Abstract
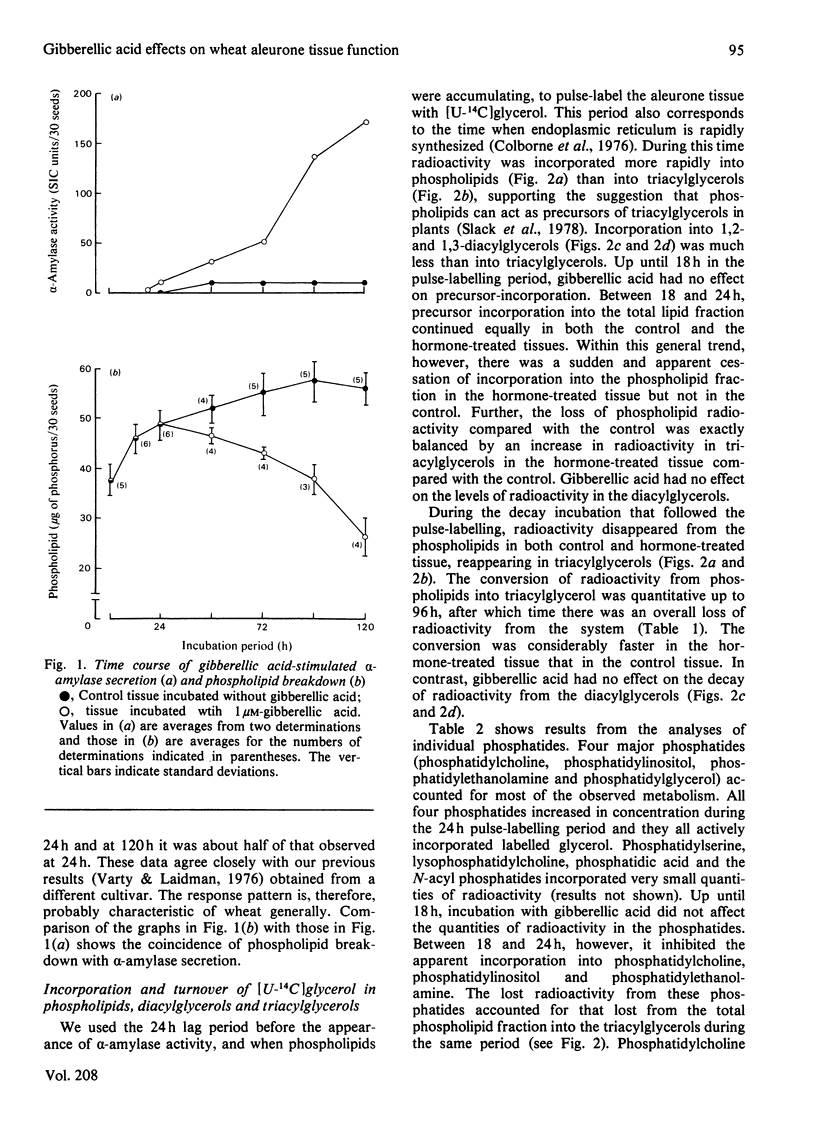
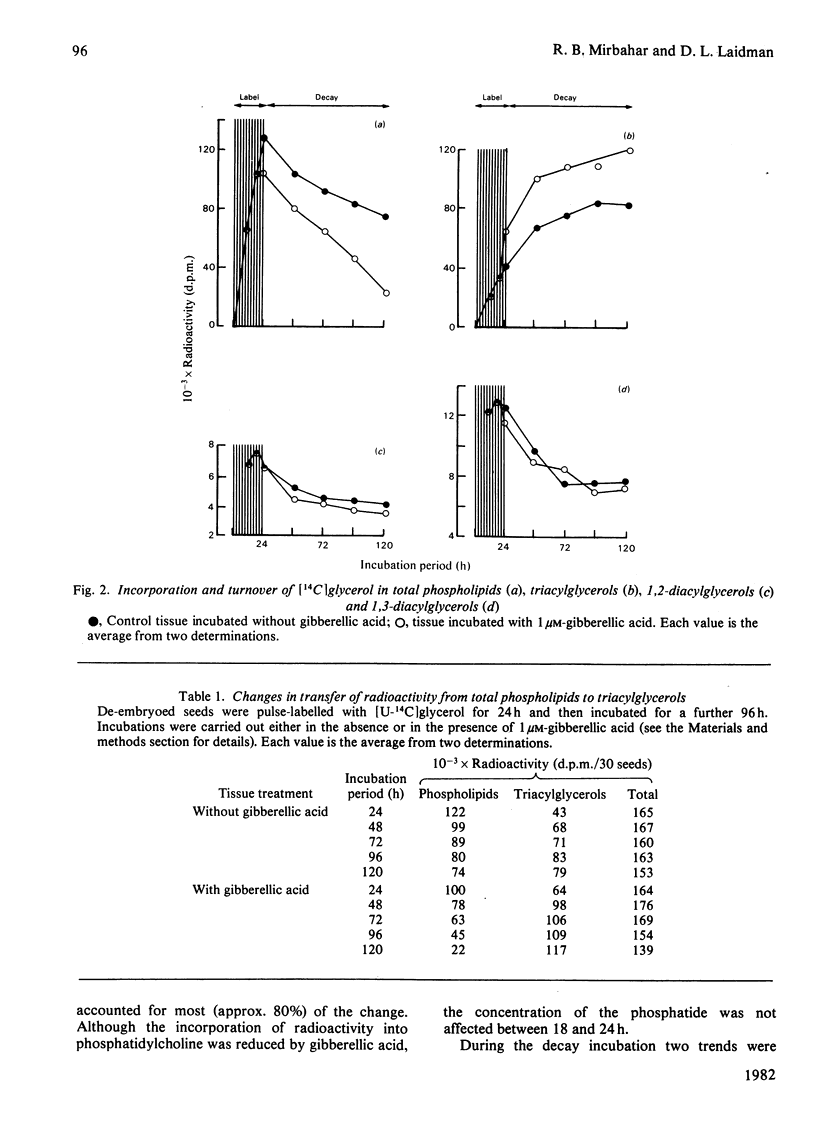
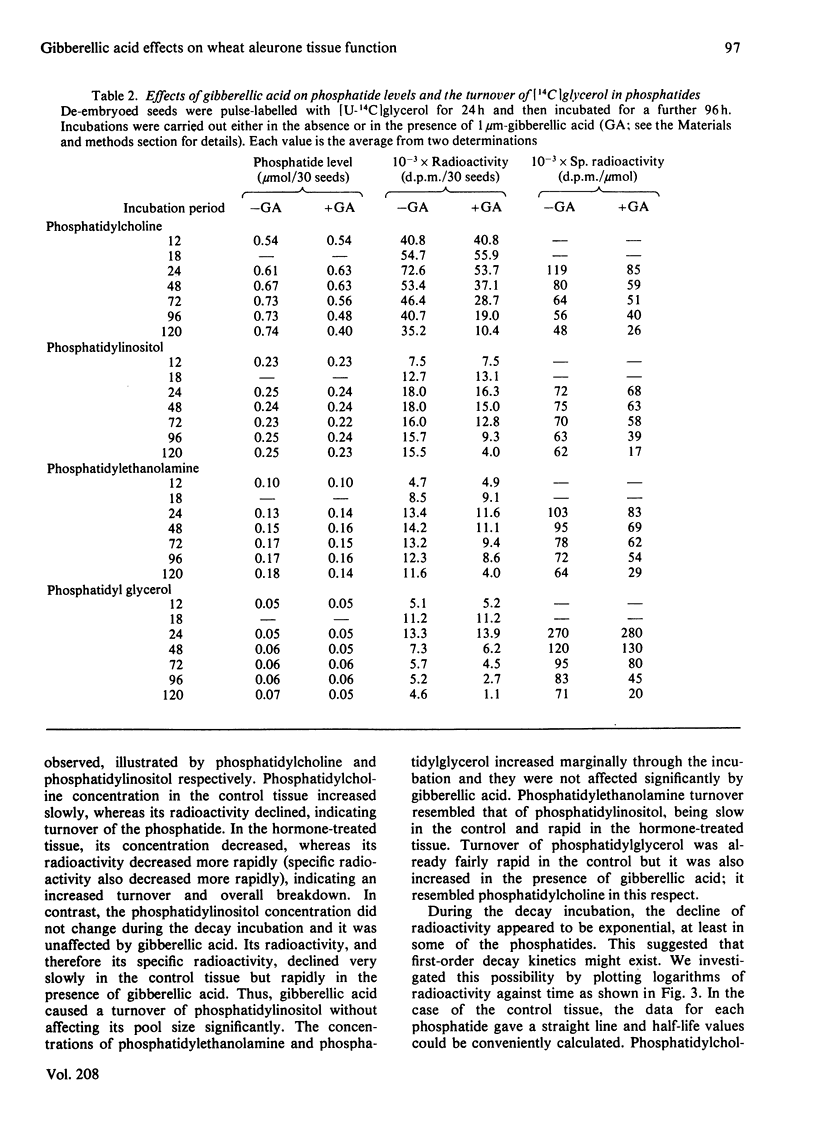
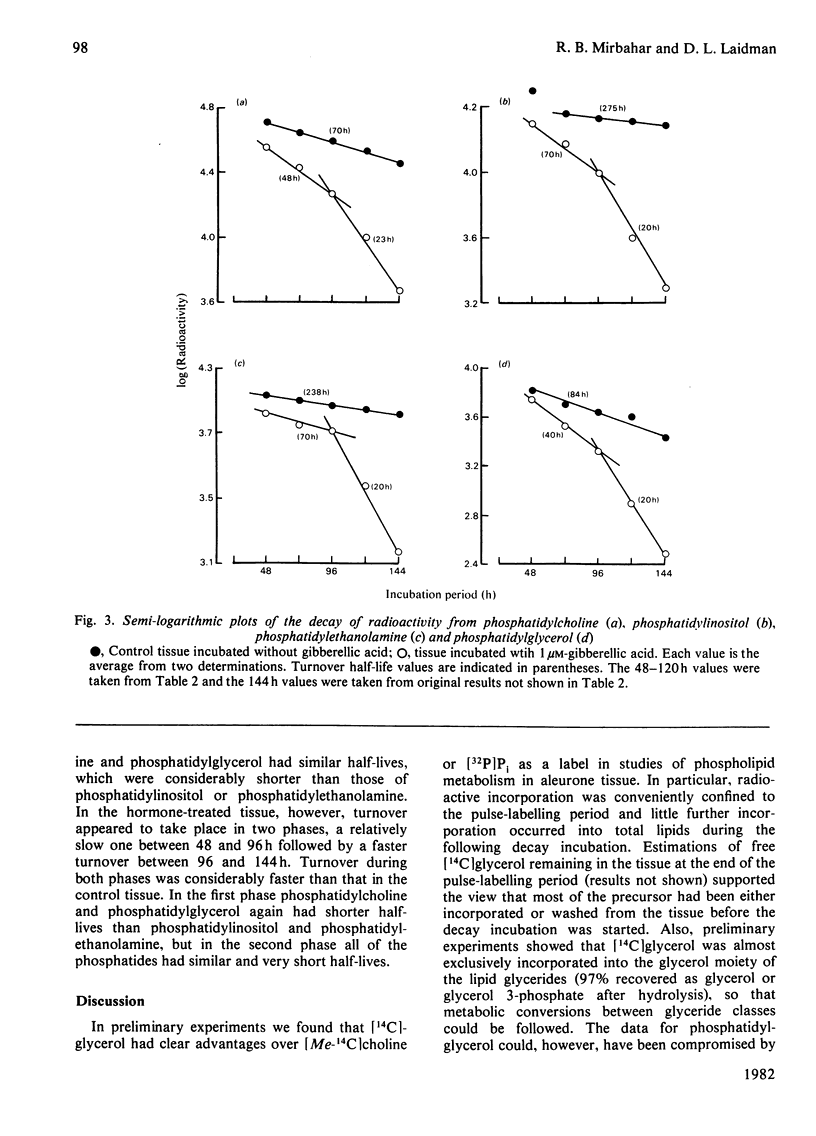
1. Turnovers of [14C]glycerol-labelled phospholipids in wheat aleurone tissue have been measured by using a pulse-decay technique. The most metabolically active phospholipids were phosphatidylcholine, phosphatidylinositol, phosphatidylethanolamine and phosphatidylglycerol. 2. Gibberellic acid action on the tissue led to breakdown of phosphatidylcholine and stimulated turnover of the other phosphatides concomitant with the secretion of alpha-amylase from the tissue. After pulse-labelling of the aleurone tissue with [14C]glycerol, radioactivity was lost from the phospholipids and appeared quantitatively in triacylglycerols, suggesting a stoichiometric metabolism of the former into the latter. Although 1,2-diacylglycerol is an expected intermediate in such a conversion, the patterns of radioactivity in diacylglycerols gave no indication of this. 3. Several aspects of the response of aleurone tissue to gibberellic acid resemble the responses of exocytotic animal tissues to external agonists. In particular, our results and previous reports in the literature suggest that endomembrane flow, exocytosis, phosphatidylinositol turnover and a requirement of Ca2+ for enzyme secretion are common to both plant and animal systems. Although considerable differences also exist between the two, the similarities are sufficient to warrant further consideration that plants and animals might have conserved a similar hormone response-secretion mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Tal Y. An early response to gibberellic Acid not requiring protein synthesis. Plant Physiol. 1974 Dec;54(6):813–816. doi: 10.1104/pp.54.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Bennett J. P., Gomperts B. D. The dependence on Ca2+ of phosphatidylinositol breakdown and enzyme secretion in rabbit neutrophils stimulated by formylmethionyl-leucylphenylalanine or ionomycin. Biochem J. 1981 Dec 15;200(3):501–508. doi: 10.1042/bj2000501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evins W. H., Varner J. E. Hormone-controlled synthesis of endoplasmic reticulum in barley aleurone cells. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1631–1633. doi: 10.1073/pnas.68.7.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P., Varner J. E., Wray J. L. Environmental or developmental changes cause many enzyme activities of higher plants to rise or fall. Science. 1969 Jul 25;165(3891):358–367. doi: 10.1126/science.165.3891.358. [DOI] [PubMed] [Google Scholar]
- Firn R. D., Kende H. Some effects of applied gibberellic Acid on the synthesis and degradation of lipids in isolated barley aleurone layers. Plant Physiol. 1974 Dec;54(6):911–915. doi: 10.1104/pp.54.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson R. A., Paleg L. G. Lysosomal nature of hormonally induced enzymes in wheat aleurone cells. Biochem J. 1972 Jun;128(2):367–375. doi: 10.1042/bj1280367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. S., Laidman D. L. The pattern and control of isoprenoid quinone and tocopherol metabolism in the germinating grain of wheat (Triticum vulgare). Biochem J. 1968 Jul;108(3):475–482. doi: 10.1042/bj1080475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawthorne J. N. Is phosphatidylinositol now out of the calcium gate? Nature. 1982 Jan 28;295(5847):281–282. doi: 10.1038/295281a0. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Dawson R. M. Phosphatidylinositol phosphodiesterase in higher plants. Biochem J. 1980 Oct 15;192(1):279–283. doi: 10.1042/bj1920279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Kende H. Hormonal Control of Lecithin Synthesis in Barley Aleurone Cells: Regulation of the CDP-Choline Pathway by Gibberellin. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2674–2677. doi: 10.1073/pnas.68.11.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler D. E., Varner J. E. Hormonal control of orthophosphate incorporation into phospholipids of barley aleurone layers. Plant Physiol. 1973 Sep;52(3):208–214. doi: 10.1104/pp.52.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Morré D. J. In vivo incorporation of radioactive metabolites by Golgi apparatus and other cell fractions of onion stem. Plant Physiol. 1970 Jun;45(6):791–799. doi: 10.1104/pp.45.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Roughan P. G., Balasingham N. Labelling of glycerolipids in the cotyledons of developing oilseeds by [1-14C] acetate and [2-3H] glycerol. Biochem J. 1978 Feb 15;170(2):421–433. doi: 10.1042/bj1700421. [DOI] [PMC free article] [PubMed] [Google Scholar]


