Abstract
Desmin is a type III intermediate filament protein specifically expressed in muscle cells, which is encoded by the DES gene. Defects in the desmin protein and cytoskeletal instability may interfere with cardiac muscle conduction signals, a fundamental mechanism for arrhythmias in patients with desmin-related myopathy. This current case report presents a female patient in her early 20s who presented with early-onset complete atrioventricular block and complete left bundle branch block over the previous decade. More recently, she had developed ventricular tachycardia, ventricular fibrillation, atrial fibrillation and other arrhythmias. Echocardiography revealed non-compaction of the ventricular myocardium and pulmonary hypertension. Whole-exome sequencing analysis identified a heterozygous missense mutation in the DES gene: c.1216C>T (p.Arg406Trp). She was eventually diagnosed with arrhythmias due to desmin-related myopathy. A literature review of international databases was undertaken to summarise the clinical characteristics of the cardiac involvement associated with this DES gene mutation.
Keywords: Desmin gene, desmin-related myopathy, arrhythmias, genic mutation, subcutaneous implantable cardioverter defibrillator, heart transplant, non-compaction of ventricular myocardium
Introduction
Desmin (OMIM: *125660) is a type III intermediate filament protein specifically expressed in muscle cells, which is encoded by the DES gene located on chromosome 2q35. Desmin is widely distributed in the atrioventricular node primordium, atrioventricular bundle, bundle branches and throughout the ventricular trabeculae, shortly after heart differentiation during embryonic development. 1 Desmin forms intracellular and intercellular networks by connecting the cytoskeletal structures (such as desmosomes and Z-discs) and organelles (such as mitochondria and nuclei) to buffer mechanical stress applied to cardiac muscle cells. It also binds to various proteins within myocardial cells and regulates related signalling pathways to maintain myocardial cell function. 2 Mutant desmin protein disrupts the normal assembly of intermediate filaments, increasing the fragility of myofibrils and causing a significant increase in cytoplasmic Ca2+ concentration, leading to myocardial cell excitability. 3 Defects in the desmin protein and cytoskeletal instability may interfere with conduction signals, a fundamental mechanism for arrhythmias in patients with desmin-related myopathy (DRM). 4 A meta-analysis of over 40 DES gene mutations revealed that more than 50% of carriers exhibited cardiomyopathy; and over 60% exhibited arrhythmia, with heart block being a significant phenotype. 5 Additionally, DRM can manifest as dilated cardiomyopathy (DCM), restrictive cardiomyopathy (RCM), arrhythmogenic cardiomyopathy (ACM) and hypertrophic cardiomyopathy (HCM).6,7 Mutations in different functional domains of the DES gene can induce different clinical phenotypes. This current case report presents a female patient experiencing various types of arrhythmias, non-compaction of ventricular myocardium (NVM) and pulmonary hypertension (PH) who eventually underwent a heart transplant. Whole-exome sequencing identified a heterozygous missense mutation in the DES gene: c.1216C>T (p.Arg406Trp).
Case report
In May 2023, a female patient in her early 20s who had syncope episodes 13 years previously experienced a recurrence 4 hours prior to admission to the Department of Cardiology, The Second Hospital of Hebei Medical University, Shijiazhuang, Hebei Province, China. Thirteen years previously, the patient experienced syncope with a heart rate at 30 beats per minute (bpm). Echocardiography undertaken at that time indicated that the heart’s structure and function were normal. She was diagnosed with intermittent complete atrioventricular block (CAVB) and complete left bundle branch block (LBBB), for which she underwent permanent cardiac pacemaker implantation (Medtronic RELIA REDR01) with a DDD pacing mode (Figure 1(a)). Postoperatively, she reported no recurrent syncope episodes.
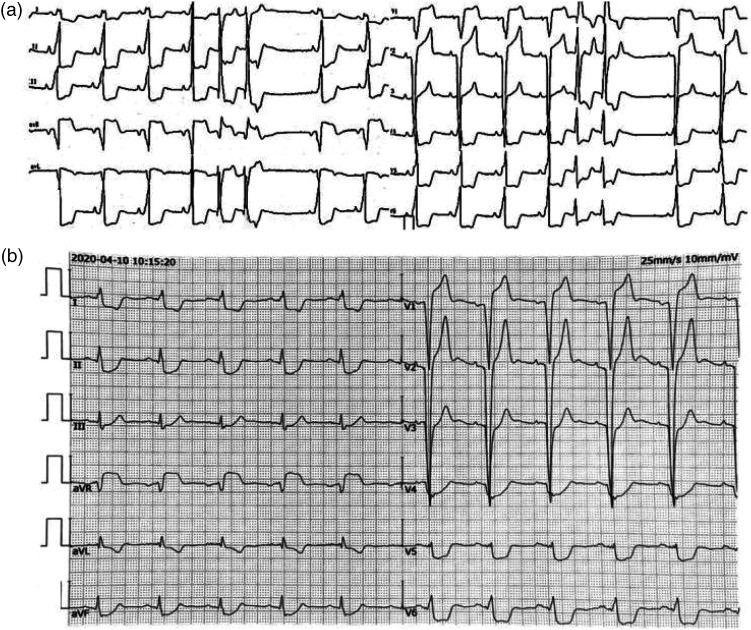
Figure 1.
Electrocardiograms of a female patient in her early 20 s who had syncope episodes 13 years previously with a heart rate of 30 beats per minute (bpm) and had then experienced a recurrence 4 hours prior to the current admission: (a) electrocardiogram taken 13 years previously before the implantation of a pacemaker showed sinus rhythm, left bundle branch block and premature atrial contractions and (b) electrocardiogram taken 3 years prior to the current admission showed pacing rhythm (atrial sensing-ventricular pacing mode) and a ventricular rate of 65 bpm.
Three years ago, she was admitted to the Department of Cardiology, The Second Hospital of Hebei Medical University, Shijiazhuang again due to battery depletion of the pacemaker. An electrocardiogram showed pacing rhythm with atrial sensing-ventricular pacing mode and a ventricular rate of 65 bpm (Figure 1(b)). Echocardiography indicated the following changes: the left atrium was enlarged at 46 mm; the left ventricular end diastolic diameter was 49 mm; the left ventricular ejection fraction was 59.3%; and the pulmonary artery systolic pressure was 67 mmHg. The left ventricular apex showed a rich trabecular structure, with a ratio of non-compacted to compacted myocardium of approximately 15:3. Brain natriuretic peptide (BNP) and troponin levels were normal. She received a dual-chamber rate-responsive pacemaker pulse generator replacement (Medtronic Sensia SENSL1). Postoperative programming showed no abnormalities.
Four hours prior to the current admission, the patient experienced syncope without any apparent cause. On the day of admission, her blood pressure was measured at 108/69 mmHg. Blood gas analysis, complete blood count, coagulation profile, liver function, kidney function and thyroid function were all within normal limits. This patient was diagnosed with NYHA classification of cardiac function II at the time of this current admission. BNP was mildly elevated as 722 pg/ml. Echocardiography indicated the following: the left ventricular ejection fraction was 59.3%; and the pulmonary artery systolic pressure was 57 mmHg. Pacemaker programming indicated that the pacing and sensing functions were satisfactory, with intermittent episodes of ventricular tachycardia (VT), atrial fibrillation and premature ventricular complex observed. During a syncope episode, intracardiac electrography suggested an episode of ventricular fibrillation (VF). Given that the patient is a young female with a slender build (body mass index: 16.5 kg/m2), a subcutaneous implantable cardioverter defibrillator (S-ICD; A209 EMBLEM™) was implanted while retaining the previous dual-chamber pacemaker.
Considering the patient’s complex disease progression, genetic testing was performed with the consent of the patient and her family. Whole-exome sequencing results revealed that the patient carries a pathogenic heterozygous mutation at exon 6 of the DES gene: c.1216C>T (p.Arg406Trp). Sanger sequencing confirmed the mutation of nucleotide 1216 in the DES gene coding region from cytosine to thymine, resulting in the substitution of arginine by tryptophan at position 406. Public database searches indicate that DES gene mutations can cause type 1 myofibrillar myopathy (OMIM: #601419) and type 1I DCM (OMIM: #604765) (Figure 2). The patient’s father died young from sudden cardiac death, but other relatives have not undergone genetic screening, which has made it difficult to determine whether this mutation is a de novo occurrence. During this current hospital stay, the patient’s serum creatine kinase levels were mildly elevated at 384 U/l (normal range, 40–200 U/l). However, no skeletal muscle activity disorders or abnormalities in muscle strength or tone were observed. The patient refused muscle biopsy and electromyography.
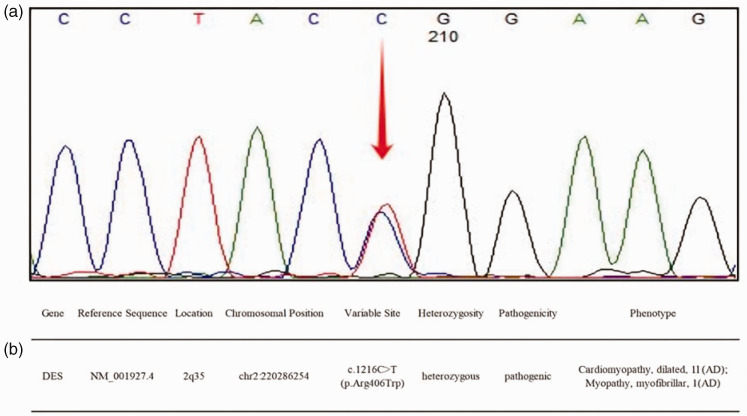
Figure 2.
Whole-exome sequencing analysis of a female patient in her early 20 s who had syncope episodes 13 years previously with a heart rate of 30 beats per minute (bpm) and had then experienced a recurrence 4 hours prior to the current admission revealed a heterozygous missense mutation in the desmin (DES) gene c.1216C>T(p.Arg406Trp): (a) Sanger sequencing DNA chromatograms of DES indicating the missense variant c.1216C>T (p.Arg406Trp). The red arrow indicates the mutation site and (b) the patient’s main genetic test result. Arg, arginine; Trp, tryptophan; AD, autosomal dominant inheritance. The colour version of this figure is available at: http://imr.sagepub.com.
The patient was discharged 1 week post-surgery. During a 6-month follow-up period, the patient received 71.25 mg metoprolol succinate sustained-release tablets oral once a day, 10 mg dapagliflozin oral once a day, 20 mg spironolactone oral twice a day and 10 mg furosemide oral once a day. All of these medications were taken continuously after hospital discharge. She experienced no further syncope episodes and the S-ICD system was functioning normally. Nine months after surgery, the S-ICD programming indicated a total of five episodes of VT/VF, all of which were converted to sinus rhythm after defibrillation and were consistent with the time of the presyncope (Figure 3). One year after surgery, the S-ICD discharged more frequently and led to patient’s extreme anxiety with a significant increase in BNP, thus she underwent a heart transplant. Pathological examination of the removed heart revealed NVM (Figure 4). The patient was ultimately diagnosed with DRM, with ventricular tachycardia, ventricular fibrillation, atrial fibrillation, NVM and PH as phenotypic expressions.
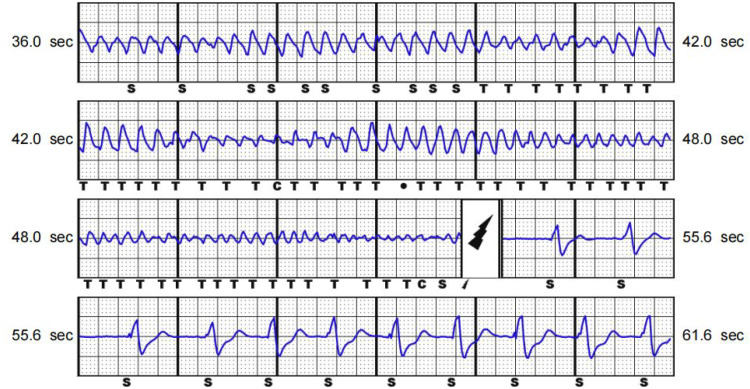
Figure 3.
Programming indicated ventricular tachycardia/ventricular tachycardia was terminated by one shock from the subcutaneous implantable cardioverter defibrillator and it took 18 seconds from automatic diagnosis, charging to discharge and the treatment was successful in a female patient in her early 20 s who had recurrent syncope episodes. The colour version of this figure is available at: http://imr.sagepub.com.
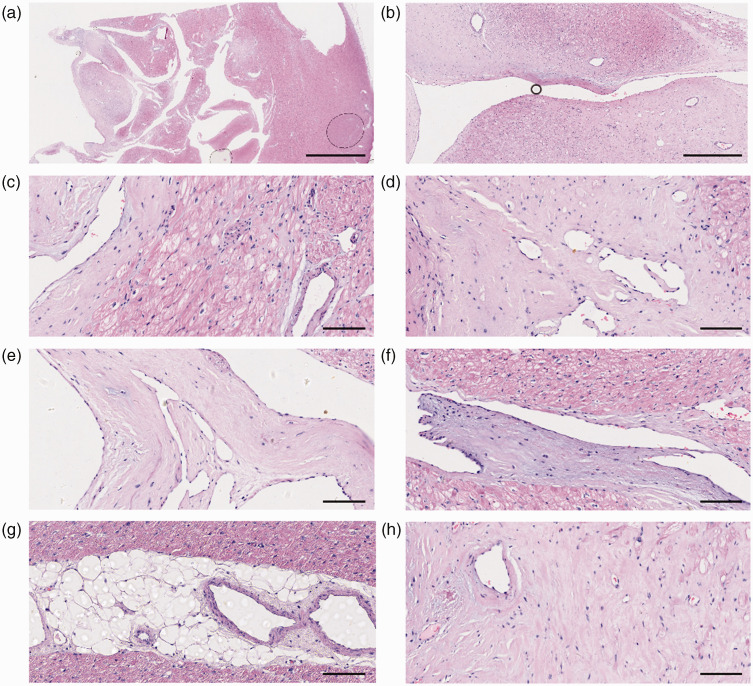
Figure 4.
Pathological examination of the heart that was removed from in a female patient in her early 20 s who had recurrent syncope episodes and subsequently had a heart transplant revealed non-compaction of the ventricular myocardium. Representative photomicrographs showing: (a) the ratio of loose to compact layers of the left ventricular myocardium was approximately 2:1.; (b) the thickened vessel walls exhibited fibrous tissue proliferation with mucinous degeneration and hyaline degeneration; (c) cardiomyocyte hypertrophy and vacuolar degeneration. Nuclei were enlarged and hyperchromatic with a box-shaped appearance; (d) myocardium interstitial fibrosis; (e) endocardial hyaline degeneration; (f) mucinous degeneration; (g) infiltration of adipose tissue in the myocardium; (h) flattened and fragmented elastic fibres. Haematoxylin and eosin staining. Scale bars: 5000 µm for (a), 625śµm for (b) and 100śµm for (c) to (h). The colour version of this figure is available at: http://imr.sagepub.com.
Written informed consent was obtained from the patient for treatment and publication of this case report. All patient details have been de-identified. The reporting of this case report conforms to the CARE guidelines. 8
Discussion
Desmin is composed of a central α-helical coiled-coil rod domain flanked by non-helical amino-terminal and carboxy-terminal domains. Mutations located in the α-helical 2B structural domain or the carboxy-terminal of desmin are often associated with arrhythmias and cardiomyopathies, specifically between amino acids 350–406. 9 A previous study observed a reduction in the transverse structures of the intercalated discs in the sinoatrial nodal pacing complex in ACM mouse models with DES gene knockout (DES–/–). 10 Compared with wild-type mice, DES–/– mice exhibited increased sympathetic nerve activity, prolonged atrial conduction time and a significant increase in ventricular and supraventricular ectopic beats, potentially related to the elevated pacemaker potential due to upregulation of T-type voltage-gated Ca2+ channels and downregulation of hyperpolarization-activated cyclic nucleotide-gated channels. 10 Another study found that desmin immunostaining in the pacing and conduction system, pulmonary vein and non-pulmonary vein myocardial sleeves was significantly stronger than in ventricular cardiomyocytes, indicating that desmin is not only distributed in the pacing regions and conduction system but also concentrated in the supraventricular regions that may trigger atrial arrhythmias. 11 In the current case, a heterozygous missense variant of the DES gene led to DRM with various types of arrhythmias, NVM and PH. The mutation residue p.Arg406Trp is located at the carboxyl terminus of the α-helical 2B structural domain within the core structure of desmin, a region that is absolutely conserved and contains a consensus sequence for intermediate filament formation.
A search of databases including PubMed®, EMBASE, Web of Science, CNKI, Chinese Medical Journal Network and Wanfang Medical Network for relevant literature from their inception to May 2024 identified 13 articles that reported the mutation p.Arg406Trp.12–24 These articles detailed 17 cases of patients with cardiac involvement (duplicate cases were excluded). Most patients developed symptoms during adolescence and 10 were females (Table 1). Apart from an early report that described a family with RCM and atrioventricular block (AVB), 15 two additional cases involved fathers confirmed to have DES germline mosaicism, the remaining cases were new mutations.13,14 Among the 10 patients with skeletal muscle involvement, common initial symptoms included fatigue and distal limb weakness, with severe cases presenting swallowing difficulties and respiratory muscle involvement. Patients with early skeletal muscle involvement had poorer prognoses, often losing the ability to perform daily activities. Among these 17 patients, 10 had cardiomyopathy (i.e. DCM, RCM, ACM, HCM), 15 had heart block (i.e. AVB, right bundle branch block, LBBB) and 13 had cardiovascular implantable electronic devices. The majority of patients were diagnosed with skeletal muscle involvement in their 20 s and some cases with cardiac involvement were as early as the first decade of life.
Table 1.
| Reference | Sex | Age, years | Ancestry | Skeletal muscle involvement |
Cardiac involvement |
Outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at onset, years | Fatigue | Distal limb weakness | Proximal limb weakness | Dysphagia | Respiratory muscle weakness | CK | EMG | Age at onset, years | Manifestation | Treatment | |||||
| Dalakas 2000 12 | F | 29 | America | 24 | + | + | + | – | – | 600 U/l | – | 25 | III AVB; syncope | PM | – |
| Park 2000 13 | F* | 29 | Greece | 24 | + | – | – | – | – | 3 times | + | 25 | III AVB; syncope | PM | Severely disabled |
| 29 | + | + | + | + | – | ||||||||||
| Dagvadorj 2004 14 | F | 28 | Spain | 22 | + | + | – | – | – | 2 times | + | 15 | III AVB; syncope | PM | Died |
| 26 | + | + | + | + | + | 21 | AFL; mild DCM | ||||||||
| F | 34 | Spain | 23 | + | + | – | – | – | 4 times | + | 23 | III AVB; syncope | PM | Severely disabled | |
| 34 | + | + | + | + | – | ||||||||||
| M* | 24 | Germany | 19 | + | + | + | – | – | – | + | 18 | AT; RBBB; MF; RCM | – | Severely disabled | |
| 19 | PVC; HF; III AVB | – | |||||||||||||
| 23 | Permanent AF; nonsustained VT; dizzy | ICD | |||||||||||||
| Arbustini 2006 15 | M | 44 | Italy | – | – | – | – | – | – | – | – | 27 | III AVB; RCM | PM | Waiting for heart transplant |
| M | 19 | Italy | 15 | – | – | – | – | – | + | + | 15 | RBBB; RCM | – | – | |
| Wahbi 2012 16 | F | 31 | France | – | + | – | – | – | – | + | – | 14 | III AVB; AF | PM | – |
| Herrmann 2020 17 | M | 19 | Caucasian | – | – | – | – | – | – | – | – | 15 | III AVB; syncope; RCM; PH; VT | PM | Heart transplant |
| 16 | MF; HF | ICD | |||||||||||||
| Kubánek 2020 18 | F | 40 | Czech | – | – | – | – | – | – | – | – | – | III AVB; ACM | – | Died |
| Cao 2023 19 | F | 32 | China | Youth | + | + | – | – | – | 839 U/L | + | 32 | Intermittent III AVB; LBBB | PM (LBBP) | Walking slowly |
| Oka 2021 20 | F | 8 | Japan | – | – | – | – | – | – | 82 U/L | – | 7 | RBBB; HCM; syncope; complete LBBB | CPR; PM; ICD | Follow-up was normal |
| Silva 2022 21 | F | 28 | Spain | 22 | + | + | – | – | – | – | – | 15 | Palpitation; AVB; bradycardia | PM | – |
| M | 34 | Spain | 14 | – | – | – | – | + | – | – | – | DCM | PM | – | |
| 20 | + | + | – | – | + | ||||||||||
| Takegami 2023 22 | M | 28 | Japan | 9 | – | – | – | + | – | – | – | 9 | HCM | PM; VAD | LVEF: 28% |
| 27 | + | + | – | + | + | 36 U/L | – | 20 | AVB; dilated-HCM; HF | ||||||
| Geryk 2024 23 | F | 22 | Europe | – | – | – | – | – | – | – | – | 9 | Sudden cardiac death | – | – |
| Xiao 2024 24 | M | 15 | China | – | – | – | – | – | – | – | – | 13 | Palpitation on exertion | – | – |
| 15 | Intermittent AVB; PVC; HCM | Diuresis; coenzyme Q10 | |||||||||||||
The father of the index case had DES germline mosaicism.
CK, creatine kinase; EMG, electromyogram; F, female; AVB, atrioventricular block; PM, pacemaker; AFL, atrial flutter; DCM, dilated cardiomyopathy; M, male; AT, atrial tachycardia; RBBB, right bundle branch block; MF, myocardial fibrosis; RCM, restrictive cardiomyopathy; PVC, premature ventricular complex; HF, heart failure; AF, atrial fibrillation; VT, ventricular tachycardia; ICD, implantable cardioverter defibrillator; PH, pulmonary hypertension; ACM, arrhythmogenic cardiomyopathy; LBBB, left bundle branch block; LBBP, left bundle branch pacing; HCM, hypertrophic cardiomyopathy; CPR, cardiopulmonary resuscitation; VAD, ventricular assist device; LVEF, left ventricular ejection fraction.
In the current case, the heterozygous missense variant of the DES gene led to DRM with multiple arrhythmias, NVM and PH. Previous research on the Arg406Trp mutation have revealed characteristic DES-positive protein aggregates and disruptions at cell junctions. 17 These disruptions include the loss of desmin and vimentin, as well as an irregular, patchy distribution of N-cadherin and desmoplakin, which affect the attachment of desmin to desmosomes and destabilize the intercalated disc. 17 These findings have been validated in genetic replacement animal models and explain the mechanisms related to early-onset conduction block and malignant arrhythmias. 17 The c.1216C>T mutation reported in this current case has previously been associated with DCM, RCM, ACM and HCM as shown in Table 1. However, in the current case, the myocardial involvement in this patient was characterized by NVM. NVM is a rare structural abnormality of the left ventricular myocardium, also known as left ventricular non-compaction (LVNC). Its aetiology remains unknown and the molecular mechanisms are yet to be elucidated. This condition is marked by left ventricular hypertrophy and hypokinetic segments with numerous prominent trabeculations and deep intertrabecular recesses. It is potentially associated with life-threatening arrhythmias and early-onset heart failure; and it shares some overlapping morphological features with other structural cardiomyopathies. Over the past few years, the association between mutations in some loci of the DES gene and NVM has been investigated. For example, DES gene missense mutations of unknown significance were described in NVM cases and involved a patient who underwent heart transplantation at the age of 10 years.25,26 A report from 2019, described a three-generation Russian family with NVM and skeletal myopathy, which led to heart transplantation and cardiogenic sudden death. 27 Genetic analysis revealed a novel small in-frame deletion within the DES gene, c.336_344del, affecting the α-helical rod domain. 27 It can be suggested that NVM due to DES gene mutations is associated with complex arrhythmias, heart failure and a severe disease course. Therefore, the DES gene should be included in the genetic screening of patients with LVNC in the future.
Interestingly, the current case has helped to broaden the clinician’s ability to choose the best treatment in other similar cases. A previous report described a young male with the DES gene mutation c.1216C>T (p.Arg406Trp). 17 He underwent pacemaker and implantable cardioverter defibrillator (ICD) implantation for RCM, intermittent CAVB and VT. 17 Ultimately, he received a heart transplant due to the progression of heart failure. 17 This previous case supports early pacemaker or ICD implantation to prevent sudden cardiac death. 17 Another study described an 8-year-old girl who was successfully resuscitated from a sudden loss of consciousness and diagnosed with HCM with CAVB. 20 She was implanted with a transvenous ICD to prevent malignant ventricular arrhythmic events. 20 No malignant ventricular arrhythmias were observed during follow-up. 20 Currently, there are no definitive pharmacological or other interventional measures for arrhythmias caused by DES gene mutation-associated myopathies. Considering that DRM-induced cardiac involvement often necessitates pacemaker implantation due to high-degree AVB, a significant proportion of right ventricular endocardial pacing can lead to intraventricular and interventricular dyssynchrony, resulting in impaired cardiac function. Physiological pacing is expected to maintain ventricular contraction synchrony. For patients with heart failure combined with complete LBBB, left bundle branch pacing optimized-cardiac resynchronization therapy and His-optimized cardiac resynchronization therapy probably achieve a good therapeutic effect. Upcoming extravenous ICDs and the fourth-generation S-ICDs will offer both bradycardia pacing and anti-tachycardia pacing functions, expanding their applicability and meeting both pacing and defibrillation needs of patients potentially with DRM.
In conclusion, the cardiac phenotype resulting from the DES c.1216C>T (p.Arg406Trp) mutation exhibits heterogeneity, as seen in this current case and previous reports. Patients with one or more manifestations of AVB, cardiomyopathy with or without skeletal muscle involvement, early onset and a family history of sudden cardiac death should be identified. Patients with early-onset arrhythmias should undergo timely dynamic electrocardiography monitoring, cardiac function evaluation and whole-genome sequencing, with annual cardiac examinations during follow-up. Relatives of patients who may carry the DES gene mutation, even without muscular involvement, should also undergo similar evaluations. For patients who have developed arrhythmias, in addition to pharmacological treatment, the prophylactic implantation of pacemakers or ICDs should be considered to prevent severe heart failure or sudden death.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605241291741 for Desmin-related myopathy manifested by various types of arrhythmias: a case report and literature review by Lu Geng, Mengxiao Wang, Keke Wang, Liang Xu, Jiaqi Li, Fan Liu and Jingchao Lu in Journal of International Medical Research
Acknowledgements
The authors would like to thank all members of the study team as well as the patient and her family.
Author contributions: Lu Geng drafted the original manuscript. Mengxiao Wang was this patient’s lead physician and responsible for data collection. Keke Wang provided critical comments on the manuscript. Liang Xu undertook the pathological examinations. Jiaqi Li assisted with the preparation of figures and the table. Fan Liu was this patient’s lead operator and contributed to the revision of the manuscript. Jingchao Lu designed the idea and guided the writing. All authors have read and agreed to the final manuscript.
The authors declare that there are no conflicts of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD: Jingchao Lu https://orcid.org/0000-0002-6946-0086
Data availability statement
The detailed information used during the current report is available from the corresponding author on reasonable request.
References
- 1.Liu HX, Jing YX, Wang JJ, et al. Expression patterns of intermediate filament proteins desmin and lamin A in the developing conduction system of early human embryonic hearts. J Anat 2020; 236: 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodehl A, Gaertner-Rommel A, Milting H. Molecular insights into cardiomyopathies associated with desmin (DES) mutations. Biophys Rev 2018; 10: 983–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen R, Qiu Z, Wang J, et al. DES mutation associated with cardiac hypertrophy and alternating bundle branch block. HeartRhythm Case Rep 2020; 7: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maggi L, Mavroidis M, Psarras S, et al . Skeletal and Cardiac Muscle Disorders Caused by Mutations in Genes Encoding Intermediate Filament Proteins. Int J Mol Sci 2021; 22: 4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Spaendonck-Zwarts KY, van Hessem L, Jongbloed JD, et al. Desmin-related myopathy. Clin Genet 2011; 80: 354–366. [DOI] [PubMed] [Google Scholar]
- 6.Taylor MR, Slavov D, Ku L, et al. Prevalence of desmin mutations in dilated cardiomyopathy. Circulation 2007; 115: 1244–1251. [DOI] [PubMed] [Google Scholar]
- 7.Hathaway J, Heliö K, Saarinen I, et al. Diagnostic yield of genetic testing in a heterogeneous cohort of 1376 HCM patients. BMC Cardiovasc Disord 2021; 21: 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. Headache 2013; 53: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 9.Su W, van Wijk SW, Brundel BJJM. Desmin variants: Trigger for cardiac arrhythmias? Front Cell Dev Biol 2022; 10: 986718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mavroidis M, Athanasiadis NC, Rigas P, et al. Desmin is essential for the structure and function of the sinoatrial node: implications for increased arrhythmogenesis. Am J Physiol Heart Circ Physiol 2020; 319: H557–H570. [DOI] [PubMed] [Google Scholar]
- 11.Kugler S, Tőkés AM, Nagy N, et al. Strong desmin immunoreactivity in the myocardial sleeves around pulmonary veins, superior caval vein and coronary sinus supports the presumed arrhythmogenicity of these regions. J Anat 2024; 244: 120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalakas MC, Park KY, Semino-Mora C, et al. Desmin myopathy, a skeletal myopathy with cardiomyopathy caused by mutations in the desmin gene. N Engl J Med 2000; 342: 770–780. [DOI] [PubMed] [Google Scholar]
- 13.Park KY, Dalakas MC, Semino-Mora C, et al. Sporadic cardiac and skeletal myopathy caused by a de novo desmin mutation. Clin Genet 2000; 57: 423–429. [DOI] [PubMed] [Google Scholar]
- 14.Dagvadorj A, Olivé M, Urtizberea JA, et al. A series of West European patients with severe cardiac and skeletal myopathy associated with a de novo R406W mutation in desmin. J Neurol 2004; 251: 143–149. [DOI] [PubMed] [Google Scholar]
- 15.Arbustini E, Pasotti M, Pilotto A, et al. Desmin accumulation restrictive cardiomyopathy and atrioventricular block associated with desmin gene defects. Eur J Heart Fail 2006; 8: 477–483. [DOI] [PubMed] [Google Scholar]
- 16.Wahbi K, Béhin A, Charron P, et al. High cardiovascular morbidity and mortality in myofibrillar myopathies due to DES gene mutations: a 10-year longitudinal study. Neuromuscul Disord 2012; 22: 211–218. [DOI] [PubMed] [Google Scholar]
- 17.Herrmann H, Cabet E, Chevalier NR, et al. Dual Functional States of R406W-Desmin Assembly Complexes Cause Cardiomyopathy With Severe Intercalated Disc Derangement in Humans and in Knock-In Mice. Circulation 2020; 142: 2155–2171. [DOI] [PubMed] [Google Scholar]
- 18.Kubánek M, Schimerová T, Piherová L, et al. Desminopathy: Novel Desmin Variants, a New Cardiac Phenotype, and Further Evidence for Secondary Mitochondrial Dysfunction. J Clin Med 2020; 9: 937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao Y, Liu Q. A case report of intermittent complete atrioventricular block caused by desmin gene mutation. Chinese Medical Care Repository 2023; 05: E03093–E03093; https://rs.yiigle.com/cmaid/1485798. [Google Scholar]
- 20.Oka H, Nakau K, Imanishi R, et al. A Case Report of a Rare Heterozygous Variant in the Desmin Gene Associated With Hypertrophic Cardiomyopathy and Complete Atrioventricular Block. CJC Open 2021; 3: 1195–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva AMS, Rodrigo P, Moreno CAM, et al. The Location of Disease-Causing DES Variants Determines the Severity of Phenotype and the Morphology of Sarcoplasmic Aggregates. J Neuropathol Exp Neurol 2022; 81: 746–757. [DOI] [PubMed] [Google Scholar]
- 22.Takegami N, Mitsutake A, Mano T, et al. The Myocardial Accumulation of Aggregated Desmin Protein in a Case of Desminopathy with a de novo DES p.R406W Mutation. Intern Med 2023; 62: 2883–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geryk M, Canac R, Forest V, et al. Generation of a patient-specific induced pluripotent stem cell line carrying the DES p.R406W mutation, an isogenic control and a DES p.R406W knock-in line. Stem Cell Res 2024; 77: 103396. [DOI] [PubMed] [Google Scholar]
- 24.Xiao H, Song L, Tao L. A case report of adolescent myofibrillar myopathy due to a de novo R406W pathogenic variant in desmin with symptoms of “hypertrophic cardiomyopathy”. Heliyon 2024; 10: e25009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miszalski-Jamka K, Jefferies JL, Mazur W, et al. Novel Genetic Triggers and Genotype-Phenotype Correlations in Patients With Left Ventricular Noncompaction. Circ Cardiovasc Genet 2017; 10: e001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Waning JI, Caliskan K, Hoedemaekers YM, et al. Genetics, Clinical Features, and Long-Term Outcome of Noncompaction Cardiomyopathy. J Am Coll Cardiol 2018; 71: 711–722. [DOI] [PubMed] [Google Scholar]
- 27.Marakhonov AV, Brodehl A, Myasnikov RP, et al. Noncompaction cardiomyopathy is caused by a novel in-frame desmin (DES) deletion mutation within the 1A coiled-coil rod segment leading to a severe filament assembly defect. Hum Mutat 2019; 40: 734–741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605241291741 for Desmin-related myopathy manifested by various types of arrhythmias: a case report and literature review by Lu Geng, Mengxiao Wang, Keke Wang, Liang Xu, Jiaqi Li, Fan Liu and Jingchao Lu in Journal of International Medical Research
Data Availability Statement
The detailed information used during the current report is available from the corresponding author on reasonable request.