Abstract
Hydrophobic chromatography on phenyl–Sepharose has revealed the decidedly hydrophobic character of several members of the group of cytolytic proteins termed `thiol-activated'. Pneumolysin, alveolysin, cereolysin, and streptolysin O were found to be equally hydrophobic, as were the oxidized and reduced forms of alveolysin. Hydrophobic chromatography has been utilized in the development of an improved procedure for the purification of pneumolysin.
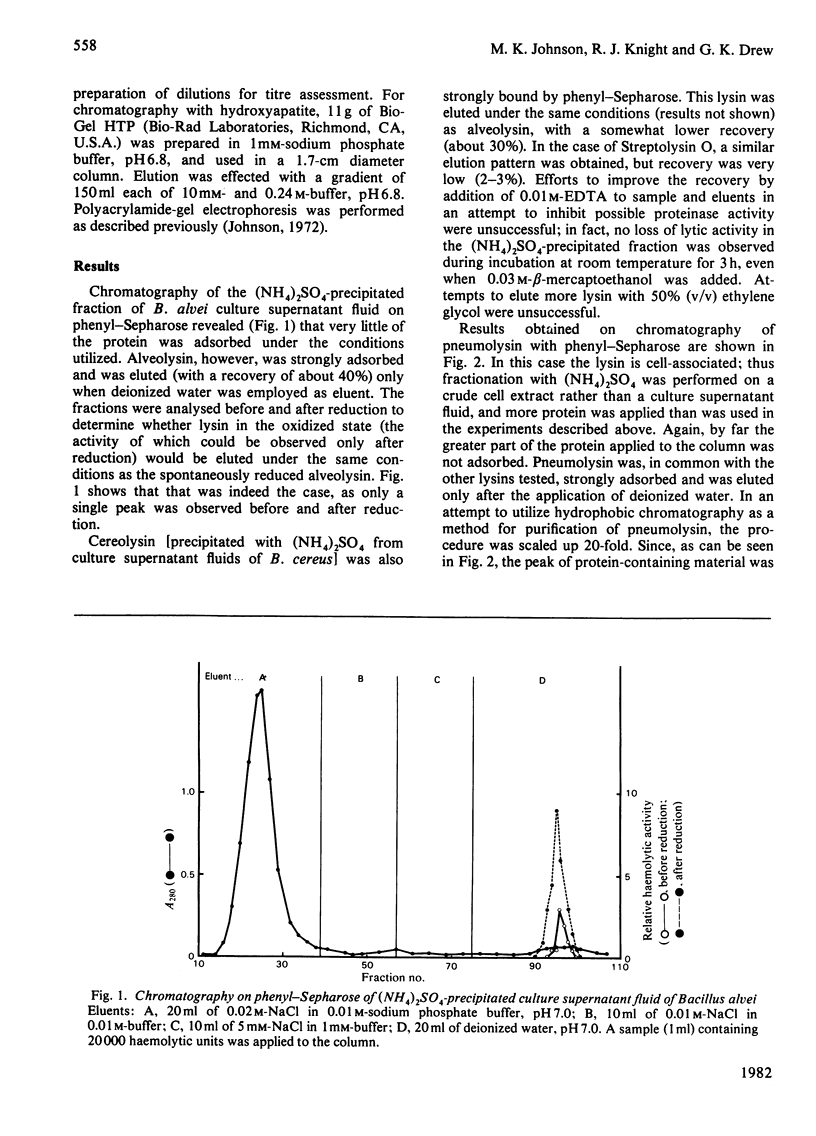
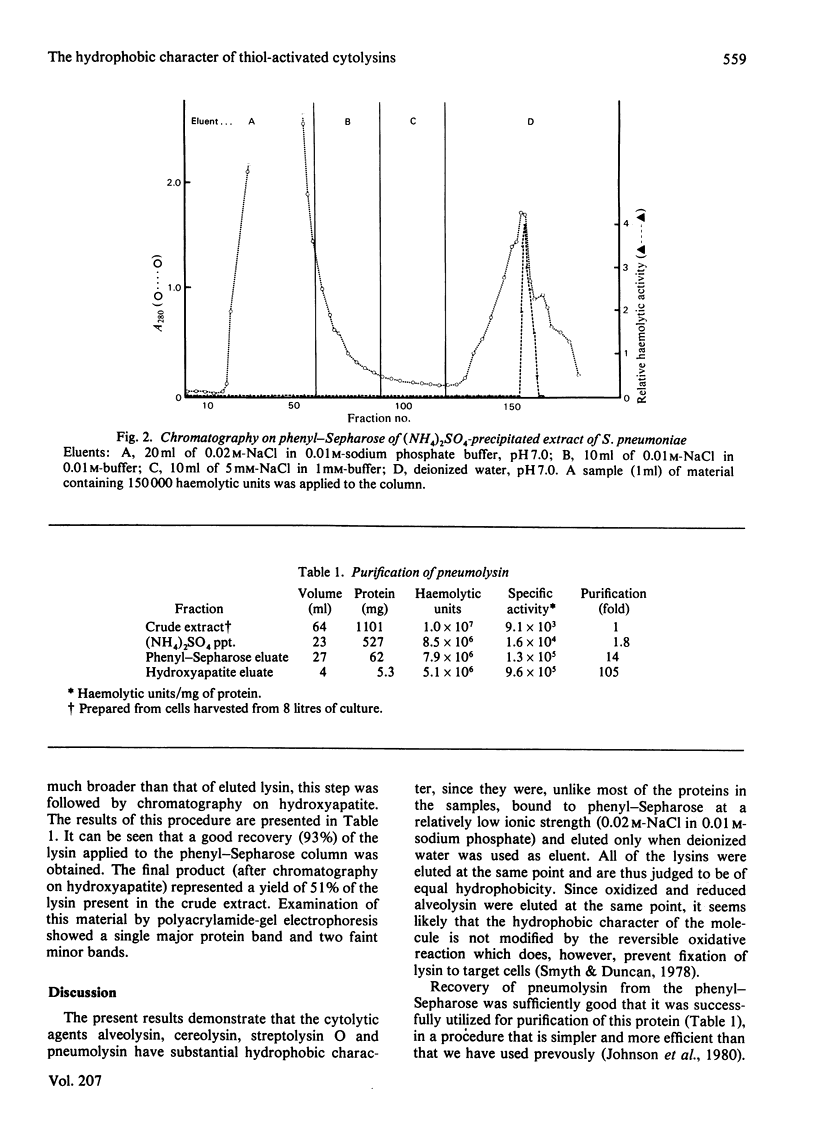
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Johnson M. K., Geoffroy C., Alouf J. E. Binding of cholesterol by sulfhydryl-activated cytolysins. Infect Immun. 1980 Jan;27(1):97–101. doi: 10.1128/iai.27.1.97-101.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K. Properties of purified pneumococcal hemolysin. Infect Immun. 1972 Nov;6(5):755–760. doi: 10.1128/iai.6.5.755-760.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]



