Abstract
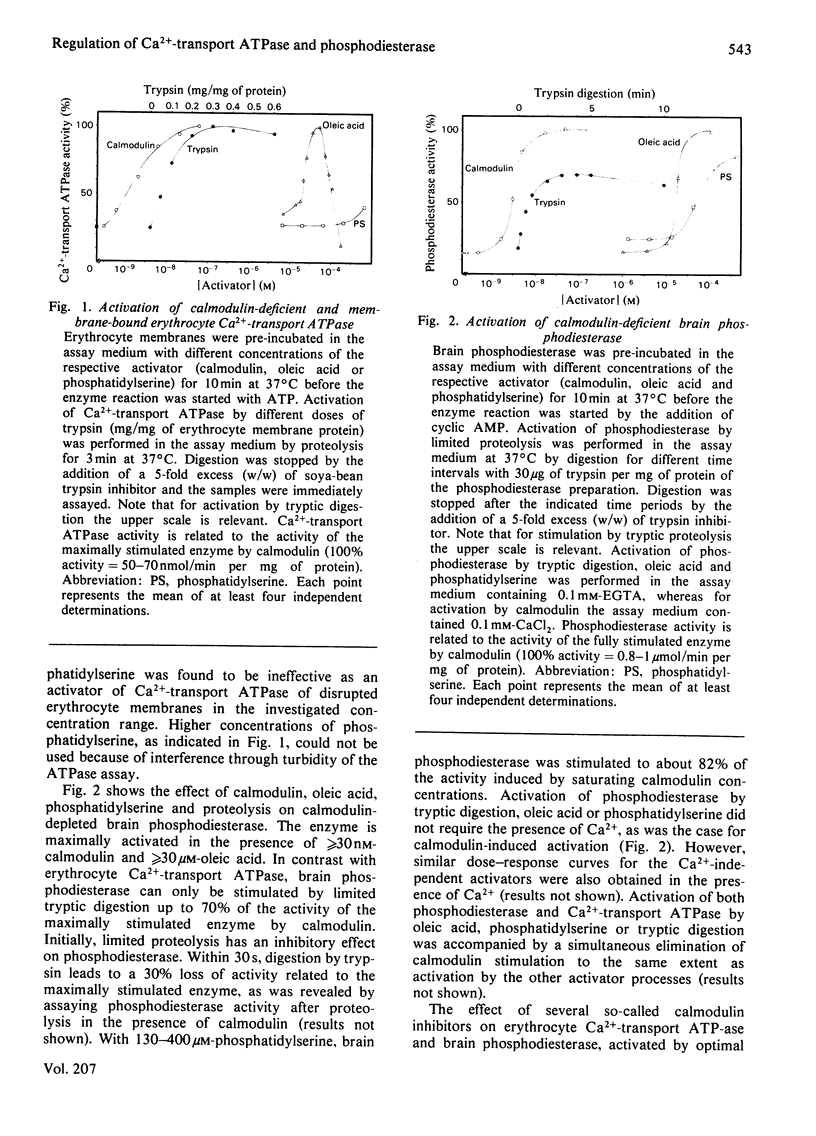
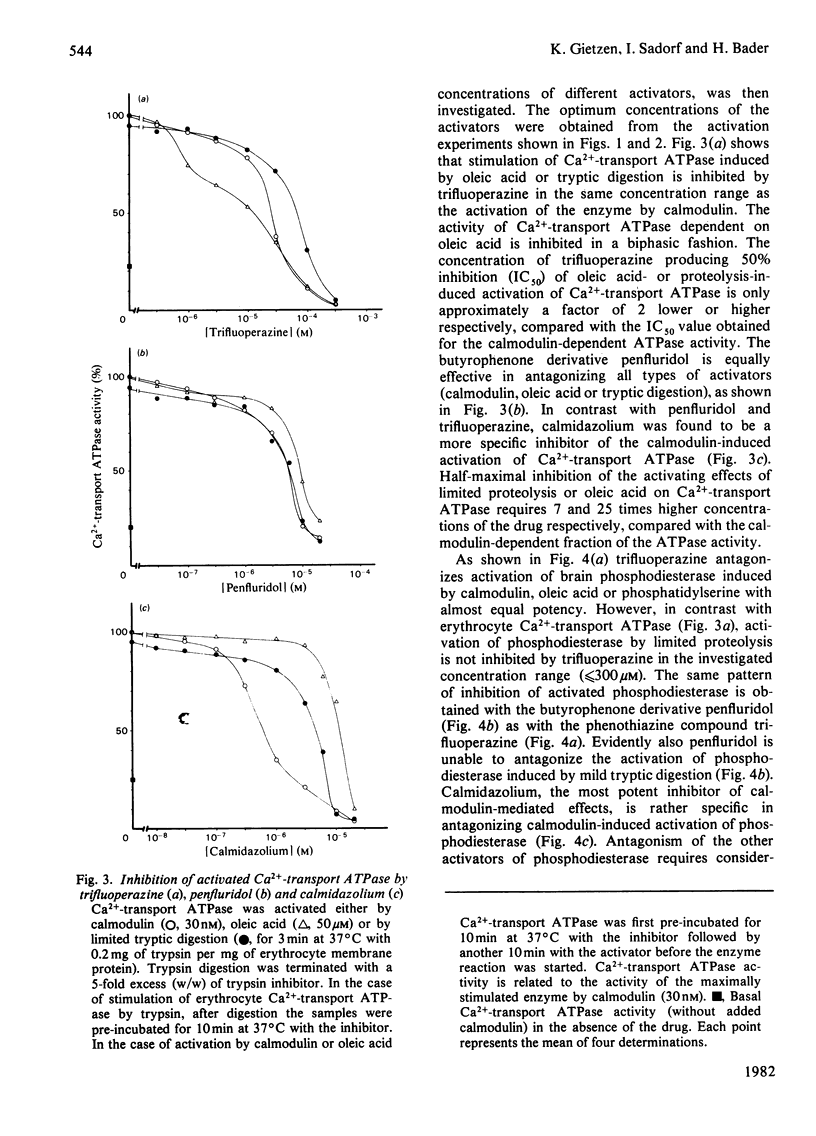
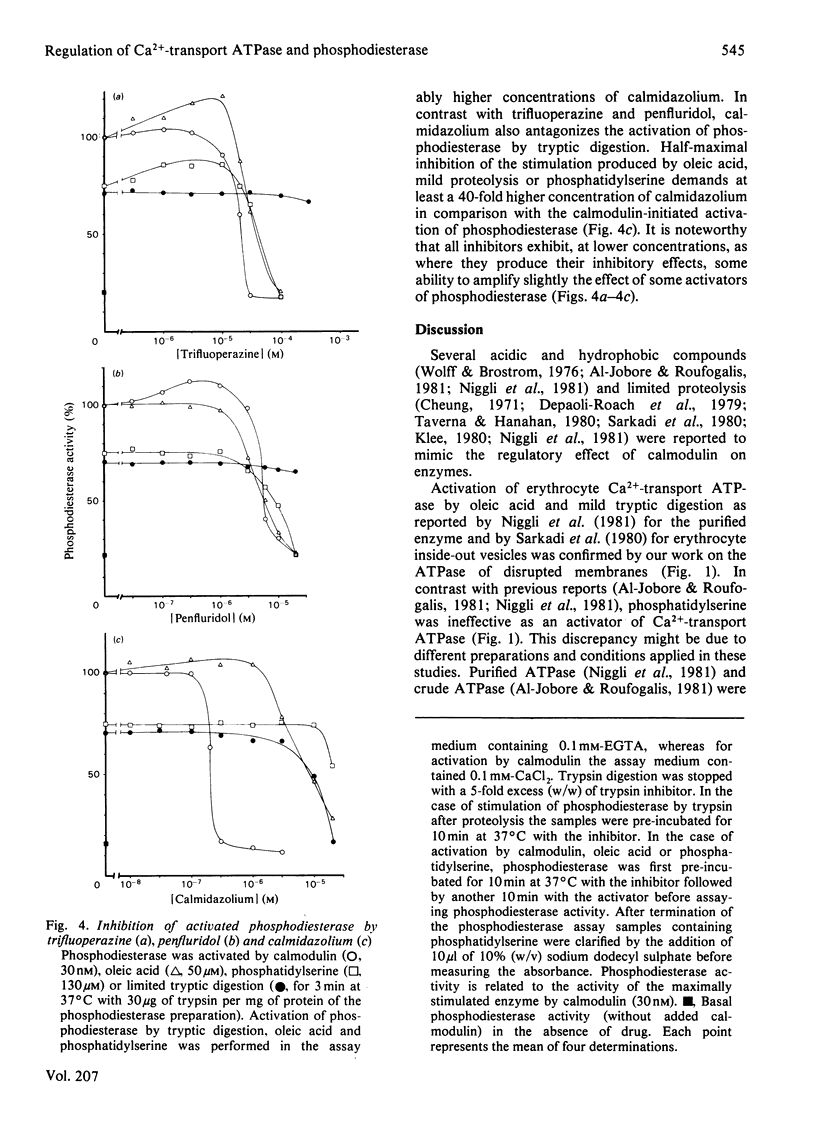
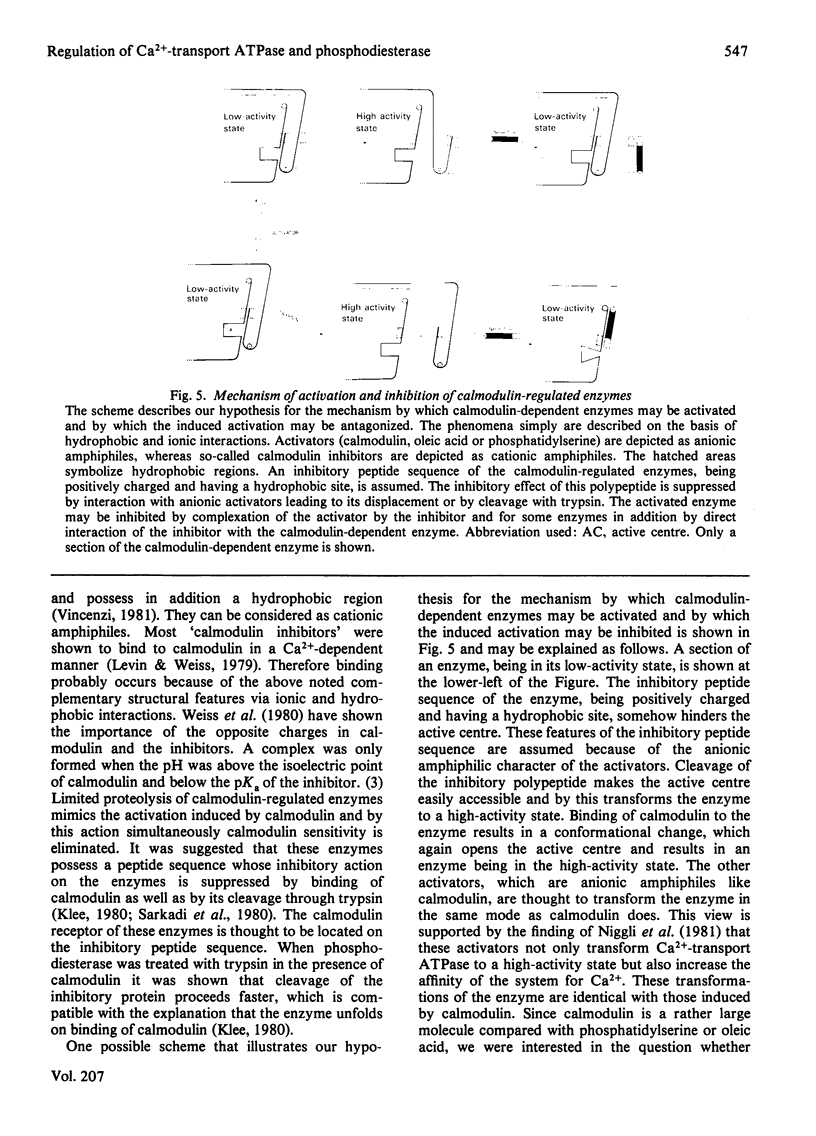
Acidic phospholipids, unsaturated fatty acids and limited proteolysis mimic the activating effect of calmodulin on erythrocyte Ca2+-transport ATPase and on brain cyclic nucleotide phosphodiesterase, as has been reported previously in several studies. Three different antagonists of calmodulin-induced activation of these enzymes were tested for their inhibitory potency on the stimulation produced by the other activators. Trifluoperazine and penfluridol were found to antagonize all the above mentioned types of activation of Ca2+-transport ATPase in the same concentration range. Both inhibitors also can reverse the activation of phosphodiesterase by oleic acid, phosphatidylserine and calmodulin at similar concentrations. However, in contrast with erythrocyte Ca2+-transport ATPase, activation of phosphodiesterase by limited tryptic digestion cannot be antagonized by penfluridol and trifluoperazine. Calmidazolium, formerly referred to as compound R 24571, was found to be a relatively specific inhibitor of calmodulin-induced activation of phosphodiesterase and Ca2+-transport ATPase, since antagonism of the other activators required much higher concentrations of the drug. The results suggest that the investigated drugs exert their inhibitory effect on calmodulin-regulated enzymes not solely via their binding to calmodulin but may also interfere directly with the calmodulin effector enzyme. In addition, a general mechanism of activation and inhibition of calmodulin-dependent enzymes is derived from our results.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Jobore A., Roufogalis B. D. Phospholipid and calmodulin activation of solubilized calcium-transport ATPase from human erythrocytes: regulation by magnesium. Can J Biochem. 1981 Nov-Dec;59(11-12):880–888. doi: 10.1139/o81-123. [DOI] [PubMed] [Google Scholar]
- Arnold A., Wolf H. U., Ackermann B. P., Bader H. An automated continuous assay of membrane-bound and solube ATPases and related enzymes. Anal Biochem. 1976 Mar;71(1):209–213. doi: 10.1016/0003-2697(76)90029-4. [DOI] [PubMed] [Google Scholar]
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Evidence for and properties of a protein activator. J Biol Chem. 1971 May 10;246(9):2859–2869. [PubMed] [Google Scholar]
- Cohen P., Burchell A., Foulkes J. G., Cohen P. T., Vanaman T. C., Nairn C. Identification of the Ca2+-dependent modulator protein as the fourth subunit of rabbit skeletal muscle phosphorylase kinase. FEBS Lett. 1978 Aug 15;92(2):287–293. doi: 10.1016/0014-5793(78)80772-8. [DOI] [PubMed] [Google Scholar]
- Depaoli-Roach A. A., Gibbs J. B., Roach P. J. Calcium and calmodulin activation of muscle phosphorylase kinase: effect of tryptic proteolysis. FEBS Lett. 1979 Sep 15;105(2):321–324. doi: 10.1016/0014-5793(79)80639-0. [DOI] [PubMed] [Google Scholar]
- Gietzen K., Mansard A., Bader H. Inhibition of human erythrocyte Ca++-transport ATPase by phenothiazines and butyrophenones. Biochem Biophys Res Commun. 1980 May 30;94(2):674–681. doi: 10.1016/0006-291x(80)91285-1. [DOI] [PubMed] [Google Scholar]
- Gietzen K., Wüthrich A., Bader H. R 24571: a new powerful inhibitor of red blood cell Ca++-transport ATPase and of calmodulin-regulated functions. Biochem Biophys Res Commun. 1981 Jul 30;101(2):418–425. doi: 10.1016/0006-291x(81)91276-6. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Sobue K., Yamazaki R., Kambayashi J., Sakon M., Kosaki G. Lack of tissue specificity of calmodulin: a rapid and high-yield purification method. FEBS Lett. 1981 Apr 20;126(2):203–207. doi: 10.1016/0014-5793(81)80242-6. [DOI] [PubMed] [Google Scholar]
- Kobayashi R., Tawata M., Hidaka H. Ca2+ regulated modulator protein interacting agents: inhibition of Ca2+-Mg2+-ATPase of human erythrocyte ghost. Biochem Biophys Res Commun. 1979 Jun 13;88(3):1037–1045. doi: 10.1016/0006-291x(79)91513-4. [DOI] [PubMed] [Google Scholar]
- LaPorte D. C., Wierman B. M., Storm D. R. Calcium-induced exposure of a hydrophobic surface on calmodulin. Biochemistry. 1980 Aug 5;19(16):3814–3819. doi: 10.1021/bi00557a025. [DOI] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Selective binding of antipsychotics and other psychoactive agents to the calcium-dependent activator of cyclic nucleotide phosphodiesterase. J Pharmacol Exp Ther. 1979 Mar;208(3):454–459. [PubMed] [Google Scholar]
- Means A. R., Dedman J. R. Calmodulin--an intracellular calcium receptor. Nature. 1980 May 8;285(5760):73–77. doi: 10.1038/285073a0. [DOI] [PubMed] [Google Scholar]
- Niggli V., Adunyah E. S., Carafoli E. Acidic phospholipids, unsaturated fatty acids, and limited proteolysis mimic the effect of calmodulin on the purified erythrocyte Ca2+ - ATPase. J Biol Chem. 1981 Aug 25;256(16):8588–8592. [PubMed] [Google Scholar]
- Taverna R. D., Hanahan D. J. Modulation of human erythrocyte Ca2+/Mg2+ ATPase activity by phospholipase A2 and proteases. A comparison with calmodulin. Biochem Biophys Res Commun. 1980 May 30;94(2):652–659. doi: 10.1016/0006-291x(80)91282-6. [DOI] [PubMed] [Google Scholar]
- Vincenzi F. F. Calmodulin pharmacology. Cell Calcium. 1981 Aug;2(4):387–409. doi: 10.1016/0143-4160(81)90027-0. [DOI] [PubMed] [Google Scholar]
- Volpi M., Sha'afi R. I., Feinstein M. B. Antagonism of calmodulin by local anesthetics. Inhibition of calmodulin-stimulated calcium transport of erythrocyte inside-out membrane vesicles. Mol Pharmacol. 1981 Sep;20(2):363–370. [PubMed] [Google Scholar]
- Wang J. H., Desai R. Modulator binding protein. Bovine brain protein exhibiting the Ca2+-dependent association with the protein modulator of cyclic nucleotide phosphodiesterase. J Biol Chem. 1977 Jun 25;252(12):4175–4184. [PubMed] [Google Scholar]
- Watanabe K., Williams E. F., Law J. S., West W. L. Specific inhibition of a calcium dependent activation of brain cyclic AMP phosphodiesterase activity by vinblastine. Experientia. 1979 Nov 15;35(11):1487–1489. doi: 10.1007/BF01962801. [DOI] [PubMed] [Google Scholar]
- Weiss B., Prozialeck W., Cimino M., Barnette M. S., Wallace T. L. Pharmacological regulation of calmodulin. Ann N Y Acad Sci. 1980;356:319–345. doi: 10.1111/j.1749-6632.1980.tb29621.x. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Brostrom C. O. Calcium-dependent cyclic nucleotide phosphodiesterase from brain identification of phospholipids as calcium-independent activators. Arch Biochem Biophys. 1976 Apr;173(2):720–731. doi: 10.1016/0003-9861(76)90310-6. [DOI] [PubMed] [Google Scholar]


