, Sugihara J, Tomita E, Sano C, Naiki T, et al. Clinical trial: extended treatment duration of peginterferon alpha2b plus ribavirin for 72 and 96 weeks in hepatitis C genotype 1‐infected late responders. Alimentary Pharmacology and Therapeutics 2009;30:343–51. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Sarrazin 2010 {published data only}
- Sarrazin C, Schwendy S, Möller B, Dikopoulos N, Buggisch P, Encke J, et al. Completely individualized treatment durations (24, 30, 36, 42, 48, 60 or 72 weeks) with peginterferon‐alfa‐2b and ribavirin in HCV genotype 1‐infected patients (INDIV‐2 study). Journal of Hepatology 2010;52:S25‐S26. [Google Scholar]
Additional references
Abergel 2004
- Abergel A, Darcha C, Chevallier M, Ughetto S, Henquell C, Pol S, et al. Histological response in patients treated by interferon plus ribavirin for hepatitis C virus‐related severe fibrosis. European Journal of Gastroenterology and Hepatology 2004;16(11):1219‐27. [DOI] [PubMed] [Google Scholar]
Alavian 2011
- Alavian SM, Tabatabaei SV, Behnava B, Mahboobi N. Optimal duration of treatment for HCV genotype 1 infection in slow responders: A meta‐analysis. Hepatitis Monthly 2011;11(8):612‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Awad 2010
- Awad T, Thorlund K, Hauser G, Stimac D, Mabrouk M, Gluud C. Peginterferon alpha‐2a is associated with higher sustained virological response than peginterferon alfa‐2b in chronic hepatitis C: systematic review of randomized trials. Hepatology 2010;51:1176‐84. [DOI] [PubMed] [Google Scholar]
Berg 2009
- Berg T, Weich V, Teuber G, Klinker H, Moller B, Rasenack J, et al. Individualized treatment strategy according to early viral kinetics in hepatitis C virus type 1‐infected patients. Hepatology 2009;50:369‐77. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
Brok 2010
- Brok J, Gluud LL, Gluud C. Ribavirin plus interferon versus interferon for chronic hepatitis C. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD005445.pub2] [DOI] [PubMed] [Google Scholar]
Buti 2003
- Buti M, Valdes A, Sanchez‐Avila F, Esteban R, Lurie Y. Extending combination therapy with peginterferon alfa‐2b plus ribavirin for genotype 1chronic hepatitis C late responders: a report of 9 cases. Hepatology 2003;37:1226‐7. [DOI] [PubMed] [Google Scholar]
CTU 2011
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. http://ctu.dk/tsa/ (accessed January 2011).
Davis 2003
- Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa‐2b plus ribavirin in patients with chronic hepatitis C. Hepatology 2003;38:645‐52. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Practical aspects in data monitoring: a brief review. Statistics in Medicine 1987;6(7):753‐60. [DOI] [PubMed] [Google Scholar]
Di Martino 2011
- Martino V, Richou C, Cervoni JP, Sanchez‐Tapias JM, Jensen DM, Mangia A, et al. Response‐guided peg‐interferon plus ribavirin treatment duration in chronic hepatitis C: Meta‐analyses of randomized, controlled trials and implications for the future. Hepatology 2011;54(3):789‐800. [DOI] [PubMed] [Google Scholar]
DiBisceglie 2011
- Bisceglie AM, Stoddard AM, Dienstag JL, Shiffman ML, Seeff LB, Bonkovsky HL, et al. Excess mortality in patients with advanced chronic hepatitis C treated with long‐term peginterferon. Hepatology 2011;53:1100‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Drusano 2004
- Drusano GL, Preston SL. A 48‐week duration of therapy with pegylated interferon alpha 2b plus ribavirin may be too short to maximize long‐term response among patients infected with genotype‐1 hepatitis C virus. Journal of Infectious Diseases 2004;189:964‐70. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Serag 2003
- El‐Serag H, Davila J, Petersen N, McGlynn K. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Annals of Internal Medicine 2003;139:817‐23. [DOI] [PubMed] [Google Scholar]
Farnik 2010
- Farnik H, Lange CM, Sarrazin C, Kronenberger B, Zeuzem S, Herrmann E. Meta‐analysis shows extended therapy improves response of patients with chronic hepatitis C virus genotype 1 infection. Clinical Gastroenterology and Hepatology 2010;8(10):884‐90. [DOI] [PubMed] [Google Scholar]
Ferenci 2005
- Ferenci P, Fried MW, Shiffman ML, Smith CI, Marinos G, Goncales FL Jr, et al. Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa‐2a (40 KD)/ribavirin. Journal of Hepatology 2005;43:425‐33. [DOI] [PubMed] [Google Scholar]
Fried 2002
- Fried MW, Shiffman M, Reddy KR, Smith C, Marinos G, Goncales FL, et al. Pefintereron alfa‐2a plus ribavirin for chronic hepatitis C virus infection. New England Journal of Medicine 2002;347:975‐82. [DOI] [PubMed] [Google Scholar]
Gamble 2005
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58(6):579‐88. [DOI] [PubMed] [Google Scholar]
Gevers 2011
- Gevers TJ, Slavenburg S, Oijen MG, Drenth JP. Treatment extension benefits HCV genotype 1 patients without rapid virological response: a systematic review. Netherlands Journal of Medicine 2011;69(5):216‐21. [PubMed] [Google Scholar]
Ghany 2009
- Ghany M, Strader D, Thomas D, Seeff L. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology 2009;49:1335‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gluud 2012
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2012, Issue 5. Art. No.: LIVER.
Hadziyannis 2004
- Hadziyannis S, Sette H Jr, Morgan T, Balan V, Diago M, Marcellin P, et al. Peginterferon‐2a and ribavirin combination therapy in chronic hepatitis C. Annals of Internal Medicine 2004;140:346‐55. [DOI] [PubMed] [Google Scholar]
Hezode 2009
- Hezode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, et al. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. New England Journal of Medicine 2009;360:1839‐50. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Colloboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice 1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Innes 2011
- Innes HA, Hutchinson SJ, Allen S, Bhattacharyya D, Bramley P, Delahooke TE, et al. Excess liver‐related morbidity of chronic hepatitis C patients, who achieve a sustained viral response, and are discharged from care. Hepatology 2011;54(5):1547‐58. [DOI] [PubMed] [Google Scholar]
Jensen 2006
- Jensen M, Morgan T, Marcellin P, Pockros P, Reddy K, Hadziyannis S, et al. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon‐2a (40 kd)/ribavirin therapy. Hepatology 2006;43:954‐60. [DOI] [PubMed] [Google Scholar]
Khuroo 2004
- Khuroo M, Khuroo M, Dahab S. Meta‐analysis: a randomized trial of peginterferon plus ribavirin for the initial treatment of chronic hepatitis C genotype 4. Alimentary Pharmacology and Therapeutics 2004;20:931‐8. [DOI] [PubMed] [Google Scholar]
Kim 2009
- Kim W, Terraul N, Pedersen R, Therneau T, Edwards E, Hindman A, et al. Trends in waiting list registration for liver transplantation for viral hepatitis in the United States. Gastroenterology 2009;137:1680–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between small and large randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Lauer 2001
- Lauer G, Walker A. Hepatits C virus infection. New England Journal of Medicine 2001;345:41‐52. [DOI] [PubMed] [Google Scholar]
Manns 2001
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa‐2b plus ribavirin compared with interferon alfa‐2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958‐65. [DOI] [PubMed] [Google Scholar]
McHutchison 2009
- McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, et al. Peginterferon alfa‐2b or alfa‐2a with ribavirin for treatment of hepatitis C infection. New England Journal of Medicine 2009;361:580‐93. [DOI] [PubMed] [Google Scholar]
McHutchison 2009b
- McHutchison JG, Everson GT, Gordon SC, Jacobson IM, Sulkowski M, Kauffman R, et al. Telaprevir with peginterferon and ribavirin for chronic HCV genotype 1 infection. New England Journal of Medicine 2009;360:1827‐38. [DOI] [PubMed] [Google Scholar]
Michaels 2010
- Michaels AJ, Nelson DR. New therapies in the management of hepatitis C virus. Current Opinions in Gastroenterology 2010;26:196‐201. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352:609‐13. [DOI] [PubMed] [Google Scholar]
Morgan 2010
- Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, Santo JL, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology 2010;52:833‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parikh 2010
- Parikh M, Singh A, Sood G. Extended treatment duration for treatment naive chronic hepatitis C genotype 1 late viral responders: a meta‐analysis comparing 48 weeks vs 72 weeks of pegylated interferon and ribavirin. Journal of Viral Hepatitis 2010; Vol. 18, issue 4:e99‐e103. [DOI: 10.1111/j.1365-2893.2010.01374.x] [DOI] [PubMed]
Poynard 1995
- Poynard T, Bedossa P, Chevallier M, Mathurin P, Lemonnier C, Trepo C, et al. A comparison of three interferon alfa‐2b regimens for the long‐term treatment of chronic non‐A, non‐B hepatitis. Multicenter study group. New England Journal of Medicine 1995;332:1457‐62. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Scherzer 2009
- Scherzer TM, Kerschner H, Beinhardt S, Rutter K, Hofer H, Steindl‐Munda P, et al. Week 8 HCV‐RNA is the optimal predictor of relapse in non‐RVR patients with genotype 1/4 randomised to 48 or 72 weeks PEG‐IFN alfa‐2A plus RBV. Journal of Hepatology 2009;50(Suppl 1):S225. [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Theodore 2006
- Theodore S, Jamal M. Epidemiology of hepatitis C virus (HCV) infection. International Journal of Medical Sciences 2006;3:41‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2005
- Thomas DL, Seeff LB. Natural history of hepatitis C. Clinical Liver Diseases 2005;9:383‐98. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual forTrial Sequential Analysis (TSA). http://ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 13 June 2012).
Ueno 2009
- Ueno Y, Sollano J, Farrell G. Prevention of hepatocellular carcinoma complicating chronic hepatitis C. Journal of Gastroenterology and Hepatology 2009;24:531‐6. [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects meta‐analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


