Abstract
In the current study, the nutritional value of various hydrolyzed feather meals (HFM) was investigated in two separate experiments (an in vitro and an in vivo experiment). The in vitro experiment was based on a completely randomized design with five replications and seven treatments including (1) Raw feather meal (RFM), (2) HFM by autoclave (Au-HFM), (3) Fermented feather meal (FFM) by Bacillus licheniformis (Bl-FFM), (4) FFM by Bacillus subtilis (Bs-FFM), (5) FFM by Aspergillus niger (An-FFM), (6) FFM by Bacillus licheniformis + Bacillus subtilis + Aspergillus niger (Co-FFM), and (7) HFM by an enzyme (En-HFM). The highest in vitro pepsin-pancreatin and apparent ileal CP digestibility were observed in Co-FFM, and the lowest amount belonged to RFM (P < 0.05). For the in-vivo experiment, 480 1-d-old male Ross 308 broilers were distributed in the experimental units in a completely randomized design with 8 treatments and 5 replicates (12 chicks/replicate). The treatments were: (1) Control diet (without feather meal (FM)), (2), (3), (4), (5), (6), (7), and (8), diets containing 4 % RFM, Au-HFM, Bl-FFM, Bs-FFM, An-FFM, Co-FFM, and En-HFM, respectively. For the in-vivo study, the birds fed control and Co-FFM diets had the highest feed intake, body weight gain, and the lowest feed conversion ratio compared to the other treatments (P < 0.05). The broilers fed the control and FFMs diets had the lowest relative weight of abdominal fat and liver compared to the other groups (P < 0.05). Therefore, our findings advise the poultry feed industry to look for Co-FFM as an effective alternative and cheaper feed ingredient to replace part of soybean meal in poultry diet.
Keywords: Broiler chicken, Co-fermentation, Feather meal, Fermentation
Highlights
-
•
The fermentation process improved the nutritional value of feather meal.
-
•
Fermented feather meal improved the performance of broilers.
-
•
Co-fermented feather meal feeding value in poultry is comparable to that of soybean meal.
1. Introduction
Up to 70 % of the total cost of commercial poultry production is spent on feed costs, partly due to shortages and the high cost of feed ingredients, particularly protein sources [1]. Feathers comprise 5–10 % of the weight of adult poultry and contain 80–90 % crude protein (CP) [2]. The yearly estimate of chicken feathers generated worldwide is around 15 billion tons [3]. The development of effective processes and appropriate strategies to recycle and improve the nutritional value of animal by-products, including poultry feathers as alternative protein sources, is becoming increasingly important from global economic and environmental perspectives [3]. Almost 90 % of the dry matter in feathers is formed of beta-keratin, a structural protein rich in the essential amino acids Thr, Arg, Val, Phe, Ile, and Leu, but low in Trp, Lys, and Met [2]. Due to the low price of feather meal (FM), it may be possible to reduce the cost of feeding monogastric animals by using it instead of other protein sources [4]. Furthermore, reduced food competition between humans and animals is achieved by feeding animals alternative or unconventional feed ingredients at lower costs [1]. However, feather keratin is insoluble in water due to hydrogen and disulfide bonds, as well as hydrophobic interactions between amino acids. Consequently, it has low digestibility with gastrointestinal enzymes such as pancreatin and pepsin [2].
There are many processing methods for the hydrolysis of FM, including autoclave, chemical hydrolysis, steam explosion, keratinolytic enzymes, and the action of keratinolytic microorganisms by fermentation process [2,5,6]. Hydrolysis of FM by autoclave is the most common process applied in the production of commercial FM. However, compared to the fermentation process, the final product obtained from hydrolyzed FM (HFM) by autoclave and chemical methods is often accompanied by a decrease in digestibility, availability, and content of amino acids [2,[7], [8], [9]]. For example, HFM by autoclave (Au-HFM) denatures amino acid structure and produces non-nutritional amino acids such as lanthionine and lysinoalanine from Cys and Lys [2,10]. Additionally, the high costs associated with both the separation and the purification of the keratinase lead to the high cost of HFM by an enzyme (En-HFM). Thus, it is necessary to use moderate hydrolysis methods to minimize the loss of valuable amino acids and increase the digestibility of FM in poultry and animal feed. Some microorganisms, such as fungi, actinomycetes, and bacillus species, are known to degrade keratinous substrates [11]. The digestibility of FM and the biological availability of its amino acids can be improved by breaking the structural bonds through the optimal way of processing methods [12,13]. Experiments indicated that compared to the other methods, the fermentation process efficiently bioconverts the FM under mild conditions, which improves digestibility and bioavailability, reduces amino acid losses, reduces hydrolyzing energy requirements, and reduces secondary pollution [8,[14], [15], [16]]. In addition, fermentation of FM is more environmentally friendly and cost-effective than other methods [15]. There is, no documented literature on the increasing fermentation time of up to 12 days for efficient improvement of the nutritional quality of FM with single-strain microorganisms and fungal-bacterial Co-fermentation to investigate their synergistic effects and comparison with the specific keratinase enzyme for feather hydrolysis. Therefore, the purpose of the current research was to determine and compare the impact of various HFM on their in vitro pepsin-pancreatin and apparent ileal CP digestibility, apparent metabolizable energy (AME), as well as AME corrected for nitrogen (AMEn), and comparison of their effect on broiler performance and carcass characteristics.
2. Materials and methods
2.1. Experiment 1
2.1.1. Preparation of various HFM
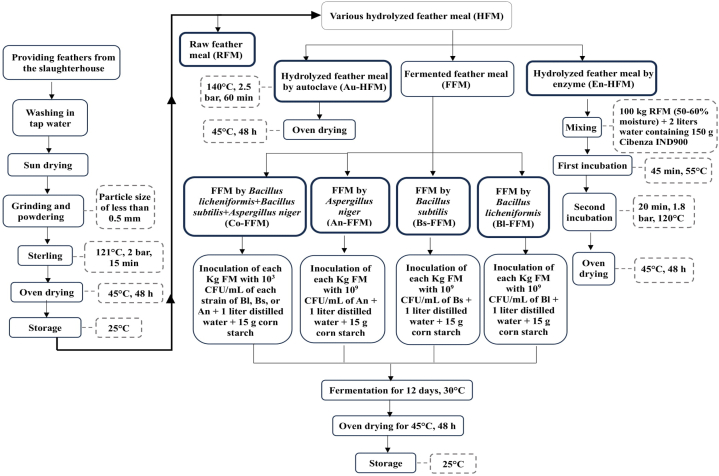
A completely randomized design with seven treatments and five replicates was used to assess the nutritional value of hydrolyzed feather meal (HFM) using different methods. Experimental treatments included (1) Raw feather meal (RFM), (2) HFM by autoclave (Au-HFM), (3) Fermented feather meal (FFM) by Bacillus licheniformis (Bl-FFM), (4) FFM by Bacillus subtilis (Bs-FFM), (5) FFM by Aspergillus niger (An-FFM), (6) FFM by Bacillus licheniformis + Bacillus subtilis + Aspergillus niger (Co-FFM), and (7) HFM by an enzyme (En-HFM). Various HFM process is shown in Fig. 1.
Fig. 1.
Flowchart of the various hydrolyzed feather meals (HFM) process.
Preparation of FM: Feathers from a local slaughterhouse were collected from white broiler chickens. After mechanically removing the feathers, the feather was hand washed in tap water to remove any dirt and finally exposed to the sun and dried. By grinding and powdering the material, a particle size of less than 0.5 mm was achieved. After the powdered feathers were sterilized in an autoclave at 121 °C, and 2 bar for 15 min, they were dried at 45 °C for 48 h in the sterile oven. Finally, for the RFM, a portion of the dried FM was kept in sterile storage. Meanwhile, the remaining material was ready for hydrolysis using an enzyme, a fermentation process, or an autoclave.
Fermentation of FM: The methodology of earlier studies was followed in the development of the solid-state fermentation process [17,18]. Below are the steps involved in fermenting FM: (1) Bacillus licheniformis (PTCC: 1595) as well as Bacillus subtilis (PTCC: 1720) bacteria, and Aspergillus niger (PTCC: 5154) fungi, were prepared from the Persian Type Culture Collection of Iranian Research Organization for Science and Technology (IROST). (2) Bacillus subtilis and Bacillus licheniformis were grown on nutrient agar (Merck, Germany) and incubated for 48 h at 30 °C and 37 °C, respectively. On a medium of potato dextrose agar (PDA; Merck, Germany), Aspergillus niger was cultivated and incubated for 72 h at 26 °C. (3) To provide carbohydrates, 15 g of corn starch and 1 L of distilled water were combined with each kilogram of FM. This mixture was then inoculated with either 109 colony-forming units (CFU)/mL of Bacillus licheniformis, Bacillus subtilis, Aspergillus niger, or Co-FFM (Co-FFM was performed with 103 CFU/mL of each strain) in FFM treatments as starter cultures. Inoculation took place in a unique tank that had a one-way valve to allow the gases produced to escape and prevent air from entering. The inoculation process lasted for 12 days at 30 °C. (4) Finally, FFM was dried at 50 °C for 2 days through a sterile oven.
Autoclave Hydrolysis: For this treatment, FM was hydrolyzed with an autoclave at 140 °C and 2.5 kg/cm2 steam pressure for 60 min, then dried at 45 °C for 48 h in the sterile oven [19].
Enzymatic Hydrolysis: Cibenza IND900, an enzyme from Novus International, Inc., was used in this experiment. The sole purpose of Cibenza IND900, a heat-stable protease, is to enhance the nutritional value of the FM [20]. According to the manufacturer, the enzyme activity of Cibenza IND900 is 65 000 U/g [20]. Below are the phases involved in FM hydrolysis by Cibenza IND900: (1) A batch of 50 kg of raw feathers with a moisture content of 50–60 % was loaded. (2) A solution containing 150 g of Cibenza IND900 was prepared by dissolving it in 2 L of water. (3) The feathers were combined with Cibenza IND900 solution and mixed thoroughly. (4) Before placing in the oven, another 50 kg of raw feathers with a moisture content of 50–60 % were added to the batch and mixed thoroughly several more times. (5) 45 min were spent on the incubation at 55 °C. (6) While stirring for 20 min, the pressure was maintained at 1.8 bar while the temperature was gradually increased to 120 °C. (7) The enzymatic HFM was dried at 45 °C during 48 h in the sterile oven [20].
2.1.2. Determination of in vitro protein digestibility
For in vitro proteolytic digestion of samples, pepsin plus pancreatin was used as outlined earlier [11,21]. Three ground samples per replicate, each weighing 1 g, were incubated for 2 h at 37 °C in 2M HCl containing 2 mg/mL pepsin (from the porcine stomach, Sigma). After incubation, 2 mg/mL pancreatin (from porcine pancreas, Sigma) was added and the pH was brought to 8.0 with 2M NaHCO3. Incubation was then continued for 16 h. Following digestion, the samples were centrifuged, and the Kjeldahl method was used to measure the amount of protein in the supernatants. The samples were measured three times in the laboratory. As shown in equation (1) the percentage of in vitro protein digestibility (D) was calculated as:
| D = ((the amount of protein released after 1 g of sample is digested)/(the amount of total protein in 1 g of sample before digestion)) × 100. | (Equation-1) |
2.1.3. Determination of apparent ileal CP digestibility
A total of 210 male broiler chickens (Ross 308) from 14 to 21 days old were used to evaluate the apparent ileal crude protein (CP) digestibility of various HFM. For the first 13 days, 700 male broilers received an FM-free basal diet consisting of corn and soybean meal which was formulated based on the broiler chicken Ross 308 nutrient requirement guideline [22]. On the 14th day, after 12 h of fasting, broilers were weighed individually. Then 210 male broilers with uniform body weight were distributed randomly to 7 treatments with 5 replicates and 6 birds per replicate in a completely randomized design. The semi-purified experimental diets were: (1) Diet containing RFM, (2) Diet containing Au-HFM, (3) Diet containing Bl-FFM, (4) Diet containing Bs-FFM, (5) Diet containing An-FFM, (6) Diet containing Co-FFM, and (7) Diet containing En-HFM. Seven iso-nitrogenous and iso-energetic diets were formulated (Table 1). Furthermore, FM was the only source of CP in each experimental diet. All diets were supplemented with 1 % celite as a source of acid-insoluble ash (AIA) as a marker. All diets were fed in the mash form, also feed and water was supplied ad libitum. On d 21, four birds from each replicate that was closest to the average pen weight were killed (euthanized by cervical vertebra dislocation), and ileal digesta were collected. To determine apparent ileal CP digestibility, the contents of the ileum, from Meckel's diverticulum to approximately 1 cm proximal to the ileocecal junction, were collected [23]. The ileal digesta was collected, freeze-dried (freeze-dryer model FD-5003-BT, Dena Vacuum, Iran), ground, and then analyzed for CP according to the method of AOAC [24]. The content of AIA in the diet and ileal digesta were determined based on De Coca-Sinova et al. [25]. Samples were analyzed in triplicate. Equation (2) was used to determine the apparent ileal CP digestibility [23]:
| Apparent ileal CP digestibility (%) = 100 – [100 × (diet AIA (%) / ileal digesta AIA (%)) × (ileal digesta CP (%) / diet CP (%))]. | (Equation-2) |
Table 1.
Ingredients and nutrient composition of the semi-purified diets during the apparent ileal crude protein digestibility experiment 14–21 days (as-fed basis).
| Ingredients (%) | Treatmentsa |
||||||
|---|---|---|---|---|---|---|---|
| RFM | Au-HFM | Bl-FFM | Bs-FFM | An-FFM | Co-FFM | En-HFM | |
| Corn starch | 66.3 | 66.04 | 64.11 | 64 | 63.93 | 63.65 | 66.2 |
| Soybean oil | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 |
| RFM | 25.08 | 0 | 0 | 0 | 0 | 0 | 0 |
| Au-HFM | 0 | 25.35 | 0 | 0 | 0 | 0 | 0 |
| Bl-FFM | 0 | 0 | 27.4 | 0 | 0 | 0 | 0 |
| Bs-FFM | 0 | 0 | 0 | 27.32 | 0 | 0 | 0 |
| An-FFM | 0 | 0 | 0 | 0 | 27.43 | 0 | 0 |
| Co-FFM | 0 | 0 | 0 | 0 | 0 | 27.74 | 0 |
| En-HFM | 0 | 0 | 0 | 0 | 0 | 0 | 25.2 |
| Acid insoluble ash (AIA) | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sodium chloride | 0.19 | 0.19 | 0.16 | 0.16 | 0.15 | 0.14 | 0.19 |
| Calcium carbonate | 1.03 | 1.03 | 1.08 | 1.09 | 1.09 | 1.09 | 1.03 |
| Dicalcium phosphate | 1.85 | 1.84 | 1.7 | 1.7 | 1.67 | 1.66 | 1.83 |
| Filler | 3.35 | 3.35 | 3.35 | 3.53 | 3.53 | 3.53 | 3.35 |
| Mineral and Vitamin Premixb | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Nutrient composition | |||||||
| Metabolizable Energy (kcal/kg) | 3200 | 3200 | 3200 | 3200 | 3200 | 3200 | 3200 |
| Crude protein (%) | 22.19 | 22.19 | 22.19 | 22.19 | 22.19 | 22.19 | 22.19 |
| Calcium (%) | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 |
| Available phosphorus (%) | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 |
RFM = raw feather meal; Au-HFM = hydrolyzed feather meal by autoclave; Bl-FFM = fermented feather meal by Bacillus Licheniformis; Bs-FFM = fermented feather meal by Bacillus Subtilis; An-FFM = fermented feather meal by Aspergillus Niger; Co-FFM = fermented feather meal by Bacillus Licheniformis + Bacillus Subtilis + Aspergillus Niger; En-HFM = hydrolyzed feather meal by an enzyme.
Supplied per kg diet: vitamin A, 11 000 U; vitamin D3, 5000 U; vitamin E, 36.75 U; vitamin K3, 3.4 mg; vitamin B1,1.98 mg; vitamin B2, 5.25 mg; pantothenic acid, 10.5 mg; niacin, 31.5 mg; vitamin B6, 2.87 mg; folic acid, 1.2 mg; vitamin B12, 0.024 mg; biotin, 0.105 mg; choline, 800 mg; manganese, 120 mg; zinc, 100 mg; iron, 50 mg; copper, 12 mg; I, 1.3 mg; selenium, 0.3 mg; antioxidant, 100 mg.
2.1.4. Determination of the metabolizable energy
A total of 280 male broiler chickens (Ross 308) with uniform body weights from 35 to 42 days were used in this experiment. The first four days were the adaptation period and the next three days were the experimental period. Birds were randomly assigned to the eight treatments with five replicates and seven birds per replicate in a completely randomized design. Each pen's solid floor was swapped out for a wire mesh floor, and plastic trays were positioned underneath to collect excrement. The apparent metabolizable energy (AME) and AME corrected for nitrogen (AMEn) values of various HFM and RFM were measured using a substitution method, as described by Azam et al. [26] and Li et al. [27]. According to this method, a corn-soybean meal basal diet was formulated as the reference diet based on the broiler chicken Ross 308 nutrient requirement guideline [22]. The amount of 30 % (w/w) of corn and soybean meal of the reference diet was substituted by the various HFM or RFM in test diets. The composition of the ingredients of the basal diet and the test diets is shown in Table 2. The following diets were used in the experiment: (1) Basal diet (without FM and based on soybean meal and corn), (2) 70 % basal diet + 30 % RFM, (3) 70 % basal diet + 30 % Au-HFM, (4) 70 % basal diet + 30 % Bl-FFM, (5) 70 % basal diet + 30 % Bs-FFM, (6) 70 % basal diet + 30 % An-FFM, (7) 70 % basal diet + 30 % Co-FFM, and (8) 70 % basal diet + 30 % En-HFM. All diets were supplemented with 1 % celite as a source of AIA as a marker. All diets were fed in mash form. Feed and water were supplied ad libitum. Excreta samples were collected daily and stored at −20 °C until analysis. Finally, before taking the sample (three samples from each replicate) for laboratory analysis, stored samples of each replicate were pooled, homogenized, and ground. In the lab, the samples were tested three times. An adiabatic bomb calorimeter (model: PARR-1356USA) was used to calculate the gross energy content of feed and excreta. The amount of AIA in the diet and excreta was determined to match with De Coca-Sinova et al. [25]. The level of AME and AMEn experimental diets and each of the various HFM, as well as RFM, were calculated using the equations number 3 to 6 [28,29]:
| AME (kcal/kg) = GE Diet – [GE Excreta × (AIADiet / AIA Excreta)] | (Equation-3) |
| AMEn (kcal/kg) = AME – 8.73 × [N Diet - (AIADiet / AIA Excreta) × N Excreta] | (Equation-4) |
| AME FM = AME basal diet - [(AME basal diet- AME test diet)/ FM inclusion rate] | (Equation-5) |
| AMEn FM = AMEn basal diet - [(AMEn basal diet- AMEn test diet)/ FM inclusion rate] | (Equation-6) |
Table 2.
Ingredient composition of the basal diet and test diets fed from 35 to 42 d of age for the determination of AME, and AMEn in broilers (as-fed basis).
| Ingredients (%) | Treatmentsa |
|||||||
|---|---|---|---|---|---|---|---|---|
| Basal diet | RFM | Au-HFM | Bl-FFM | Bs-FFM | An-FFM | Co-FFM | En-HFM | |
| Corn | 58.13 | 48.13 | 48.13 | 48.13 | 48.13 | 48.13 | 48.13 | 48.13 |
| Soybean meal (CP: 44 %) | 32.04 | 12.04 | 12.04 | 12.04 | 12.04 | 12.04 | 12.04 | 12.04 |
| Soybean oil | 5.04 | 5.04 | 5.04 | 5.04 | 5.04 | 5.04 | 5.04 | 5.04 |
| RFM | 0 | 30 | 0 | 0 | 0 | 0 | 0 | 0 |
| Au-HFM | 0 | 0 | 30 | 0 | 0 | 0 | 0 | 0 |
| Bl-FFM | 0 | 0 | 0 | 30 | 0 | 0 | 0 | 0 |
| Bs-FFM | 0 | 0 | 0 | 0 | 30 | 0 | 0 | 0 |
| An-FFM | 0 | 0 | 0 | 0 | 0 | 30 | 0 | 0 |
| Co-FFM | 0 | 0 | 0 | 0 | 0 | 0 | 30 | 0 |
| En-HFM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 30 |
| Acid insoluble ash (AIA) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Dicalcium phosphate | 1.52 | 1.52 | 1.52 | 1.52 | 1.52 | 1.52 | 1.52 | 1.52 |
| Calcium carbonate | 0.81 | 0.81 | 0.81 | 0.81 | 0.81 | 0.81 | 0.81 | 0.81 |
| Sodium bicarbonate | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Sodium chloride | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 | 0.13 |
| Mineral and Vitamin Premix b | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| DL-Met | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| L-Lys | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| L-Thr | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
Basal diet = diet based on corn and soybean meal, without FM; RFM = raw feather meal; Au-HFM = hydrolyzed feather meal by autoclave; Bl-FFM = fermented feather meal by Bacillus Licheniformis; Bs-FFM = fermented feather meal by Bacillus Subtilis; An-FFM = fermented feather meal by Aspergillus Niger; Co-FFM = fermented feather meal by Bacillus Licheniformis + Bacillus Subtilis + Aspergillus Niger; En-HFM = hydrolyzed feather meal by an enzyme.
Supplied per kg diet: vitamin A, 11 000 U; vitamin D3, 5000 U; vitamin E, 36.75 U; vitamin K3, 3.4 mg; vitamin B1,1.98 mg; vitamin B2, 5.25 mg; pantothenic acid, 10.5 mg; niacin, 31.5 mg; vitamin B6, 2.87 mg; folic acid, 1.2 mg; vitamin B12, 0.024 mg; biotin, 0.105 mg; choline, 800 mg; manganese, 120 mg; zinc, 100 mg; iron, 50 mg; copper, 12 mg; I, 1.3 mg; selenium, 0.3 mg; antioxidant, 100 mg.
AME: apparent metabolizable energy (kcal/kg); AMEn: apparent metabolizable energy corrected for nitrogen (kcal/kg); FM: feather meal; GE: gross energy (kcal/kg); N: nitrogen; AIA: acid insoluble ash; and 8.73: nitrogen correction factor.
2.2. Experiment 2
2.2.1. Experimental birds and diets
A completely randomized design consisting of eight dietary treatments and five replicates of twelve birds each was applied to 480 one-day-old male broilers (Ross 308) purchased from the commercial hatchery and weighed individually before being placed in replicates. Broilers were vaccinated against Gumboro and Newcastle Disease in the hatchery, also birds were subcutaneously vaccinated in the back of the neck against Newcastle-influenza (H9N2 subtype) with 0.3 ml per chick at 8 d of age. In addition, birds were orally vaccinated against Newcastle disease (Lasota) at 18 d of age. The birds were kept in pens (1 m wide, 1.60 m long, and 1 m high), equipped with a feeder, a nipple drinker, and wood shavings as litter. Environmental conditions in the house, photoperiod, temperature, and relative humidity, were set according to the Ross guidelines [22]. The chemical composition analysis of RFM and various HFM from the first experiment reported in our previous study [30] was used for diet formulation. The nutritional requirements guideline for Ross 308 broilers was followed when formulating the diets. The following were the experimental diets: (1) Control diet (without FM and based on soybean meal and corn), (2) Diet containing 4 % RFM, (3) Diet containing 4 % Au-HFM, (4) Diet containing 4 % Bl-FFM, (5) Diet containing 4 % Bs-FFM, (6) Diet containing 4 % An-FFM, (7) Diet containing 4 % Co-FFM, and (8) Diet containing 4 % En-HFM. Table 3, Table 4, Table 5 present the ingredients and chemical composition of starter (1–10 days), grower (11–24 days) and finisher (25–42 days) diets in that order. During the study, birds had ad libitum access to feed and water, and all diets were given in mash form. The study was conducted for 42 days.
Table 3.
Ingredients and nutrient composition of the starter (1–10 days) diets.
| Ingredients (%) | Treatmentsa |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | RFM | Au-HFM | Bl-FFM | Bs-FFM | An-FFM | Co-FFM | En-HFM | |
| Corn | 52.97 | 59.98 | 59.83 | 58.93 | 58.99 | 58.94 | 58.76 | 59.93 |
| Soybean meal (CP: 44 %) | 40.12 | 30.58 | 30.71 | 31.58 | 31.56 | 31.6 | 31.8 | 30.65 |
| Soybean oil | 2.44 | 0.82 | 0.86 | 0.99 | 0.95 | 0.97 | 1.01 | 0.83 |
| RFM | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Au-HFM | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| Bl-FFM | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 |
| Bs-FFM | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| An-FFM | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| Co-FFM | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| En-HFM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Dicalcium phosphate | 1.9 | 1.89 | 1.88 | 1.86 | 1.86 | 1.86 | 1.86 | 1.88 |
| Calcium carbonate | 0.95 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Sodium bicarbonate | 0.21 | 0.23 | 0.23 | 0.21 | 0.2 | 0.2 | 0.19 | 0.23 |
| Sodium chloride | 0.23 | 0.15 | 0.15 | 0.16 | 0.16 | 0.16 | 0.16 | 0.15 |
| Mineral and Vitamin Premixb | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| DL-Met | 0.34 | 0.32 | 0.31 | 0.3 | 0.3 | 0.3 | 0.3 | 0.31 |
| L-Lys | 0.24 | 0.44 | 0.43 | 0.4 | 0.39 | 0.39 | 0.37 | 0.43 |
| L-Thr | 0.11 | 0.1 | 0.1 | 0.08 | 0.08 | 0.08 | 0.05 | 0.1 |
| Nutrient composition | ||||||||
| MEc (kcal/kg) | 2900 | 2900 | 2900 | 2900 | 2900 | 2900 | 2900 | 2900 |
| Crude protein (%) | 22.23 | 22.23 | 22.23 | 22.23 | 22.23 | 22.23 | 22.23 | 22.23 |
| Lys (%) | 1.39 | 1.39 | 1.39 | 1.39 | 1.39 | 1.39 | 1.39 | 1.39 |
| Met + Cys (%) | 1.04 | 1.04 | 1.04 | 1.04 | 1.04 | 1.04 | 1.04 | 1.04 |
| Thr (%) | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 |
| Calcium (%) | 0.93 | 0.93 | 0.93 | 0.93 | 0.93 | 0.93 | 0.93 | 0.93 |
| Available phosphorus (%) | 0.46 | 0.46 | 0.46 | 0.46 | 0.46 | 0.46 | 0.46 | 0.46 |
| Sodium (%) | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
Control = diet based on corn and soybean meal, without FM; RFM = raw feather meal; Au-HFM = hydrolyzed feather meal by autoclave; Bl-FFM = fermented feather meal by Bacillus Licheniformis; Bs-FFM = fermented feather meal by Bacillus Subtilis; An-FFM = fermented feather meal by Aspergillus Niger; Co-FFM = fermented feather meal by Bacillus Licheniformis + Bacillus Subtilis + Aspergillus Niger; En-HFM = hydrolyzed feather meal by an enzyme.
Supplied per kg diet: vitamin A, 11 000 U; vitamin D3, 5000 U; vitamin E, 36.75 U; vitamin K3, 3.4 mg; vitamin B1,1.98 mg; vitamin B2, 5.25 mg; pantothenic acid, 10.5 mg; niacin, 31.5 mg; vitamin B6, 2.87 mg; folic acid, 1.2 mg; vitamin B12, 0.024 mg; biotin, 0.105 mg; choline, 800 mg; manganese, 120 mg; zinc, 100 mg; iron, 50 mg; copper, 12 mg; I, 1.3 mg; selenium, 0.3 mg; antioxidant, 100 mg.
ME = metabolizable Energy.
Table 4.
Ingredients and nutrient composition of the grower (11–24 days) diets.
| Ingredients (%) | Treatmentsa |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | RFM | Au-HFM | Bl-FFM | Bs-FFM | An-FFM | Co-FFM | En-HFM | |
| Corn | 56.51 | 62.96 | 63.05 | 62.43 | 62.52 | 62.48 | 62.15 | 63.22 |
| Soybean meal (CP: 44 %) | 35.25 | 26.74 | 26.42 | 26.74 | 26.69 | 26.73 | 27.22 | 26.22 |
| Corn gluten meal | 1 | 0.31 | 0.62 | 1 | 1 | 1 | 0.8 | 0.71 |
| Soybean oil | 3.18 | 1.82 | 1.74 | 1.74 | 1.70 | 1.72 | 1.82 | 1.68 |
| RFM | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Au-HFM | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| Bl-FFM | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 |
| Bs-FFM | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| An-FFM | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| Co-FFM | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| En-HFM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Dicalcium phosphate | 1.7 | 1.69 | 1.68 | 1.67 | 1.66 | 1.65 | 1.65 | 1.69 |
| Calcium carbonate | 0.88 | 0.91 | 0.92 | 0.92 | 0.93 | 0.93 | 0.93 | 0.92 |
| Sodium bicarbonate | 0.19 | 0.2 | 0.21 | 0.19 | 0.19 | 0.18 | 0.17 | 0.21 |
| Sodium chloride | 0.24 | 0.17 | 0.16 | 0.17 | 0.17 | 0.17 | 0.17 | 0.16 |
| Mineral and Vitamin Premixb | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| DL-Met | 0.28 | 0.26 | 0.25 | 0.24 | 0.24 | 0.24 | 0.24 | 0.25 |
| L-Lys | 0.2 | 0.37 | 0.38 | 0.35 | 0.36 | 0.36 | 0.33 | 0.38 |
| L-Thr | 0.07 | 0.07 | 0.06 | 0.04 | 0.04 | 0.04 | 0.02 | 0.06 |
| Nutrient composition | ||||||||
| MEc (kcal/kg) | 3000 | 3000 | 3000 | 3000 | 3000 | 3000 | 3000 | 3000 |
| Crude protein (%) | 20.81 | 20.81 | 20.81 | 20.81 | 20.81 | 20.81 | 20.81 | 20.81 |
| Lys (%) | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 |
| Met + Cys (%) | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 |
| Thr (%) | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 | 0.85 |
| Calcium (%) | 0.84 | 0.84 | 0.84 | 0.84 | 0.84 | 0.84 | 0.84 | 0.84 |
| Available phosphorus (%) | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 |
| Sodium (%) | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
Control = diet based on corn and soybean meal, without FM; RFM = raw feather meal; Au-HFM = hydrolyzed feather meal by autoclave; Bl-FFM = fermented feather meal by Bacillus Licheniformis; Bs-FFM = fermented feather meal by Bacillus Subtilis; An-FFM = fermented feather meal by Aspergillus Niger; Co-FFM = fermented feather meal by Bacillus Licheniformis + Bacillus Subtilis + Aspergillus Niger; En-HFM = hydrolyzed feather meal by an enzyme.
Supplied per kg diet: vitamin A, 11 000 U; vitamin D3, 5000 U; vitamin E, 36.75 U; vitamin K3, 3.4 mg; vitamin B1,1.98 mg; vitamin B2, 5.25 mg; pantothenic acid, 10.5 mg; niacin, 31.5 mg; vitamin B6, 2.87 mg; folic acid, 1.2 mg; vitamin B12, 0.024 mg; biotin, 0.105 mg; choline, 800 mg; manganese, 120 mg; zinc, 100 mg; iron, 50 mg; copper, 12 mg; I, 1.3 mg; selenium, 0.3 mg; antioxidant, 100 mg.
ME = metabolizable Energy.
Table 5.
Ingredients and nutrient composition of the finisher (25–42 days) diets.
| Ingredients (%) | Treatmentsa |
|||||||
|---|---|---|---|---|---|---|---|---|
| Control | RFM | Au-HFM | Bl-FFM | Bs-FFM | An-FFM | Co-FFM | En-HFM | |
| Corn | 60.24 | 67.24 | 67.08 | 66.18 | 66.26 | 66.19 | 66.02 | 67.14 |
| Soybean meal (CP: 44 %) | 31.68 | 22.15 | 22.29 | 23.16 | 23.12 | 23.17 | 23.36 | 22.23 |
| Soybean oil | 4.34 | 2.72 | 2.76 | 2.89 | 2.85 | 2.88 | 2.91 | 2.75 |
| RFM | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Au-HFM | 0 | 0 | 4 | 0 | 0 | 0 | 0 | 0 |
| Bl-FFM | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 0 |
| Bs-FFM | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| An-FFM | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 |
| Co-FFM | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0 |
| En-HFM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Dicalcium phosphate | 1.52 | 1.5 | 1.5 | 1.48 | 1.48 | 1.48 | 1.48 | 1.5 |
| Calcium carbonate | 0.82 | 0.86 | 0.86 | 0.87 | 0.87 | 0.86 | 0.86 | 0.86 |
| Sodium bicarbonate | 0.3 | 0.33 | 0.32 | 0.29 | 0.29 | 0.3 | 0.28 | 0.34 |
| Sodium chloride | 0.13 | 0.05 | 0.06 | 0.07 | 0.07 | 0.06 | 0.07 | 0.06 |
| Mineral and Vitamin Premix b | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| DL-Met | 0.26 | 0.23 | 0.23 | 0.22 | 0.22 | 0.22 | 0.21 | 0.23 |
| L-Lys | 0.17 | 0.37 | 0.36 | 0.32 | 0.32 | 0.32 | 0.3 | 0.36 |
| L-Thr | 0.05 | 0.04 | 0.04 | 0.02 | 0.02 | 0.02 | 0 | 0.04 |
| Nutrient composition | ||||||||
| MEc (kcal/kg) | 3100 | 3100 | 3100 | 3100 | 3100 | 3100 | 3100 | 3100 |
| Crude protein (%) | 18.89 | 18.89 | 18.89 | 18.89 | 18.89 | 18.89 | 18.89 | 18.89 |
| Lys (%) | 1.12 | 1.12 | 1.12 | 1.12 | 1.12 | 1.12 | 1.12 | 1.12 |
| Met + Cys (%) | 0.88 | 0.88 | 0.88 | 0.88 | 0.88 | 0.88 | 0.88 | 0.88 |
| Thr (%) | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 |
| Calcium (%) | 0.77 | 0.77 | 0.77 | 0.77 | 0.77 | 0.77 | 0.77 | 0.77 |
| Available phosphorus (%) | 0.38 | 0.38 | 0.38 | 0.38 | 0.38 | 0.38 | 0.38 | 0.38 |
| Sodium (%) | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
Control = diet based on corn and soybean meal, without FM; RFM = raw feather meal; Au-HFM = hydrolyzed feather meal by autoclave; Bl-FFM = fermented feather meal by Bacillus Licheniformis; Bs-FFM = fermented feather meal by Bacillus Subtilis; An-FFM = fermented feather meal by Aspergillus Niger; Co-FFM = fermented feather meal by Bacillus Licheniformis + Bacillus Subtilis + Aspergillus Niger; En-HFM = hydrolyzed feather meal by an enzyme.
Supplied per kg diet: vitamin A, 11 000 U; vitamin D3, 5000 U; vitamin E, 36.75 U; vitamin K3, 3.4 mg; vitamin B1,1.98 mg; vitamin B2, 5.25 mg; pantothenic acid, 10.5 mg; niacin, 31.5 mg; vitamin B6, 2.87 mg; folic acid, 1.2 mg; vitamin B12, 0.024 mg; biotin, 0.105 mg; choline, 800 mg; manganese, 120 mg; zinc, 100 mg; iron, 50 mg; copper, 12 mg; I, 1.3 mg; selenium, 0.3 mg; antioxidant, 100 mg.
ME = metabolizable Energ
2.2.2. Performance and carcass characteristics
The body weight and feed intake (FI) of the birds were recorded weekly to calculate the body weight gain (BWG), FI, and feed conversion ratio (FCR). On day 42, at the end of the study, three birds selected whose weight was closest to the average weight of their pen were weighed, slaughtered, and de-feathered to study carcass traits. The weight of carcass, heart, gizzard, thymus, abdominal fat, liver, spleen, pancreas, and bursa of Fabricius was measured in gram and expressed as a percent of live body weight, and the weight of the breast and thighs, which were weighed and expressed as a percentage of eviscerated carcass weight.
2.3. Statistical analysis
Homogeneity of variance and distribution normality were assessed using the Levene and Shapiro-Wilk tests, respectively. The General Linear Model (GLM) procedures of SAS software version 9.2 [31] were used to analyze the data in a completely randomized design, using one-way ANOVA test. The significant differences in treatment means were found using Tukey's post hoc multiple-range tests. The significance level was set at P < 0.05.
3. Results
3.1. Experiment 1
3.1.1. Determination of in vitro, and in vivo CP digestibility, and metabolizable energy of various HFM
As shown in Table 6, compared with the raw feather meal (RFM), significantly higher in vitro pepsin-pancreatin crude protein (CP) digestibility and apparent ileal CP digestibility were in FFM by Bacillus licheniformis + Bacillus subtilis + Aspergillus niger (Co-FFM), fermented feather meal (FFM) by single strains (each of FFM by Bacillus licheniformis (Bl-FFM), FFM by Bacillus subtilis (Bs-FFM), and FFM by Aspergillus niger (An-FFM)), hydrolyzed feather meal (HFM) by an enzyme (En-HFM), and HFM by autoclave (Au-HFM) respectively (P < 0.05). There was no significant difference in apparent metabolizable energy (AME) and AME corrected for nitrogen (AMEn) between various HFM and RFM (P > 0.05).
Table 6.
In vitro pepsin-pancreatin crude protein digestibility (%), apparent ileal crude protein digestibility at 21 d of age (%), AME, and AMEn.
| Treatmentsa | Parametersb |
|||
|---|---|---|---|---|
| Pepsin-pancreatin crude protein digestibility | Apparent ileal crude protein digestibility | AME (kcal/kg) | AMEn (kcal/kg) | |
| RFM | 42.4e | 35.5e | 3013 | 2920 |
| Au-HFM | 53.1d | 51.1d | 3003 | 2918 |
| Bl-FFM | 81.3b | 79.6 b | 3039 | 2957 |
| Bs-FFM | 83.4b | 80.4 b | 3066 | 2991 |
| An-FFM | 83.7b | 80.2 b | 3046 | 2973 |
| Co-FFM | 94.1a | 90.4a | 3056 | 2982 |
| En-HFM | 63.8c | 59.8c | 3023 | 2932 |
| SEM | 3.44 | 3.57 | 16.42 | 16.47 |
| P-value | 0.001 | 0.001 | 0.863 | 0.631 |
a-e Means within a column with different superscripts differ significantly (P < 0.05).
RFM = raw feather meal; Au-HFM = hydrolyzed feather meal by autoclave; Bl-FFM = fermented feather meal by Bacillus Licheniformis; Bs-FFM = fermented feather meal by Bacillus Subtilis; An-FFM = fermented feather meal by Aspergillus Niger; Co-FFM = fermented feather meal by Bacillus Licheniformis + Bacillus Subtilis + Aspergillus Niger; En-HFM = hydrolyzed feather meal by an enzyme.
AME = apparent metabolizable energy; AMEn = apparent metabolizable energy corrected for nitrogen.
3.2. Experiment 2
3.2.1. Performance of broilers chicken
The effect of various HFM on feed intake (FI), body weight gain (BWG), and feed conversion ratio (FCR) are summarized in Table 7. Results revealed that FI and BWG were increased in birds fed the diet containing Co-FFM and the control diet in comparison with the other treatments (P < 0.05). The FI and BWG were not significantly different among birds fed An-FFM, Bs-FFM, and Bl-FFM (P > 0.05). Broilers fed Bl-FFM, and An-FFM had significantly higher FI than those fed En-HFM (P < 0.05). Bl-FFM, Bs-FFM, and An-FFM had significantly higher BWG than birds fed the diet containing RFM, Au-HFM, or En-HFM (P < 0.05). Birds fed En-HFM had higher FI and BWG than those fed diets containing RFM, and Au-HFM (P < 0.05). The FCR of birds fed the diet containing Co-FFM or the control diet was significantly lower than all of the other treatments (P < 0.05). Although no significant difference was found among birds fed the diet containing Bl-FFM, Bs-FFM, and An-FFM (P > 0.05), they showed significantly lower FCR compared to broilers fed the diet containing Au-HFM, En-HFM, and RFM (P < 0.05). Birds fed the diet containing En-HFM had lower FCR than birds fed RFM (P < 0.05).
Table 7.
The effect of various hydrolyzed feather meals on the performance of broilers in 1–42 days old.
| Treatmentsa | Parameters |
||
|---|---|---|---|
| Feed intake (g/Chick/day) | Body weight gain (g/Chick/day) | Feed conversion ratio (g/g) | |
| Control | 119a | 67.3a | 1.77d |
| RFM | 103d | 49.5e | 2.08a |
| Au-HFM | 104d | 52.1d | 2.01ab |
| Bl-FFM | 112b | 60.2b | 1.86c |
| Bs-FFM | 111bc | 59.7b | 1.87c |
| An-FFM | 112b | 60.2b | 1.86c |
| Co-FFM | 118a | 66.4a | 1.78d |
| En-HFM | 109c | 56.0c | 1.94b |
| SEM | 1.004 | 1.060 | 0.047 |
| P-value | 0.001 | 0.001 | 0.001 |
a-e Means within a column with different superscripts differ significantly (P < 0.05).
Control = diet based on corn and soybean meal, without FM; RFM = raw feather meal; Au-HFM = hydrolyzed feather meal by autoclave; Bl-FFM = fermented feather meal by Bacillus Licheniformis; Bs-FFM = fermented feather meal by Bacillus Subtilis; An-FFM = fermented feather meal by Aspergillus Niger; Co-FFM = fermented feather meal by Bacillus Licheniformis + Bacillus Subtilis + Aspergillus Niger; En-HFM = hydrolyzed feather meal by an enzyme.
3.2.2. Carcass characteristics of broilers chicken
Data on the effect of various HFM on broiler carcass characteristics are presented in Table 8. The relative weight of the liver and abdominal fat in broilers fed on diets containing FFM and control diet was significantly lower than those fed diets containing RFM, Au-HFM, and En-HFM (P < 0.05). Birds fed diets containing FFM had a significantly higher relative weight of the pancreas compared to the control and the other groups (P < 0.05). There was no significant difference in the relative weight of the carcass, breast, thigh, heart, gizzard, thymus, spleen, and bursa of Fabricius between different treatments (P > 0.05).
Table 8.
The effect of various hydrolyzed feather meals on carcass characteristics of broilers at 42 d of age.
| Treatmentsa | Parameters |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Carcassb | Breastc | Thighc | Heartb | Gizzardb | Thymusb | Spleenb | Bursa of Fabriciusb | Liverb | Abdominal fatb | Pancreasb | |
| Control | 66.2 | 24.4 | 21.6 | 0.552 | 1.51 | 0.351 | 0.145 | 0.206 | 1.68d | 1.46d | 0.210b |
| RFM | 62.2 | 21.2 | 19.8 | 0.534 | 1.48 | 0.312 | 0.126 | 0.183 | 2.44a | 2.45a | 0.211b |
| Au-HFM | 62.4 | 22.4 | 19.8 | 0.536 | 1.48 | 0.312 | 0.127 | 0.186 | 2.23b | 2.26b | 0.211b |
| Bl-FFM | 65.1 | 23.6 | 21.1 | 0.540 | 1.51 | 0.341 | 0.140 | 0.195 | 1.71d | 1.47d | 0.288a |
| Bs-FFM | 64.1 | 23.3 | 20.6 | 0.551 | 1.49 | 0.334 | 0.142 | 0.201 | 1.71d | 1.48d | 0.278a |
| An-FFM | 64.0 | 24.1 | 20.6 | 0.550 | 1.51 | 0.334 | 0.136 | 0.200 | 1.70d | 1.48d | 0.292a |
| Co-FFM | 65.3 | 24.3 | 21.5 | 0.543 | 1.50 | 0.342 | 0.143 | 0.193 | 1.70d | 1.47d | 0.292a |
| En-HFM | 64.0 | 22.5 | 20.2 | 0.537 | 1.49 | 0.324 | 0.135 | 0.194 | 2.00c | 2.11c | 0.225b |
| SEM | 0.543 | 0.258 | 0.214 | 0.003 | 0.005 | 0.003 | 0.001 | 0.002 | 0.035 | 0.050 | 0.005 |
| P-value | 0.578 | 0.164 | 0.062 | 0.526 | 0.636 | 0.080 | 0.095 | 0.511 | 0.001 | 0.001 | 0.001 |
a-d Means within a column with different superscripts differ significantly (P < 0.05).
Control = diet based on corn and soybean meal, without FM; RFM = raw feather meal; Au-HFM = hydrolyzed feather meal by autoclave; Bl-FFM = fermented feather meal by Bacillus Licheniformis; Bs-FFM = fermented feather meal by Bacillus Subtilis; An-FFM = fermented feather meal by Aspergillus Niger; Co-FFM = fermented feather meal by Bacillus Licheniformis + Bacillus Subtilis + Aspergillus Niger; En-HFM = hydrolyzed feather meal by an enzyme.
Percent of live body weight.
Percent of eviscerated carcass weight.
4. Discussion
4.1. Experiment 1
4.1.1. Determination of in vitro CP digestibility
The quality of the feather meal (FM) was evaluated by its digestibility in pepsin and pancreatin solution under in vitro digestion conditions. It has been reported that to use FM in poultry diets, at least 75 % of its crude protein (CP) must be pepsin digestible [32]. In the present research, in vitro pepsin-pancreatin CP digestibility of fermented feather meal (FFM) was significantly higher than unfermented FM. The results of the present research are consistent with the findings of many researchers who reported that the value of in vitro protein digestibility significantly increased in FFM by Bacillus sp [8,11,16,33,34]. Similarly, it has been reported that the use of hydrolyzed feather meal (HFM) by autoclave (Au-HFM) led to a decrease in in vitro protein digestibility [35,36]. The fermentation of FM can break down disulfide bonds, increase solubility, and open the feather structure. This increases access to sites to which the pepsin and pancreatin have a pre-existing affinity and consequently increases the digestibility of the FFM. The results of the present research also are in agreement with the findings of Rutkowski et al. [9], who showed that enzymatically HFM compared to Au-HFM significantly increased in vitro protein digestibility. Novus International claims that, compared to raw feather meal (RFM) and Au-HFM, Cibenza IND900 increases FM's nutritional value, reduces the amount of heat required for the rendering process, and increases profitability [20]. In contrast, Adler et al. [2] reported that the processing of FM by Cibenza IND900 did not affect in vitro pepsin protein digestibility.
However, in the current study, the in vitro pepsin-pancreatin CP digestibility of FFM by a single microorganism and Co-FFM was significantly higher than unfermented FM. The fungal-bacterial Co-fermented feather meal (Co-FFM) achieved significantly the highest in vitro pepsin-pancreatin CP digestibility compared to other treatments. Co-fermentation is a technique in which two or more different populations of microorganisms are grown together to improve fermenting, study interactions, establish synergistic interactions between populations, or enhance the efficiency of specific processes [37]. Over 50 g of feathers are difficult to biodegrade for a single strain of microorganisms due to their complex keratin structure [37]. Hydrolysis of feathers through Co-fermentation allows more efficient conversion than individual strains of microorganisms and isolated enzymes [37]. To effectively degrade feather keratin, at least three types of keratinolytic microorganisms or keratinases are required, including exo-acting, oligopeptide-acting, and endo-acting [38]. In addition, the keratinases or various keratinolytic microorganisms work synergistically with the sulfur bridge degradation of the feather keratin structure to loosen the molecular structure, allowing the enzyme to access both the protein structure and its substrate [38]. Regarding this, it has been documented that keratinolytic enzymes such as keratinase could not hydrolyze FM efficiently in the absence of live microorganisms [37]. Complete feather hydrolysis is initiated by microorganisms’ colonization to open the complex feather structure and activate redox mechanisms such as the production of disulfide reductase [37]. These factors are actioned together to achieve higher biodegradation efficiency and improved degradative effects on FFM during the Co-fermentation process.
4.1.2. Determination of apparent ileal CP digestibility
Improving the digestibility of FM could make it a useful feed ingredient for the poultry industry. In the current study, the apparent ileal CP digestibility of FFM was higher than in the other groups. The current result is in line with the outcome of He et al. [15], who reported that microorganisms such as Bacillus subtilis, Aspergillus niger, and Bacillus licheniformis are highly efficient in degrading feather keratin. In the present study, greater protein digestibility, at least 75 % required for poultry feed, was obtained by fermentation of FM. It is inferred that during the fermentation process, disulfide bonds of the Cys by microorganisms are broken, leading to the secondary structure of feather keratin. Then poultry gastrointestinal proteases act on the porous structure of keratin, allowing the feathers to be easily digested. Furthermore, the metabolic activity of microorganisms through the generation of enzymes, peptides, and other unidentified products during the fermentation process can improve nutrient utilization. Which in turn increases the amount of soluble and readily available proteins [39]. The digestibility of FM varied depending on the technique of processing. It has been reported that autoclave and chemical treatments are expensive, and potentially cause protein denaturation thus damaging nutrients such as amino acids, which is characterized by a change in the form, and a decrease in digestibility of FM [4,40]. One of the main disadvantages of enzymatic hydrolysis is its limited stability, and the high cost of the enzymes is another disadvantage [41].
In the current study, the apparent ileal CP digestibility of various FFM was significantly higher than unfermented FM. However, the highest apparent ileal CP digestibility of FM was mediated successfully by the synergistic action of keratinases and a variety of other proteases produced by several keratinolytic microorganisms in the fungal-bacterial Co-FFM. The primary purpose of the Co-fermentation process is to fully biodegrade the substrate to develop new feed resources, improve the digestibility and nutritional value of fermented feed, decrease anti-nutritional factors, and enhance the flavor and palatability of fermented feed [42]. Which could be a suitable alternative for dietary protein sources in the poultry industry. Single strains can hydrolyze feathers individually but have less efficiency in growth, conversion to fermented product, enzyme production, and biodegradation conditions [37]. Complete biodegradation of FM can be achieved by the synergistic action of keratinolytic protease, disulfide reductase, and lipase which may be produced by the different keratinolytic microorganisms in Co-fermentation processes [37]. In this case, it has been reported that keratin-rich materials are broken down in nature through the cooperation of bacteria, fungi, and other microorganisms [37].
4.1.3. Determination of AME and AMEn
Based on our results, apparent metabolizable energy (AME), and AME corrected for nitrogen (AMEn) did not differ significantly between the different treatments. The findings of our study are consistent with the results of Eaksuree et al. [43], who showed that there were no significant differences in AMEn between FFM by Bacillus licheniformis (Bl-FFM) and RFM. Although the AME and AMEn in the FFM were numerically higher than other treatments, there are several possible reasons for no significant difference in AME and AMEn between FFM and unfermented FM. (1) The FM's nutrient digestibility and metabolizable energy content are affected by many factors, including origin, processing methods, physicochemical characteristics, levels of feeding, and methods for measuring digestibility [44]. (2) Some microbiota species at the end of the ileum and cecum can ferment undigested components of ileal digesta with significant energy consumption, consequently decreasing energy excretion and energy availability for birds [45]. Therefore, AME and AMEn were falsely increased in broilers fed unfermented FM. (3) Considering the negative correlation between the amount of FI and the passage time of digesta, the increased feed intake (FI) in broilers fed FFM may shorten digesta passage time and impair the ability of the gut microbiota to break down complex carbohydrates, potentially leading to an increase in the energy content of excreta. (4) Increased FI and digesta passage rate in broilers fed FFM may increase endogenous energy in excreta, which in turn could influence AME and AMEn levels [45]. (5) In FFM, the digestibility of CP is high; However, the CP higher than the bird's requirement leads to the excretion of nitrogen in the form of uric acid with significant energy consumption, affecting AME and AMEn levels. However, the effect of various HFM on AME and AMEn in broilers needs further investigation.
4.2. Experiment 2
4.2.1. Performance of broiler chickens
In the current study, broilers fed the control diet and the diet containing Co-FFM had the lowest feed conversion ratio (FCR), highest FI, and highest body weight gain (BWG) compared to the other treatments. Likewise, numerous studies have shown that broiler performance can be improved by fermented feed [39,[46], [47], [48]]. It has been reported that the broilers fed with biologically processed FM showed significantly improved performance compared to those fed with soybean meal. Also resulted in the product having an improved overall amino acid availability, digestibility, and absorption [16].
Similarly, it has been reported that broilers fed with commercial keratinase enzyme (including Cibenza DP100) supplemented FM significantly increased BWG and decreased FCR compared to those fed diets containing RFM without keratinase [[49], [50], [51]]. The lower growth rate and higher FCR of the broilers fed RFM, Au-HFM, and HFM by enzyme (En-HFM) groups may be attributed to the lower FI and poor digestibility of FM as well as the non-palatable FM. Ajayi and Akoma [49], reported that since FM is indigestible and its amino acids are unavailable for poultry, this could also reduce FI. Whereas, fermentation processing improves feed nutritional indicators, and feed palatability [52]. In the current study, changes in feather keratin structure by fermentation, facilitate the work of digestive enzymes and thus improve nutrient digestibility and palatability of FFM. This is reflected in the improvement in the growth performance and increase in FI by FFM compared to unfermented FM. In other words, the rate of nutrient digestion and absorption has a direct effect on the digesta passage rate in the digestive tract [53]. Consequently, the positive correlation between the digesta passage rate and the amount of FI has increased the broiler's FI in FFM groups. Furthermore, our research demonstrated that broiler performance was improved by feeding FFM by a single microorganism or a combination thereof as opposed to unfermented FM. However, only the performance of broilers fed Co-FFM was comparable to the control diet. The higher hydrolysis rate of FM in the fermentation process by the coculture system is because of higher keratinase and protease activities [54], which could be an effective method of FM processing on a commercial scale for the poultry industry.
Disulfide bond reductase can be produced by some keratinase-producing microorganisms. It has been reported that both disulfide reductase and keratinase activities are present in Bacillus subtilis [55,56], and Bacillus licheniformis [57]. The disulfide bond of feather keratin can be broken by disulfide bond reductase, exposing the cleavage sites within the keratin molecules to enzymatic attack and loosening the stable keratin structure. This increases the accessibility of digestive enzymes including pepsin and trypsin to the stable keratin structure and improves the digestibility of FFM [58]. Disulfide reductase breaks the disulfide bridges responsible for the stringency of keratin in feathers [56]. Microorganisms can break many peptide bonds in feather keratin during the fermentation process, increasing the number of polar groups (such as –NH2+, and COO−). This increases the hydrophilicity and solubility of the keratin, which in turn increases the digestibility of FFM [58]. Feeding of fermented feeds in broilers by increasing the height of the intestinal villous as well as the villous height to crypt depth ratio increases digestion and absorption of nutrients [52]. As a result of current research, fermentation represents a practical process for hydrolysis of FM and is an effective technique for improving the digestibility of amino acids and protein, reducing feed costs, and improving the growth performance of poultry.
4.2.2. Carcass characteristics
In the current study, the highest relative weight of abdominal fat and the liver percentage was found in broilers fed diets containing RFM, and the highest pancreas relative weight was in broilers fed with FFM. Many studies have reported that fermented feed increases the secretion of digestive enzymes from the pancreas [46]. In broilers, high secretion of enzymes and continuous stimulation of the pancreas could increase the size of the pancreas [59]. In this regard, in the current study, it seems that increasing the secretion of the digestive enzyme from the pancreas of broilers fed FFM, increases the activity of their pancreas and consequently increases its size and weight. Diets with low protein digestibility and amino acid imbalances, reduce protein production and increase the breakdown of protein and amino acids in the liver. The breakdown of amino acids in the liver increases the size and weight of the liver, as well as causes the excretion of nitrogen (in the form of uric acid). Therefore, their carbon skeleton is converted to fat [60,61]. The higher abdominal fat percentage observed in broilers fed diets containing unfermented FM was associated with the quality of the protein [47]. It has been reported that broilers fed with a low-protein diet had a significantly higher percentage of abdominal fat, which has been attributed to a higher calorie-to-absorbable protein ratio [[62], [63], [64]]. Excessive fat accumulation in broilers is detrimental, resulting in an added expense for both the broiler industry and consumers. In addition, it has been reported that feeding fermented feed reduces the amount of abdominal fat in broilers. This could be due to increased lipolysis of abdominal fat cells, mainly through increased β-oxidation of fatty acids as well as hydrolysis of triglycerides [65,66]. Therefore, in the current study, bioactive metabolites (produced during fermentation) and high digestibility and availability of FFM might be responsible for lower abdominal fat content. This could lead to the production of meat with low fat, which is preferred by both commercial poultry producers and poultry meat consumers.
5. Conclusion
According to the results of the first experiment, it can be concluded that among different feather hydrolysis methods, the fermentation process improved the nutritional value of feather meal (FM) by increasing its in vitro and apparent ileal crude protein (CP) digestibility. The results of the second experiment confirmed the findings of the first experiment. The in vivo study showed that the fermentation process improved the performance of broilers by increasing the nutritional value of FM. Also, fermented feather meal (FFM) resulted in an increase in carcass quality due to a decrease in abdominal fat percentage. Various fermentation processes improved the nutritional value of FM. Also, the positive effect of various FFM, especially Co-fermented feather meal (Co-FFM) on the broilers' performance, and carcass traits suggests that the fermentation process could eliminate the negative effects of raw feather meal (RFM). According to our results, the Co-fermentation process can improve the nutritional value of FM, making it as valuable as soybean meal for poultry feed.
CRediT authorship contribution statement
Hassan Safari: Writing – review & editing, Writing – original draft, Software, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Ardeshir Mohit: Writing – review & editing, Writing – original draft, Software, Investigation, Formal analysis, Data curation. Maziar Mohiti-Asli: Writing – review & editing, Writing – original draft, Software, Formal analysis, Data curation.
Data availability statement
Data will be made available on request.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Safari H., Mohit A., Mohiti-Asli M. Influence of dietary supplementation of dried purslane (Portulaca oleracea L.) powder on growth performance and susceptibility of chicken thigh muscle to lipid oxidation during frozen storage conditions. Indian J. Anim. Nutr. 2016;33(2):169–175. doi: 10.5958/2231-6744.2016.00029.3. [DOI] [Google Scholar]
- 2.Adler S.A., Slizyte R., Honkapää K., Løes A.-K. In vitro pepsin digestibility and amino acid composition in soluble and residual fractions of hydrolyzed chicken feathers. Poult. Sci. 2018;97(9):3343–3357. doi: 10.3382/ps/pey175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abd-Aziz S., Gozan M., Ibrahim M.F., Phang L.Y. John Wiley and Sons; 2023. Chemical Substitutes from Agricultural and Industrial By-Products: Bioconversion, Bioprocessing, and Biorefining; pp. 123–144. [Google Scholar]
- 4.Said M., Yuliati F., Sukma M. The effects of acidic and alkaline hydrolysis process on some physical and chemical properties of broiler chicken feathers, Iran. J. Appl. Anim. Sci. 2019;9(3) 529-450. [Google Scholar]
- 5.Gupta R., Rajput R., Sharma R., Gupta N. Biotechnological applications and prospective market of microbial keratinases. Appl. Microbiol. Biotechnol. 2013;97:9931–9940. doi: 10.1007/s00253-013-5292-0. [DOI] [PubMed] [Google Scholar]
- 6.Lasekan A., Bakar F.A., Hashim D. Potential of chicken by-products as sources of useful biological resources. Waste Manag. 2013;33(3):552–565. doi: 10.1016/j.wasman.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Caisin L., Vrancean V., Anton V., Bivol L. 2017. The Effect of Replacement of Fishmeal with Feather Meal on the Performance of Broiler Chickens, 52 Hrvatski I 12 Međunarodni Simpozij Agronoma; p. 484. [Google Scholar]
- 8.Maciel J.L., Werlang P.O., Daroit D.J., Brandelli A. Characterization of protein-rich hydrolysates produced through microbial conversion of waste feathers. Waste biomass valorization. 2017;8:1177–1186. doi: 10.1007/s12649-016-9694-y. [DOI] [Google Scholar]
- 9.Rutkowski A., Jozefiak D., Fratczak M., Wiaz M. A note on the nutritional value of enzymatically hydrolyzed feather meal for broiler chickens. J. Anim. Feed Sci. 2003;12(2):299–306. doi: 10.22358/jafs/67707/2003. [DOI] [Google Scholar]
- 10.Karthikeyan R., Balaji S., Sehgal P. Industrial applications of keratins–A review. J. Sci. Ind. Res. 2007;66:710–715. [Google Scholar]
- 11.Fakhfakh N., Ktari N., Haddar A., Mnif I.H., Dahmen I., Nasri M. Total solubilisation of the chicken feathers by fermentation with a keratinolytic bacterium, Bacillus pumilus A1, and the production of protein hydrolysate with high antioxidative activity. Process Biochem. 2011;46(9):1731–1737. doi: 10.1016/j.procbio.2011.05.023. [DOI] [Google Scholar]
- 12.Zhang Y., Yang R., Zhao W. Improving digestibility of feather meal by steam flash explosion. J. Agric. Food Chem. 2014;62(13):2745–2751. doi: 10.1021/jf405498k. [DOI] [PubMed] [Google Scholar]
- 13.Safari H., Mohit A., Mohiti-Asli M. Fermented feather meal improves the antioxidant status, meat quality, and immune response of broilers. Iranian J. Vet. Med. 2024 doi: 10.22059/IJVM.2024.369109.1005483. In Press. [DOI] [Google Scholar]
- 14.Sironi P.B., Mazotto A.M., de Lima M.F., Nogueira R.I., Miguel Â.S.M., Vermelho A.B. Hydrolyzed feather keratin obtained by microbial fermentation encapsulated with maltodextrin–A sustainable approach to increase digestible protein in feed. Biocatal. Agric. Biotechnol. 2022;40 doi: 10.1016/j.bcab.2022.102297. [DOI] [Google Scholar]
- 15.He Z., Sun R., Tang Z., Bu T., Wu Q., Li C., Chen H. Biodegradation of feather waste keratin by the keratin-degrading strain Bacillus subtilis 8. J. Microbiol. Biotechnol. 2018;28:314–322. doi: 10.4014/jmb.1708.08077. [DOI] [PubMed] [Google Scholar]
- 16.Lakshmi P.J., Lakshmi V. Enhancement in nutritive value and in vitro digestability of keratinse treated feather meal. Intern. J. Sci. Eng. Res. 2015;6(2):36–40. [Google Scholar]
- 17.Adejumo I.O., Adetunji C.O. Production and evaluation of biodegraded feather meal using immobilised and crude enzyme from Bacillus subtilis on broiler chickens. Braz. J. Biol. Sci. 2018;5(10):405–416. doi: 10.21472/bjbs.051017. [DOI] [Google Scholar]
- 18.Belewu M., Asafa A., Ogunleke F. Processing of feather meal by solid state fermentation. Biotechnology. 2008;7(3):589–591. doi: 10.3923/biotech.2008.589.591. [DOI] [Google Scholar]
- 19.Wiradimadja R., Rusmana D., Widjastuti T., Mushawwir A. Chicken slaughterhouse waste utilization (chicken feather meal treated) as a source of protein animal feed ingredients in broiler chickens. Lucr. Stiint. Ser. Zooteh. 2014;62:120–124. [Google Scholar]
- 20.Novus I. 2013. CIBENZA IND900 Improve Feather Digestibility with IND900 Batch Process NS 2931; pp. 1–2. [Google Scholar]
- 21.Grazziotin A., Pimentel F., De Jong E., Brandelli A. Nutritional improvement of feather protein by treatment with microbial keratinase. Anim. Feed Sci. Technol. 2006;126(2):135–144. doi: 10.1016/j.anifeedsci.2005.06.002. [DOI] [Google Scholar]
- 22.Aviagen Ross 308. 2019. Broiler Nutrition Specifications; pp. 1–10. [Google Scholar]
- 23.Kim E., Utterback P., Applegate T., Parsons C. Comparison of amino acid digestibility of feedstuffs determined with the precision-fed cecectomized rooster assay and the standardized ileal amino acid digestibility assay. Poult. Sci. 2011;90(11):2511–2519. doi: 10.3382/ps.2011-01400. [DOI] [PubMed] [Google Scholar]
- 24.AOAC . AOAC International; Washington DC, USA: 2005. Official Methods of Analysis of the Association of Official Analytical Chemists; p. 18. [Google Scholar]
- 25.De Coca-Sinova A., Mateos G.G., González-Alvarado J., Centeno C., Lázaro R., Jiménez-Moreno E. Comparative study of two analytical procedures for the determination of acid insoluble ash for evaluation of nutrient retention in broilers. Span. J. Agric. Res. 2011;9(3):761–768. doi: 10.5424/sjar/20110903-439-10. [DOI] [Google Scholar]
- 26.Azam F., Qaisrani S., Khalique A., Bibi F., Akram C., Naveed S., Pasha T. Exploring nutritive profile, metabolizable energy, protein, and digestible amino acids contents of indigenous protein sources of different locations for male broilers. Poultry Sci. 2019;98(10):4664–4672. doi: 10.3382/ps/pez167. [DOI] [PubMed] [Google Scholar]
- 27.Li Y., Li S., Li C., Chang W., Cai H., Liu G. Effects of fermentation on the apparent metabolizable energy and standardized ileal digestibility of amino acids in soybean meal fed to broiler chickens. Fermentation. 2022;9(1):23. doi: 10.3390/fermentation9010023. [DOI] [Google Scholar]
- 28.Dozier W., III, Gehring C., Corzo A., Olanrewaju H. Apparent metabolizable energy needs of male and female broilers from 36 to 47 days of age. Poult. Sci. 2011;90(4):804–814. doi: 10.3382/ps.2010-01132. [DOI] [PubMed] [Google Scholar]
- 29.Scott T., Hall J. Using acid insoluble ash marker ratios (diet: digesta) to predict digestibility of wheat and barley metabolizable energy and nitrogen retention in broiler chicks. Poult. Sci. 1998;77(5):674–679. doi: 10.1093/ps/77.5.674. [DOI] [PubMed] [Google Scholar]
- 30.Safari H., Mohit A., Mohiti-Asli M. Feather meal processing methods impact the production parameters, blood biochemical indices, gut function, and hepatic enzyme activity in broilers. J. Anim. Sci. 2024;102 doi: 10.1093/jas/skae068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.SAS, Institute . SAS Institute Inc.; Cary, NC, USA: 2009. SAS Statistics User's Guide. [Google Scholar]
- 32.Okonkwo V., Obih T., Mbachu M. The performance of broiler finisher birds fed varying levels of feather meal as replacement for soya bean meal. J. Agric. Food Sci. 2016;14(2):33–40. doi: 10.4314/jafs.v14i2.4. [DOI] [Google Scholar]
- 33.Rahayu S., Bata M. Quality of chicken feather processed in different conditions. Anim. Prod. 2015;16(3):170–175. doi: 10.20884/1.jap.2014.16.3.464. [DOI] [Google Scholar]
- 34.Bertsch A., Coello N. A biotechnological process for treatment and recycling poultry feathers as a feed ingredient. Bioresour. Technol. 2005;96(15):1703–1708. doi: 10.1016/j.biortech.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 35.Sinhorini M.R., Balbinot-Alfaro E., de Aguiar W., da Trindade Alfaro A. Influence of process parameters and raw material on the characteristics of hydrolyzed feather meal. Waste Biomass Valorization. 2021;12:2469–2476. doi: 10.1007/s12649-020-01203-1. [DOI] [Google Scholar]
- 36.Bellagamba F., Caprino F., Mentasti T., Vasconi M., Moretti V.M. The impact of processing on amino acid racemization and protein quality in processed animal proteins of poultry origin. Ital. J. Anim. Sci. 2015;14(2):3770. doi: 10.4081/ijas.2015.3770. [DOI] [Google Scholar]
- 37.Verma P. CRC Press; 2023. Enzymes in the Valorization of Waste; pp. 183–212. CRC Press. [DOI] [Google Scholar]
- 38.Lange L., Huang Y., Busk P.K. Microbial decomposition of keratin in nature—a new hypothesis of industrial relevance. Appl. Microbiol. Biotechnol. 2016;100(5):2083–2096. doi: 10.1007/s00253-015-7262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shabani A., Jazi V., Ashayerizadeh A., Barekatain R. Inclusion of fish waste silage in broiler diets affects gut microflora, cecal short-chain fatty acids, digestive enzyme activity, nutrient digestibility, and excreta gas emission. Poult. Sci. 2019;98(10):4909–4918. doi: 10.3382/ps/pez244. [DOI] [PubMed] [Google Scholar]
- 40.Park C.S., Naranjo V.D., Htoo J.K., Adeola O. Comparative amino acid digestibility between broiler chickens and pigs fed different poultry by-products and meat and bone meal. J. Anim. Sci. 2020;98(7) doi: 10.1093/jas/skaa223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D'Este M., Alvarado-Morales M., Angelidaki I. Amino acids production focusing on fermentation technologies–A review. Biotechnol. Adv. 2018;36(1):14–25. doi: 10.1016/j.biotechadv.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Li W., Cheng P., Zhang J., Zhao L., Ma Y., Ding K. Synergism of microorganisms and enzymes in solid-state fermentation of animal feed- A review. J. Anim. Feed Sci. 2021;30(1):3–10. doi: 10.22358/jafs/133151/2021. [DOI] [Google Scholar]
- 43.Eaksuree W., Prachayakitti A., Upathanpreecha T., Taharnklaew R., Nitisinprasert S., Keawsompong S. In vitro and in vivo evaluation of protein quality of enzymatic treated feather meals. SpringerPlus. 2016;5:1–6. doi: 10.1186/s40064-016-2626-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zarei A., Mohammadi M., Hemmati B. Metabolizable energy and chemical composition of poultry by-product meal. Iranian J. Appl. Anim. Sci. 2014;4(4):849–853. [Google Scholar]
- 45.Song M., Wang Y., Liu Y., Ren C., Yan L., Xie J., Jinliang L., Guilian Z., Yong L., Feng Z. The age-related metabolizable energy of cereal grains, oilseed meals, corn gluten meals, and feather meals for broilers. J. Anim. Sci. 2023;101 doi: 10.1093/jas/skad051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soumeh E., Mohebodini H., Toghyani M., Shabani A., Ashayerizadeh A., Jazi V. Synergistic effects of fermented soybean meal and mannan-oligosaccharide on growth performance, digestive functions, and hepatic gene expression in broiler chickens. Poult. Sci. 2019;98(12):6797–6807. doi: 10.3382/ps/pez409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jazi V., Boldaji F., Dastar B., Hashemi S., Ashayerizadeh A. Effects of fermented cottonseed meal on the growth performance, gastrointestinal microflora population and small intestinal morphology in broiler chickens. Br. Poult. Sci. 2017;58(4):402–408. doi: 10.1080/00071668.2017.1315051. [DOI] [PubMed] [Google Scholar]
- 48.Sun H., Tang J-w., Yao X-h., Wu Y-f., Wang X., Feng J. Effects of dietary inclusion of fermented cottonseed meal on growth, cecal microbial population, small intestinal morphology, and digestive enzyme activity of broilers. Trop. Anim. Health Prod. 2013;45:987–993. doi: 10.1007/s11250-012-0322-y. [DOI] [PubMed] [Google Scholar]
- 49.Ajayi H., Akoma O. Performance and carcass characteristics of broiler chickens fed low protein diets containing hydrolyzed feather meal, with or without protease supplementation. BIU. J. Basic Appl. Sci. 2017;3(1):20–30. [Google Scholar]
- 50.Yan F., Dibner J., Knight C., Vazquez-Anon M. Effect of carbohydrase and protease on growth performance and gut health of young broilers fed diets containing rye, wheat, and feather meal. Poult. Sci. 2017;96(4):817–828. doi: 10.3382/ps/pew300. [DOI] [PubMed] [Google Scholar]
- 51.Odetallah N., Wang J., Garlich J., Shih J. Keratinase in starter diets improves growth of broiler chicks. Poult. Sci. 2003;82(4):664–670. doi: 10.1093/ps/82.4.664. [DOI] [PubMed] [Google Scholar]
- 52.Chang Y., Omer S.H.S., Li G., Lian H., Liu Y. Research advance on application of microbial fermented fodder in broilers production: a short review. Open J. of Anim. Scie. 2022;12(2):200–209. doi: 10.4236/ojas.2022.122015. [DOI] [Google Scholar]
- 53.Safari H., Mohiti-Asli M., Mohammadpour F. Effect of purslane powder on performance, quality and oxidative stability of meat and some blood metabolites in fattening lambs. Animal Production Research. 2016;5(1):15–26. [Google Scholar]
- 54.Peng Z., Mao X., Zhang J., Du G., Chen J. Effective biodegradation of chicken feather waste by co-cultivation of keratinase producing strains. Microb. Cell Fact. 2019;18(1):1–11. doi: 10.1186/s12934-019-1134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rahayu S., Syah D., Suhartono M.T. Degradation of keratin by keratinase and disulfide reductase from Bacillus sp. MTS of Indonesian origin. Biocatal. Agric. Biotechnol. 2012;1(2):152–158. doi: 10.1016/j.bcab.2012.02.00. [DOI] [Google Scholar]
- 56.Gupta S., Singh S.P., Singh R. Synergistic effect of reductase and keratinase for facile synthesis of protein-coated gold nanoparticles. J. Microbiol. Biotechnol. 2015;25(5):612–619. doi: 10.4014/jmb.1411.11022. [DOI] [PubMed] [Google Scholar]
- 57.Ramnani P., Singh R., Gupta R. Keratinolytic potential of Bacillus licheniformis RG1: structural and biochemical mechanism of feather degradation. Can. J. Microbiol. 2005;51(3):191–196. doi: 10.1139/w04-123. [DOI] [PubMed] [Google Scholar]
- 58.Zhou L., Xie X., Wu T., Chen M., Yao Q., Zhu H., Zou W. Compound enzymatic hydrolysis of feather waste to improve the nutritional value. Biomass Convers. Biorefin. 2020;12:278–298. doi: 10.1007/s13399-020-00643-y. [DOI] [Google Scholar]
- 59.Bryan D.D., Abbott D.A., Van Kessel A.G., Classen H.L. The influence of indigestible protein on broiler digestive tract morphology and caecal protein fermentation metabolites. J. Anim. Physiol. Anim. Nutr. 2020;104(3):847–866. doi: 10.1111/jpn.13256. [DOI] [PubMed] [Google Scholar]
- 60.Farrell D., Atmamihardja S., Pym R. Calorimetric measurements of the energy and nitrogen metabolism of Japanese quail. Br. Poult. Sci. 1982;23(5):375–382. doi: 10.1080/00071688208447971. [DOI] [PubMed] [Google Scholar]
- 61.Golian A., Azghadi A., Pilevar M. Influence of various levels of energy and protein on performance and humoral immune responses in broiler chicks. Glob. Vet. 2010;4(5):434–440. [Google Scholar]
- 62.Jabbar A., Tahir M., Alhidary I.A., Abdelrahman M.A., Albadani H., Khan R.U. Impact of microbial protease enzyme and dietary crude protein levels on growth and nutrients digestibility in broilers over 15–28 days. Animals. 2021;11(9):2499. doi: 10.3390/ani11092499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Law F.L., Zulkifli I., Soleimani A.F., Liang J.B., Awad E.A. The effects of low-protein diets and protease supplementation on broiler chickens in a hot and humid tropical environment. Asian-Australas. J. Anim. Sci. 2018;31(8):1291. doi: 10.5713/ajas.17.0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Namroud N., Shivazad M., Zaghari M. Effects of fortifying low crude protein diet with crystalline amino acids on performance, blood ammonia level, and excreta characteristics of broiler chicks. Poult. Sci. 2008;87(11):2250–2258. doi: 10.3382/ps.2007-00499. [DOI] [PubMed] [Google Scholar]
- 65.Sugiharto S., Yudiarti T., Isroli I. Growth performance, haematological parameters, intestinal microbiology, and carcass characteristics of broiler chickens fed two-stage fermented cassava pulp during finishing phase. Trop. Anim. Sci. J. 2019;42(2):113–120. doi: 10.5398/tasj.2019.42.2.113. [DOI] [Google Scholar]
- 66.Nie C.-x., Zhang W-j., Wang Y-q., Liu Y-f., Ge W-x., Liu J-c. Tissue lipid metabolism and hepatic metabolomic profiling in response to supplementation of fermented cottonseedmeal in the diets of broiler chickens. J. Zhejiang Univ. - Sci. B. 2015;16(6):447. doi: 10.1631/jzus.B1400255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.