Abstract
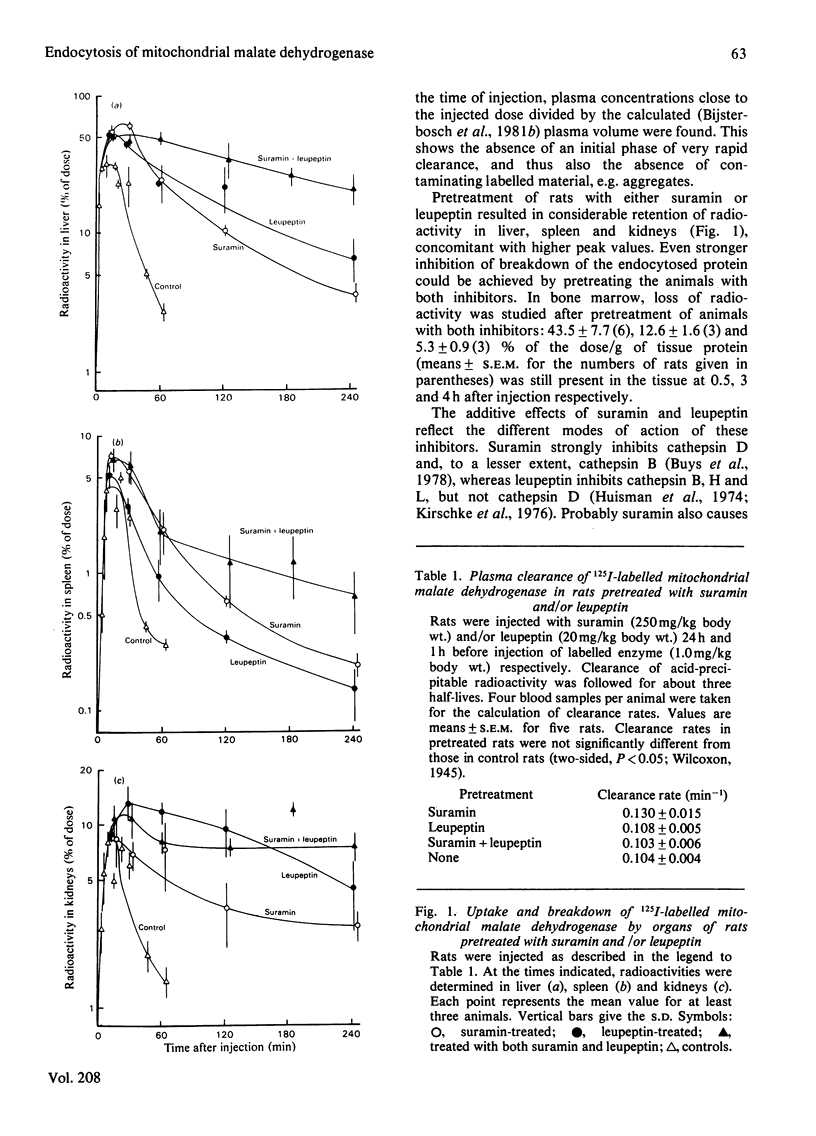
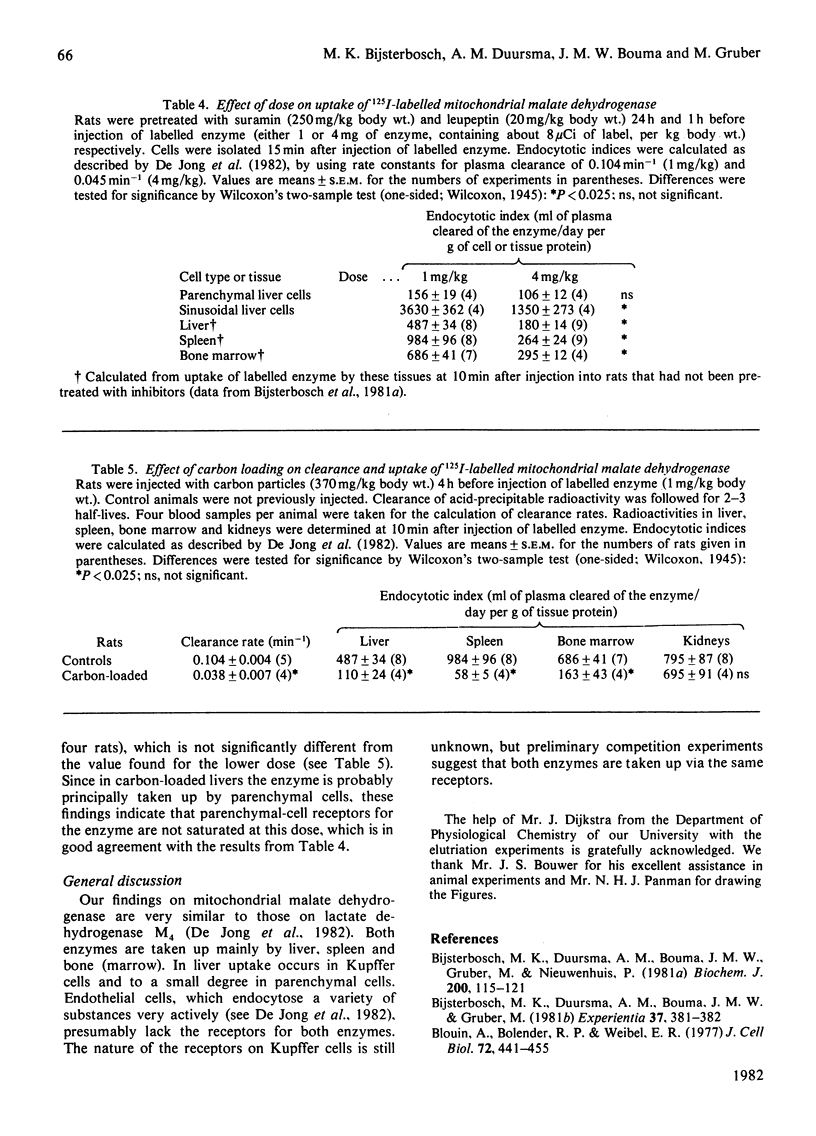
1. The plasma clearance of intravenously injected 125I-labelled mitochondrial malate dehydrogenase (half-life 7 min) was not influenced by previous injection of suramin and/or leupeptin (inhibitors of intralysosomal proteolysis). 2. Pretreatment with both inhibitors considerably delayed degradation of endocytosed enzyme in liver, spleen, bone marrow and kidneys. 3. The tissue distribution of radioactivity was determined at 30 min after injection, when only 3% of the dose was left in plasma. All injected radioactivity was still present in the carcass. The major part of the injected dose was found in liver (49%), spleen (5%), kidneys (13%) and bone, including marrow (11%). 4. Liver cells were isolated 15 min after injection of labelled enzyme. We found that Kupffer cells and parenchymal cells had endocytosed the enzyme at rates corresponding to 9530 and 156 ml of plasma/day per g of cell protein respectively. Endothelial cells do not significantly contribute to uptake of the enzyme. 5. Uptake by Kupffer cells was saturable, whereas uptake by parenchymal cells was not. This suggests that these cell types endocytose the enzyme via different receptors. 6. Previous injection of carbon particles greatly decreased uptake of the enzyme by liver, spleen and bone marrow.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bijsterbosch M. K., Duursma A. M., Bouma J. M., Gruber M., Nieuwenhuis P. Plasma clearance and endocytosis of mitochondrial malate dehydrogenase in the rat. Biochem J. 1981 Oct 15;200(1):115–121. doi: 10.1042/bj2000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin A., Bolender R. P., Weibel E. R. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977 Feb;72(2):441–455. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buys C. H., Bouma J. M., Gruber M., Wisse E. Induction of lysosomal storage by suramin. Naunyn Schmiedebergs Arch Pharmacol. 1978 Sep;304(2):183–190. doi: 10.1007/BF00495555. [DOI] [PubMed] [Google Scholar]
- Buys C. H., Elferink M. G., Bouma J. M., Gruber M., Nieuwenhuis P. Proteolysis of formaldehyde-treated albumin in Kupffer cells and its inhibition by suramin. J Reticuloendothel Soc. 1973 Aug;14(2):209–223. [PubMed] [Google Scholar]
- De Jong A. S., Bouma J. M., Gruber M. O-(4-Diazo-3,5-di[125I]iodobenzoyl)sucrose, a novel radioactive label for determining organ sites of catabolism of plasma proteins. Biochem J. 1981 Jul 15;198(1):45–51. doi: 10.1042/bj1980045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jong A. S., Duursma A. M., Bouma J. M., Gruber M., Brouwer A., Knook D. L. Endocytosis of lactate dehydrogenase isoenzyme M4 in rats in vivo. Experiments with enzyme labelled with O-(4-diazo-3,5-di[125I]iodobenzoyl)sucrose. Biochem J. 1982 Mar 15;202(3):655–660. doi: 10.1042/bj2020655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W. A., LaBadie J. H., Aronson N. N., Jr Inhibition of 125I-asialofetuin catabolism by leupeptin in the perfused rat liver and in vivo. J Biol Chem. 1979 May 25;254(10):4191–4196. [PubMed] [Google Scholar]
- Friedel R., Trautschold I., Gärtner K., Helle-Feldmann M., Gaudssuhn D. Einfluss verschiedener Methoden zur Blutgewinnung auf Enzym-Aktivitäten im Serum kleiner Laboratoriumstiere. Z Klin Chem Klin Biochem. 1975 Nov;13(11):499–505. [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Interference with normal phagosome-lysosome fusion in macrophages, using ingested yeast cells and suramin. Nature. 1975 Jul 3;256(5512):47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- Huisman W., Lanting L., Doddema H. J., Bouma J. M., Gruber M. Role of individual cathepsins in lysosomal protein digestion as tested by specific inhibitors. Biochim Biophys Acta. 1974 Nov 25;370(1):297–307. doi: 10.1016/0005-2744(74)90054-0. [DOI] [PubMed] [Google Scholar]
- Kirschke H., Langner J., Wiederanders B., Ansorge S., Bohley P., Broghammer U. Intrazellulärer Proteinabbau. VII. Kathepsin L und H: Zwei neue Proteinasen aus Rattenleberlysosomen. Acta Biol Med Ger. 1976;35(3-4):285–299. [PubMed] [Google Scholar]
- Knook D. L., Sleyster E. C. Separation of Kupffer and endothelial cells of the rat liver by centrifugal elutriation. Exp Cell Res. 1976 May;99(2):444–449. doi: 10.1016/0014-4827(76)90605-4. [DOI] [PubMed] [Google Scholar]
- Kooistra T., Duursma A. M., Bouma J. M., Gruber M. Endocytosis and breakdown of ribonuclease oligomers by sinusoidal rat liver cells in vivo. I. Effect of size. Biochim Biophys Acta. 1979 Oct 4;587(2):282–298. doi: 10.1016/0304-4165(79)90361-1. [DOI] [PubMed] [Google Scholar]
- Munniksma J., Noteborn M., Kooistra T., Stienstra S., Bouma J. M., Gruber M., Brouwer A., Praaning-van Dalen Dalen D., Knook D. L. Fluid endocytosis by rat liver and spleen. Experiments with 125I-labelled poly(vinylpyrrolidone) in vivo. Biochem J. 1980 Nov 15;192(2):613–621. doi: 10.1042/bj1920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman R. C., Attie A. D., Carew T. E., Steinberg D. Tissue sites of degradation of low density lipoprotein: application of a method for determining the fate of plasma proteins. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5345–5349. doi: 10.1073/pnas.76.10.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praaning-Van Dalen D. P., Knook D. L. Quantitative determination of in vivo endocytosis by rat liver Kupffer and endothelial cells facilitated by an improved cell isolation method. FEBS Lett. 1982 May 17;141(2):229–232. doi: 10.1016/0014-5793(82)80054-9. [DOI] [PubMed] [Google Scholar]
- Praaning-van Dalen D. P., Brouwer A., Knook D. L. Clearance capacity of rat liver Kupffer, Endothelial, and parenchymal cells. Gastroenterology. 1981 Dec;81(6):1036–1044. [PubMed] [Google Scholar]
- Sinke J., Bouma J. M., Kooistra T., Gruber M. Endocytosis and breakdown of 125I-labelled lactate dehydrogenase isoenzyme M4 by rat liver and spleen in vivo. Biochem J. 1979 Apr 15;180(1):1–9. doi: 10.1042/bj1800001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zile J., Henderson L. A., Baynes J. W., Thorpe S. R. [3H]Raffinose, a novel radioactive label for determining organ sites of catabolism of proteins in the circulation. J Biol Chem. 1979 May 10;254(9):3547–3553. [PubMed] [Google Scholar]
- van Berkel T. J., van Tol A. In vivo uptake of human and rat low density and high density lipoprotein by parenchymal and nonparenchymal cells from rat liver. Biochim Biophys Acta. 1978 Aug 25;530(2):299–304. doi: 10.1016/0005-2760(78)90015-2. [DOI] [PubMed] [Google Scholar]


