Abstract
1. Methods using t.l.c. and high-pressure liquid chromatography (h.p.l.c.) have been used to separate the complex variety of substances possessing a carbonyl function that are produced during lipid peroxidation. 2. The major type of lipid peroxidation studied was the ADP-Fe2+-stimulated peroxidation of rat liver microsomal phospholipids. Preliminary separation of the polar and non-polar products was achieved by t.l.c.: further separation and identification of individual components was performed by h.p.l.c. Estimations were performed on microsomal pellets and the supernatant mixture after incubation of microsomes for 30 min at 37 degrees C. 3. The polar fraction was larger than the non-polar fraction when expressed as nmol of carbonyl groups/g of liver. In the non-polar supernatant fraction the major contributors were n-alkanals (31% of the total), alpha-dicarbonyl compounds (22%) and 4-hydroxyalkenals (37%) with the extraction method used. 4. Major individual contributors to the non-polar fraction were found to be propanal, 4-hydroxynonenal, hexanal and oct-2-enal. Other components identified include butanal, pent-2-enal, hex-2-enal, hept-2-enal, 4-hydroxyoctenal and 4-hydroxyundecenal. The polar carbonyl fraction was less complex than the non-polar fraction, although the identities of the individual components have not yet been established. 5. Since these carbonyl compounds do not react significantly in the thiobarbituric acid reaction, which largely demonstrates the presence of malonaldehyde, it is concluded that considerable amounts of biologically reactive carbonyl derivatives are released in lipid peroxidation and yet may not be picked up by the thiobarbituric acid reaction.
Full text
PDF




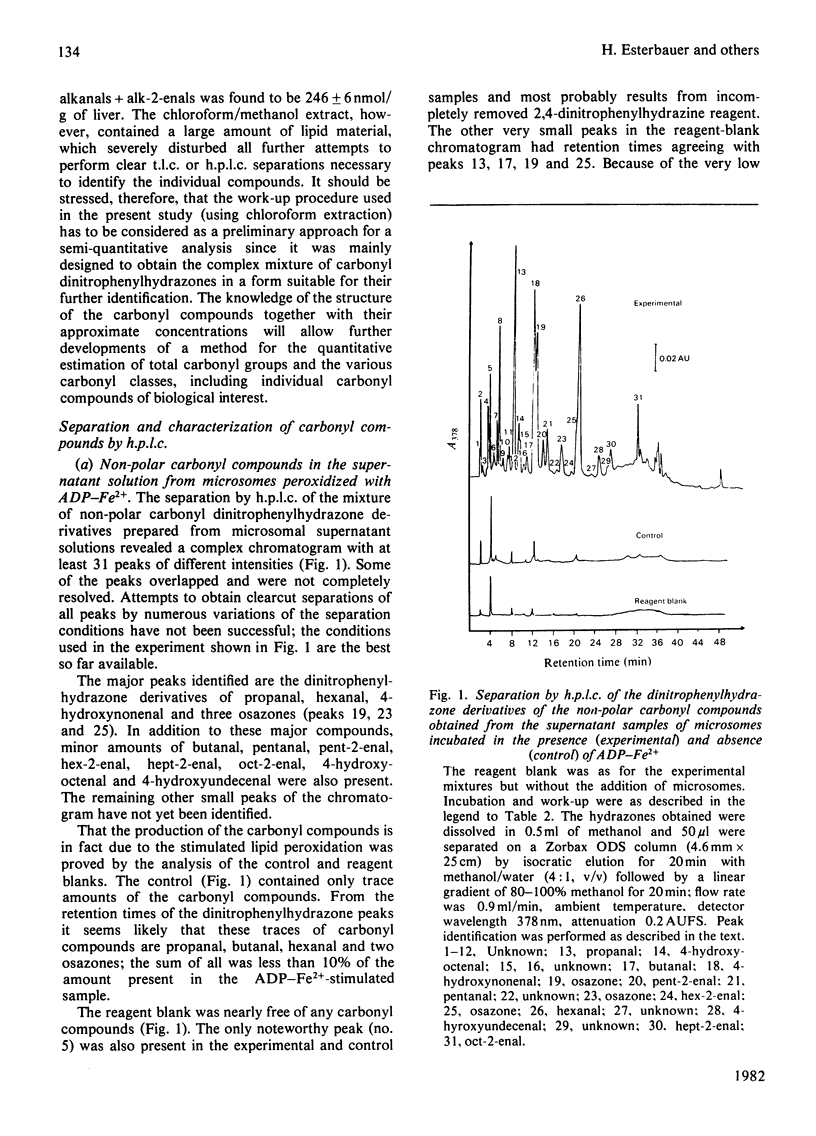
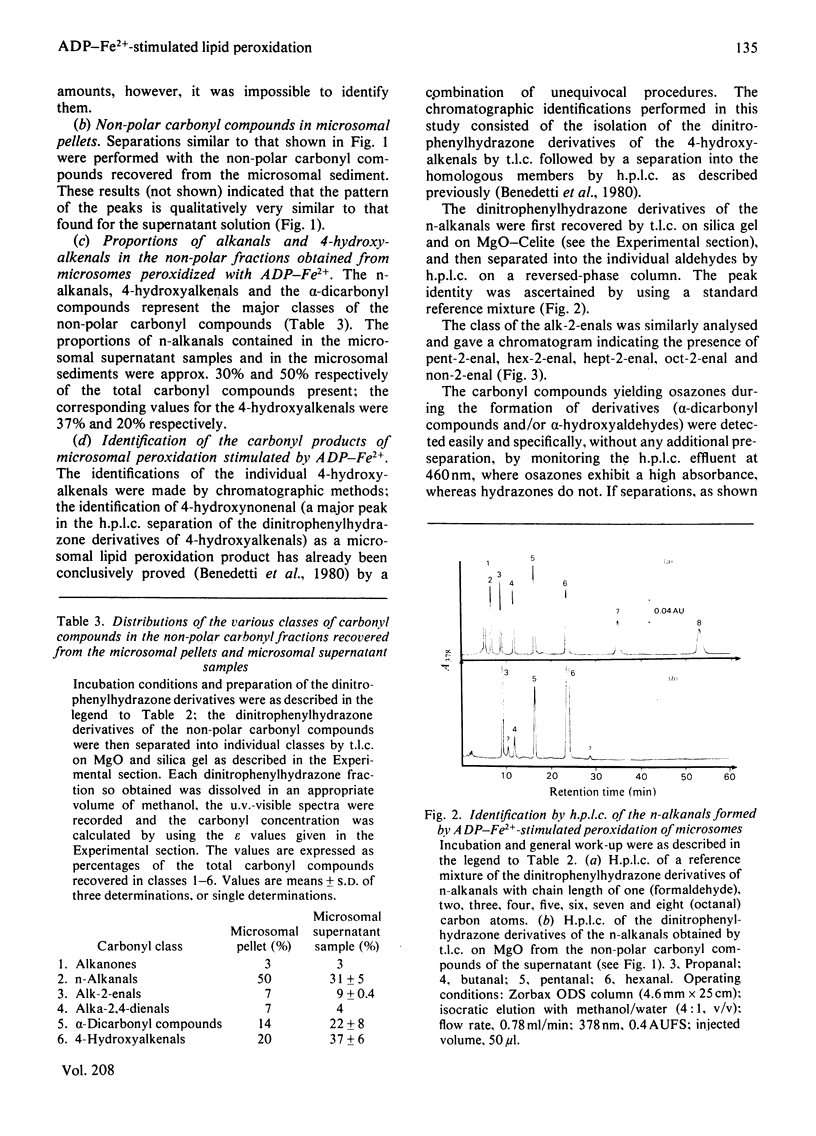
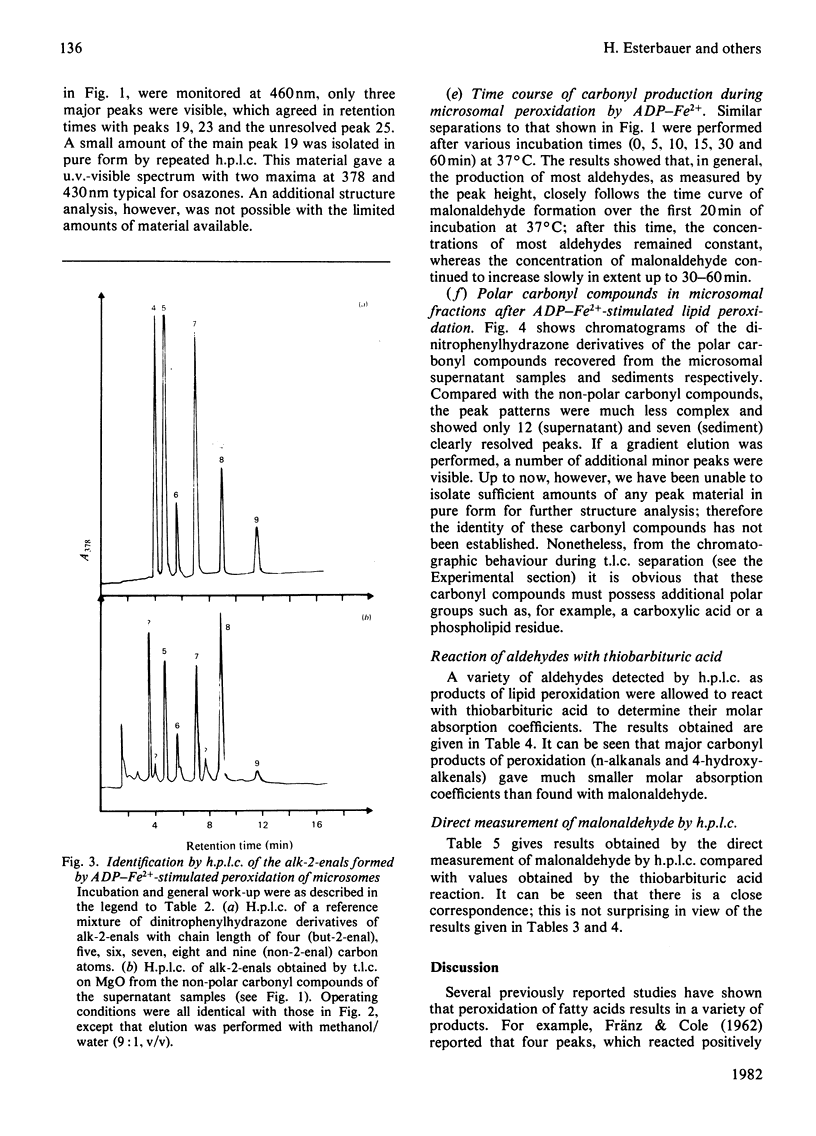
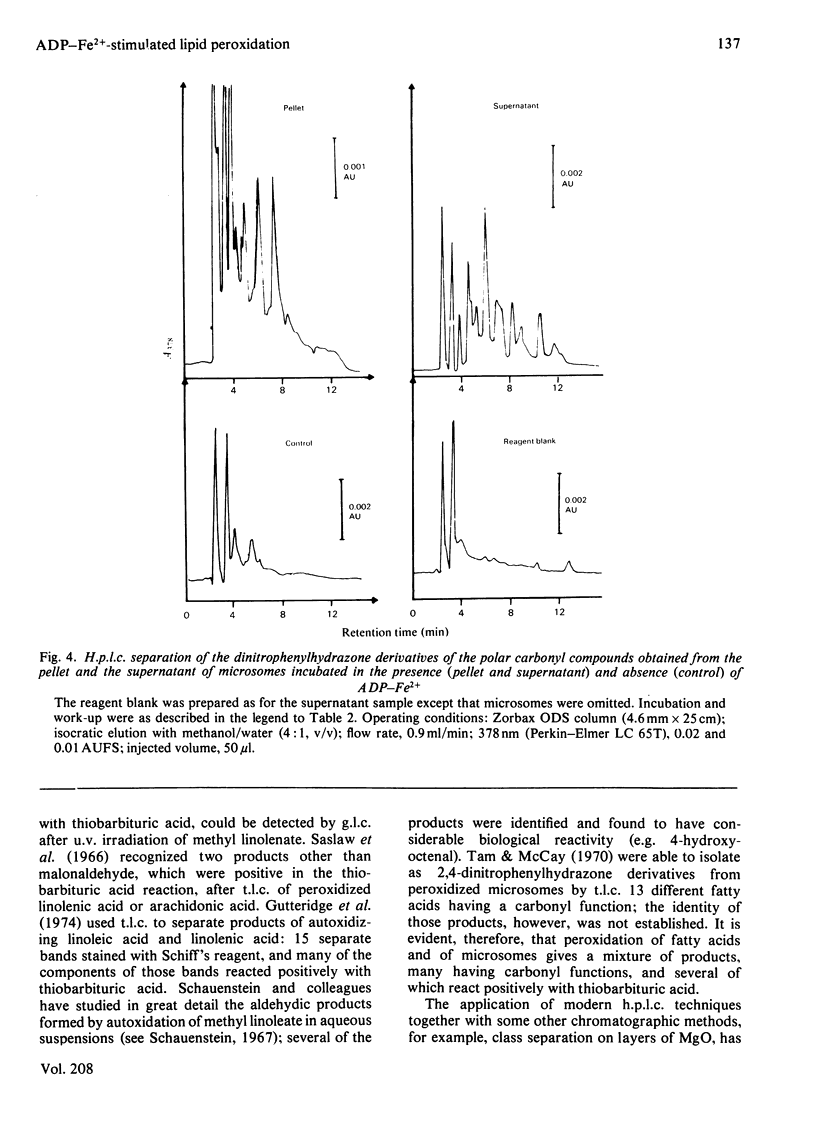


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedetti A., Barbieri L., Ferrali M., Casini A. F., Fulceri R., Comporti M. Inhibition of protein synthesis by carbonyl compounds (4-hydroxyalkenals) originating from the peroxidation of liver microsomal lipids. Chem Biol Interact. 1981 Jun;35(3):331–340. doi: 10.1016/0009-2797(81)90008-9. [DOI] [PubMed] [Google Scholar]
- Benedetti A., Comporti M., Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta. 1980 Nov 7;620(2):281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- Comporti M., Saccocci C., Dianzani M. U. Effect of CCl-4 in vitro and in vivo on lipid peroxidation of rat liver homogenates and subcellular fractions. Enzymologia. 1965 Nov 6;29(3):185–204. [PubMed] [Google Scholar]
- Conroy P. J., Nodes J. T., Slater F., White G. W. Carcinostatic activity of 4-hydroxy-2-pent-en-1-al against transplantable murine tumour lines. Eur J Cancer. 1975 Apr;11(4):231–240. doi: 10.1016/0014-2964(75)90003-1. [DOI] [PubMed] [Google Scholar]
- Dostal V., Schauenstein E., Kulnigg P., Schmeller E. Der Einfluss von 4-Hydroxy-2.3-pentenal (HPE) auf die Vermehrung von Vaccinia-Virus in Hühnerfibroblastenkulturen. Z Naturforsch C. 1974 Jan-Feb;29(1):76–81. [PubMed] [Google Scholar]
- Esterbauer H., Zollner H., Scholz N. Reaction of glutathione with conjugated carbonyls. Z Naturforsch C. 1975 Jul-Aug;30(4):466–473. doi: 10.1515/znc-1975-7-808. [DOI] [PubMed] [Google Scholar]
- FRANZ J., COLE B. T. Effects of ultraviolet-irradiated methyl linolenate on cell division and respiration in Saccharomyces cerevisiae. Arch Biochem Biophys. 1962 Feb;96:382–385. doi: 10.1016/0003-9861(62)90424-1. [DOI] [PubMed] [Google Scholar]
- Ferrali M., Fulceri R., Benedetti A., Comporti M. Effects of carbonyl compounds (4-hydroxyalkenals) originating from the peroxidation of liver microsomal lipids on various microsomal enzyme activities of the liver. Res Commun Chem Pathol Pharmacol. 1980 Oct;30(1):99–112. [PubMed] [Google Scholar]
- Glende E. A., Jr, Hruszkewycz A. M., Recknagel R. O. Critical role of lipid peroxidation in carbon tetrachloride-induced loss of aminopyrine demethylase, cytochrome P-450 and glucose 6-phosphatase. Biochem Pharmacol. 1976 Oct 1;25(19):2163–2170. doi: 10.1016/0006-2952(76)90128-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Stocks J., Dormandy T. L. Thiobarbituric acid-reacting substances derived from autoxidizing linoleic and linolenic acids. Anal Chim Acta. 1974 May;70(1):107–111. doi: 10.1016/S0003-2670(01)82915-9. [DOI] [PubMed] [Google Scholar]
- HOCHSTEIN P., ERNSTER L. ADP-ACTIVATED LIPID PEROXIDATION COUPLED TO THE TPNH OXIDASE SYSTEM OF MICROSOMES. Biochem Biophys Res Commun. 1963 Aug 14;12:388–394. doi: 10.1016/0006-291x(63)90111-6. [DOI] [PubMed] [Google Scholar]
- Jordan R. A., Schenkman J. B. Relationship between malondialdehyde production and arachidonate consumption during NADPH-supported microsomal lipid peroxidation. Biochem Pharmacol. 1982 Apr 1;31(7):1393–1400. doi: 10.1016/0006-2952(82)90034-x. [DOI] [PubMed] [Google Scholar]
- MORRISON W. R., SMITH L. M. PREPARATION OF FATTY ACID METHYL ESTERS AND DIMETHYLACETALS FROM LIPIDS WITH BORON FLUORIDE--METHANOL. J Lipid Res. 1964 Oct;5:600–608. [PubMed] [Google Scholar]
- May H. E., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. I. Nature of the lipid alterations. J Biol Chem. 1968 May 10;243(9):2288–2295. [PubMed] [Google Scholar]
- McDonald-Gibson R. G., Young M. The use of an automatic solids injection system for quantitative determination of plasma long chain non-esterified fatty acids by gas-liquid chromatography. Clin Chim Acta. 1974 May 31;53(1):117–126. doi: 10.1016/0009-8981(74)90359-3. [DOI] [PubMed] [Google Scholar]
- O'brien P. J., Rahimtula A. Involvement of cytochrome P-450 in the intracellular formation of lipid peroxides. J Agric Food Chem. 1975 Mar-Apr;23(2):154–158. doi: 10.1021/jf60198a045. [DOI] [PubMed] [Google Scholar]
- Orrenius S., Dallner G., Ernster L. Inhibition of the TPNH-linked lipid peroxidation of liver microsomes by drugs undergoing oxidative demethylation. Biochem Biophys Res Commun. 1964;14:329–334. doi: 10.1016/s0006-291x(64)80005-x. [DOI] [PubMed] [Google Scholar]
- Plaa G. L., Witschi H. Chemicals, drugs, and lipid peroxidation. Annu Rev Pharmacol Toxicol. 1976;16:125–141. doi: 10.1146/annurev.pa.16.040176.001013. [DOI] [PubMed] [Google Scholar]
- Placer Z. A., Cushman L. L., Johnson B. C. Estimation of product of lipid peroxidation (malonyl dialdehyde) in biochemical systems. Anal Biochem. 1966 Aug;16(2):359–364. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- Poyer J. L., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. IV. Dependence on Fe3+. J Biol Chem. 1971 Jan 10;246(1):263–269. [PubMed] [Google Scholar]
- Rechnagel R. O., Glende E. A., Jr Carbon tetrachloride hepatotoxicity: an example of lethal cleavage. CRC Crit Rev Toxicol. 1973 Nov;2(3):263–297. doi: 10.3109/10408447309082019. [DOI] [PubMed] [Google Scholar]
- Saslaw L. D., Corwin L. M., Waravdekar V. S. Production of chromophoric substances in the thiobarbituric acid test. Arch Biochem Biophys. 1966 Apr;114(1):61–66. doi: 10.1016/0003-9861(66)90305-5. [DOI] [PubMed] [Google Scholar]
- Schauenstein E. Autoxidation of polyunsaturated esters in water: chemical structure and biological activity of the products. J Lipid Res. 1967 Sep;8(5):417–428. [PubMed] [Google Scholar]
- Seeber S., Warnecke P., Weser U. Zur Wirkungsweise des 4-Hydroxy-pentenals bei der Nucleinsäurebiosynthese. Z Krebsforsch. 1969;72(2):137–143. [PubMed] [Google Scholar]
- Slater T. F. Necrogenic action of carbon tetrachloride in the rat: a speculative mechanism based on activation. Nature. 1966 Jan 1;209(5018):36–40. doi: 10.1038/209036a0. [DOI] [PubMed] [Google Scholar]
- Slater T. F., Sawyer B. C. The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem J. 1971 Aug;123(5):805–814. doi: 10.1042/bj1230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater T. F. The inhibitory effects in vitro of phenothiazines and other drugs on lipid-peroxidation systems in rat liver microsomes, and their relationship to the liver necrosis produced by carbon tetrachloride. Biochem J. 1968 Jan;106(1):155–160. doi: 10.1042/bj1060155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIELE E. H., HUFF J. W. THIOBARBITURIC REACTING SUBSTANCE(S) PRODUCED I N NORMAL LIVER FRACTIONS. Arch Biochem Biophys. 1964 Mar;104:468–472. doi: 10.1016/0003-9861(64)90490-4. [DOI] [PubMed] [Google Scholar]
- Tam B. K., McCay P. B. Reduced triphosphopyridine nucleotide oxidase-catalyzed alterations of membrane phospholipids. 3. Transient formation of phospholipid peroxides. J Biol Chem. 1970 May 10;245(9):2295–2300. [PubMed] [Google Scholar]
- Weddle C. C., Hornbrook K. R., McCay P. B. Lipid peroxidation and alteration of membrane lipids in isolated hepatocytes exposed to carbon tetrachloride. J Biol Chem. 1976 Aug 25;251(16):4973–4978. [PubMed] [Google Scholar]


