Abstract
Introduction
RET rearrangements occur in 1% to 2% NSCLCs. Since no clinically validated RET antibody is currently available, fluorescence in situ hybridization (FISH) is often used as a screening tool to identify patients likely to benefit from RET-targeted therapy. In this study, we performed a comprehensive review of publications in which RET-rearrangement testing was performed by FISH and compared the methods and results with our data.
Methods
The findings of an electronic search for publications using RET-FISH in lung cancer were compared with the results obtained at the Grenoble University Hospital where 784 EGFR-, KRAS-, ALK-, and ROS1-negative NSCLCs were tested by RET break-apart FISH and confirmed by RNA-sequencing (RNA-seq).
Results
Out of the 85 publications using RET-FISH analysis, 52 pertained to patients with lung cancer. The most often used positivity threshold was 15%. Six publications compared RET-FISH with at least one other molecular technique on at least eight samples, and the concordance was variable, from 5.9% to 66.7% for FISH-positive cases. Regarding our data, out of the 784 analyzed samples, 32 (4%) were positive by RET-FISH. The concordance between RET-FISH and RNA-seq in RET-FISH positive samples was 69%.
Conclusions
Overall, both existing literature and our data suggest that RET-FISH testing can be used for rapid screening of RET rearrangements in NSCLC. Nevertheless, using an orthogonal technique such as RNA-seq to confirm RET-FISH-positive cases is essential for ensuring that only patients likely to benefit from RET-target therapy receive the treatment.
Keywords: RET, Fusion gene, Lung cancer, FISH, RNA-sequencing, Targeted therapy
Introduction
Kinase gene fusions are the product of chromosomal rearrangements, an important class of oncogenic drivers associated with many solid tumors and hematologic malignancies.1, 2, 3, 4 The RET gene (located at chromosome 10q11.21) encodes a single-pass transmembrane tyrosine kinase receptor. Under normal circumstances, this receptor interacts with its ligands by means of glial cell-line-derived neurotrophic factor family receptor-α co-receptors and mediates cellular processes such as proliferation, differentiation, survival, migration, and metabolism, playing important roles in the development and maintenance of the enteric nervous and genitourinary systems, and various other tissue types, such as the nervous and neuroendocrine tissues.5 Fusions involving the RET receptor tyrosine kinase are created by the in-frame chromosomal fusion of the 3′ tyrosine kinase domain of the RET proto-oncogene to the 5′ regions of various heterologous partner genes. These fusions have been identified in various cancers,6 but occur predominantly in non-medullary thyroid carcinomas (TCs), including 10% to 20% of all papillary TCs and to a lesser extent in follicular TCs, and anaplastic (undifferentiated) TCs.7, 8, 9 RET gene fusions also occur in 1% to 2% of NSCLCs, mainly in adenocarcinomas.10, 11, 12, 13, 14 Targeted therapy with multikinase inhibitors has shown modest clinical activity, with objective response rates ranging from 0% to 50% in patients with NSCLC with RET fusions,15 lower than the rates obtained with ALK and ROS1 small molecule inhibitors (up to 83 and 77%, respectively).16,17 Nevertheless, in early-phase clinical trials, novel selective RET inhibitors, such as LOXO-292 (selpercatinib) and BLU-667 (pralsetinib) revealed objective response rates of 61%18 and 64%,19 respectively, in pretreated patients with NSCLC, and of 84%18 and 72%,19 respectively, in patients who were treatment-naive. LOXO-292 and BLU-667 have been approved since 2020 by the American Food and Drug Administration for the treatment of advanced RET-driven NSCLC, and other potent and selective RET inhibitors, such as BOS17273820 and KL590586 (NCT05265091) are undergoing clinical evaluation. In addition, RET rearrangements have been reported as a resistance mechanism in patients with EGFR-mutated NSCLC treated with tyrosine kinase inhibitors.21
Collectively, these data highlight the importance of implementing robust and practical screening methods to identify patients who are likely to benefit from RET-targeted therapy.
In many Pathology laboratories, fluorescence in situ hybridization (FISH), is often used for screening of RET gene rearrangements in patients with NSCLC, since RET immunohistochemistry (IHC) shows low sensitivity and specificity.21, 22, 23, 24 The turn-around time of the technique is short (1–2 d) and small amounts of tissue are needed.
In this study, we performed an up-to-date comprehensive review of publications in which FISH was used to detect RET rearrangements to understand the testing environment and the potential utility of this technique when compared with other molecular diagnostic tools. We then contrasted the results obtained with our own data.
Materials and Methods
Literature Review
We performed a systematic literature review by 'pearl growing', citation chasing, and PubMed search for studies published between 2000 and 2022 mentioning RET-FISH in their methodology. A total of 86 publications were identified (Supplementary Table 1), and out of these, 52 were lung cancer-related, the rest concerned other tumor types, such as TC (Supplementary Table 2).
Patients
From February 2013 to February 2021, 784 specimens of primary NSCLC were sent to the Grenoble University Hospital cancer molecular genetics platform for routine lung cancer biomarker testing. These specimens were either formalin- or AFA-fixed (the nature of the fixative was not systematically specified), and paraffin-embedded. They included small biopsies (bronchial, transthoracic, or liver biopsies) and surgical specimens (lung resections, lymph node, pleural, or pericardial surgical biopsies). All 784 specimens were tested at least for EGFR and KRAS mutations and immunohistochemical expression of ALK and ROS1 proteins, and were all negative.
This study was conducted according to the European General Data Protection Regulation. The data used are derived from an aggregated, non-individualized database. No personal data allowing identification of subjects was used in this work.
RET-FISH
FISH was performed on unstained 4 μm formalin- or AFA-fixed paraffin-embedded tumor tissue sections with the use of a RET break-apart probe set (ZytoLight SPEC RET Dual Color Break Apart Probe, ZytoVision, Clinisciences, France) using a paraffin pretreatment reagent kit (Vysis, Abbott Molecular or Dako, Agilent Technologies, France). Assays were performed following the manufacturers’ instructions. Nuclei were counterstained with 4',6-diamidino-2-phenylindole-Vectashield (Vektor Laboratories, Ab-Cys, Paris, France). Sections were analyzed with a GSL10 Leica slide scanning system (Leica, France) under a 63× oil immersion objective with a fluorescence microscope equipped with appropriate filters, a charge-coupled device camera, and the FISH imaging and capturing software CytoVision (Leica Biosystems, Nanterre, France). Signals were enumerated with the CytoVision software (Leica Biosystems). Non-rearranged (negative) RET-FISH revealed fusion signals or very close apposition of the probes adjacent to the 5′ (orange) and the 3′ (green) ends of the gene. Rearranged RET-FISH appeared as split 3′ and 5′ (with a gap between the 5′ and 3′ signals being greater than the largest of the two signal diameters), or isolated 3′ (green) signals. Tumor tissues were considered RET-FISH positive (RET-FISH rearranged) if at least 15% of tumor cells were positive in at least 60 tumor cells, on the basis of the criteria used for ALK FISH.25 Samples with less than 60 analyzable tumor cells were considered not interpretable. Otherwise, the samples were considered as being RET-FISH negative.
All RET-FISH positive samples were checked by targeted RNA-sequencing (RNA-seq) for the presence of a RET fusion transcript.
Targeted RNA-Seq and Data Analysis
Library preparation was performed using either the RNA Fusion Lung Cancer panel from 10 ng of total RNA (Thermo Fisher Scientific, Illkirch, France) or the FusionPlex Lung panel from 200 ng of total nucleic acids (ArcherDx, Boulder, Colorado), following the manufacturers’ instructions. Libraries were sequenced on a Thermo Fisher sequencer (Ion PGM or Ion S5). The latest versions of the Ion Reporter (Thermo Fisher Scientific) or Archer Analysis (ArcherDx) software were used to identify fusion gene products from raw sequence data.
Results
Literature Review Findings
Reported Concordance Between RET-FISH And Other Molecular Techniques
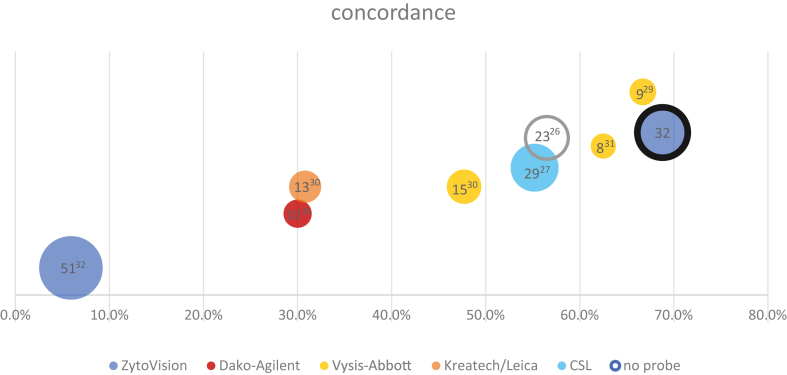
Out of the 52 publications identified using FISH for RET-rearrangement detection in lung cancers, 31 studies reported positive cases by break-apart FISH, which were also tested by at least one other molecular technique (mostly reverse transcription polymerase chain reaction [RT-PCR], next-generation sequencing [NGS], or NanoString) (Tables 1 and 2).11,14,22,23,26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 Studies that did not compare RET-FISH positive cases with another molecular technique, for which comparison data were incomplete, or which were case reports, were not included. In nine of these 31 studies, RET-FISH was used as the initial method or screening method and the results were compared with at least one other molecular technique, leading to very variable concordances, and sometimes very few samples (Table 1).11,14,22,23,26, 27, 28,30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 For the six studies where FISH was used as the screening method and the concordance with another technique was assessed on more than eight samples,26,27,30, 31, 32,53 the concordances varied from 5.9% to 66.7% for FISH-positive cases (Table 111,14,22,23,26, 27, 28,30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53 and Fig. 1). The rest of the studies (22) used FISH as a validation technique for RT-PCR, NanoString or NGS assays. Notably, one additional study reported two cases that were not conclusive by FISH but were also found not conclusive by NanoString because of pre-analytical issues.54
Table 1.
Concordance Between RET-FISH and Other Molecular Techniques Performed on Lung Cancer Reported in the Literature: Cases Initially Screened by FISH
| Study | Number of RET-FISH+ Samples Reported | Number of RET-FISH+ Samples Compared With Other Techniques | Number and % of Concordant Samples Between FISH and Other Techniques | Comments |
|---|---|---|---|---|
| Kim et al.,32 2018 | 51 | 51 | 3 (5.9) [NanoString] | |
| Radonic et al.,30 2021 | 48 | 30 | 9 (30) [RNA-seq] | |
| Tsuta et al.,27 2014 | 50 | 29 | 16 (55.2) [RT-PCR] | RT-PCR analysis only when RNA was available (29) and 14 KIF5B::RET fusions and 2 CCDC6::RET fusions were confirmed. |
| Takeuchi et al.,26 2012 | 22 | 22 | 12 (54.5) [RT-PCR] | |
| Tan et al.,29 2020 | 30 | 9 | 6 (66.7) [NGS] | 2 equivocal FISH samples (10-15% positive cells) were also positive by RNA-seq. |
| Baker et al.,31 2021 | 8 | 8 | 5 (62.5) [RNA-seq] | |
| Go et al.,33 2013 | 3 | 3 | 3 (100) [PCR] 3 (100%) [WTS] |
|
| Rogers et al.,28 2017 | 1 | 1 | 0 (0) [NanoString] 1 (100) [Agena] 0 (0) [RNA-seq] |
The only RET-FISH-positive case in this study was also the most degraded sample, failing to be detected by NanoString and ThermoFisher RNA-seq, and was borderline positive with Agena allele-specific assay. |
| Piton et al.,34 2018 | 1 | 1 | 1 (100) [ligation-dependent RT-PCR] | FISH-positive cases with rearranged nuclei between 15 and 20% were excluded because it was a high risk of a false-positive result, as the authors did not want to test LD-RT-PCR on these unsure 'positive' cases. |
FISH, fluorescence in situ hybridization; LD-RT-PCR, ligation-dependent reverse transcription polymerase chain reaction; NGS, next generation sequencing; PCR, polymerase chain reaction; RNA-seq, RNA-sequencing; RT-PCR, reverse transcription polymerase chain reaction; WTS, whole transcriptome sequencing.
Table 2.
Concordance Between RET-FISH and Other Molecular Techniques Performed on Lung Cancer Reported in the Literature: Cases Initially Screened by Another Method Where FISH Was Used as a Confirmatory/Validation Technique for the Chosen Screening Technique
| Study | Number of RET-FISH+ Samples Reported | Number of RET-FISH+ Samples Compared With Other Techniques | Number and % of Concordant Samples Between FISH and Other Techniques |
|---|---|---|---|
| Takeuchi et al.,36 2021 | 34 | 34 | 4 (100) [RT-PCR] 30 (100) [RNA-seq] |
| Yang et al.,23 2021 | 27 lung cancers (subset of the 171 samples with a RET structural variant) | 27 | 27 (100) [DNA-NGS] 25 (88.9) [RNA-seq] |
| Feng et al.,49 2022 | 25 | 25 | 25 (100) [RNA-seq] |
| Shang et al.,47 2019 | 20 | 20 | 20 (100) [RT-qPCR] |
| Yoh et al.,51 2017 | 19 | 19 | 19 (100) [RT-PCR] |
| Lira et al.,45 2014 | 15 | 15 | 15 (100) [NanoString] |
| Pan et al.,39 2014 | 15 | 15 | 15 (100) [RT-PCR] |
| Lee et al.,22 2015 | 14 | 14 | 14 (100) [NanoString] |
| Wang et al.,14 2012 | 13 | 13 | 13 (100) [RT-PCR] |
| Song et al.,37 2016 | 11 | 11 | 11 (100) [RT-PCR] 11 (100) [RNA-seq] |
| Chen et al.,48 2020 | 10 | 10 | 10 (100) [DNA-NGS] |
| Radonic et al.,30 2021 | 9 | 9 | 9 (100) [RNA-seq] |
| Kim et al.,40 2015 | 9 | 9 | 9 (100) [RT-PCR] |
| Kohno et al.,11 2012 | 6 | 6 | 6 (100) [RT-PCR] |
| Tanaka et al.,43 2017 | 4 | 4 | 4 (100) [RT-PCR] |
| Sokolova et al.,52 2020 | 3 | 3 | 3 (100) [validated method] |
| Sasaki et al.,35 2012 | 2 | 2 | 2 (100) [RT-PCR] |
| Reguart et al.,42 2017 | 2 | 2 | 2 (100) [NanoString] |
| Song et al.,41 2017 | 2 | 2 | 2 (100) [RT-PCR] |
| Ambrosini-Spaltro et al.,50 2022 | 2 | 2 | 2 (100) [RNA-seq] |
| Suehara et al.,44 2012 | 1 | 1 | 1 (100) [NanoString] |
| Borrelli et al.,38 2013 | 1 | 1 | 1 (100) [RT-PCR] |
| Velizheva et al.,46 2018 | 1 | 1 | 1 (100) [RNA-seq] |
DNA-NGS, DNA next generation sequencing; FISH, fluorescence in situ hybridization; RNA-seq, RNA-sequencing; RT-PCR, reverse transcription polymerase chain reaction; RT-qPCR, reverse transcription quantitative polymerase chain reaction.
Figure 1.
Concordance values between FISH and another molecular technique. On this graph, bubble size is the number of RET-FISH+ samples compared with another technique. The highlighted bubble represents our data (local cohort). The concordance values are based on the data in Table 1. FISH, fluorescence in situ hybridization.
RET-FISH Probes And Methodology Used In The Literature
A wide range of probes was used across publications (Supplementary Table 2), targeting various lengths and locations within the 5′ and 3′ regions of the RET gene. Out of the 52 publications, 40 mentioned the use of commercial break-apart probes (one publication mentioned four probes30). Among the commercial probes (provided by 12 different manufacturers), the most often used (39%) was the ZytoLight SPEC RET Dual Color Break (ZytoVision GmbH) (Supplementary Table 3).
Scoring
Signal Patterns
Regarding the scoring, positivity was considered when only a separation of the 5′ and 3′ signals (split signal) was found, or when a split signal or single 3′ signals or both, were present by an equal proportion of authors (Supplementary Table 4). The split signal was considered positive when the gap between the 5′ and 3′ signals was either at least more than one signal diameter or in some cases, a separation greater than twice the signal diameter.23,55 In general, a “complex” or “atypical” pattern was defined as a rearrangement with any pattern that could not be classified using the usual split or single 3′ patterns. Some authors considered complex or atypical patterns as potentially positive, and a confirmatory test was always initiated if sufficient tissue was available. Single 5′ patterns were reported sometimes but were mostly considered clinically negative, because of the potential loss of the RET kinase domain.30 Notably, one publication38 considered single 5′ RET signals to be positive.
Cutoff Value
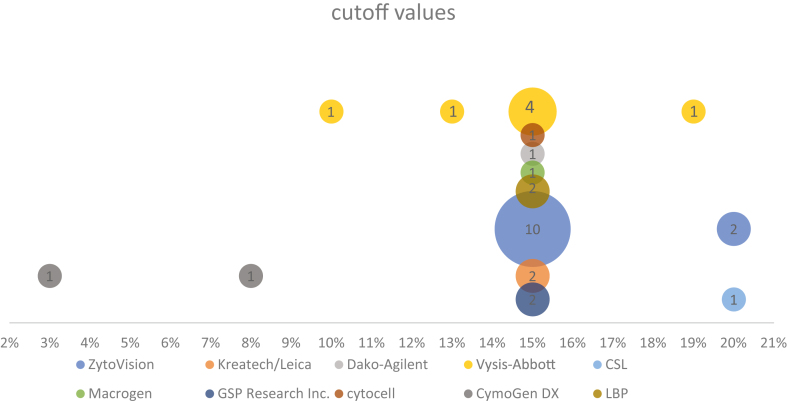
Since there are no standard guidelines to date regarding the cutoff value for RET-FISH positivity in lung tumors, we found several approaches and different cutoff values cited in the literature, ranging from 3% to 20% of cells with RET-positive patterns. Nevertheless, as shown in Figure 2, in most studies a threshold of at least 15% FISH-positive tumor nuclei was chosen to define a RET-FISH-positive tumor. In other studies, to consider a sample positive for a RET-rearrangement, the cutoff was determined as the mean value of positive cells +3 S.D. in known RET-negative31,56 or RET-positive samples.55,57 Remarkably, 20 articles of 52 did not specify the cutoff used.
Figure 2.
RET-FISH cutoff values used in the literature. Graph showing the number of times a cutoff value was mentioned in the literature for RET-FISH (31 publications identified). Bubble size is the number of mentions for that probe (33 mentions in total). FISH, fluorescence in situ hybridization.
RET Testing Results on Our NSCLC Cohort
RET-FISH Results
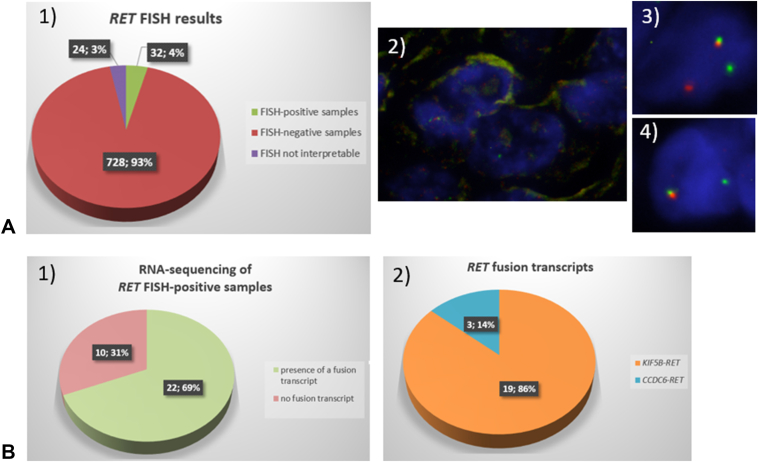
Out of the 784 samples analyzed by RET-FISH, 24 (3%) were not interpretable, either because of the absence or very poor quality of the hybridization signals (16 samples) or because less than 60 analyzable tumor cells were present on the tissue section analyzed (eight samples) (Fig. 3). Out of the 760 samples for which the RET-FISH analysis yielded an interpretable result, 32 (4%) were positive by FISH (≥15% of tumor cells with separated 5′ and 3′ signals or isolated 3′ signals or both) and 728 / 760 (96%) were negative (<15% positive tumor cells) (Fig. 3 and Table 3). Notably, no samples harbored a FISH pattern showing isolated 5′ (orange) signals. Nineteen patients were tested twice: 13 patients were analyzed at diagnosis and then at progression 1 to 3 years later, five patients had RET-FISH analysis performed first on a biopsy (n = 3) or a cytologic specimen (n = 2) and then on a resection sample, and one patient had two biopsies from two different metastatic sites. For all 19 patients, RET-FISH analyses were concordant: negative on all samples.
Figure 3.
RET-FISH results on the 784 NSCLC samples analyzed and targeted RNA-seq results on the 32 RET-FISH positive samples. (A) RET-FISH results on the 784 NSCLC samples of our NSCLC cohort. (1) Repartition of the RET-FISH positive, negative, and not interpretable samples. (2) Example of a non-interpretable result (signal intensity too low), (3) Example of a RET-FISH positive nucleus showing a split signal, (4) Example of a RET-FISH positive nucleus showing an isolated 3′ (green) signal. (B) Targeted RNA-seq results on the 32 RET-FISH positive samples. (1) Number and proportion of RET-FISH positive cases for which a fusion transcript was present or absent. (2) Nature and repartition of the fusion transcripts detected. FISH, fluorescence in situ hybridization.
Table 3.
Clinical, Histopathologic, and FISH Data of the 760 RET-FISH-Positive and -Negative NSCLC Samples
| N = 760 | RET-FISH-Positive Samples (≥15%) n = 32 | RET-FISH-Negative Samples (<15%) n = 728 |
|---|---|---|
| Patient gender, n (%) | ||
| M | 18 (56) | 493 (68) |
| F | 14 (44) | 235 (32) |
| Patient age, mean, median (range) | ||
| M | 70, 71 (51–86) | 68, 69 (21–94) |
| F | 67, 65 (48–82) | 66, 66 (29–93) |
| Histology, n (%) | ||
| Adenocarcinoma | 24 (75) | 583 (80.1) |
| Squamous cell carcinoma | 0 | 7 (1.0) |
| Sarcomatoid carcinoma | 0 | 3 (0.4) |
| (large cell) neuroendocrine carcinoma | 1 (3.1) | 6 (0.8) |
| Other type of carcinoma | 7 (21.9) | 129 (17.7) |
| Type of sample, n (%) | ||
| Lung resection | 7 (21.9) | 123 (16.9) |
| Resection of metastatic site | 4 (12.5) | 70 (9.6) |
| Lung biopsy | 13 (40.6) | 344 (47.3) |
| Biopsy of metastatic site | 5 (15.6) | 132 (18.1) |
| Cytologic sample | 3 (9.4) | 59 (8.1) |
| % of tumor cells in the analyzed sample, n (%) | ||
| <20% | 5 (15.6) | 86 (11.8) |
| 20%–50% | 20 (62.5) | 434 (59.6) |
| >50% | 7 (21.9) | 203 (27.9) |
| Not specified | 0 | 5 (0.7) |
| Number of nuclei analyzed by FISH, mean (range) | 105 (84–128) | 105 (60–311) |
| % of RET-FISH positive nuclei mean, median (range) | 55.3%, 57.7% (17.7%–94%) | 4.4%, 3.9% (0–14.3%) |
F, female; FISH, fluorescence in situ hybridization; M, male.
The mean and median percentages of FISH-positive cells in the RET-FISH-negative samples were 4.4% and 3.9%, respectively, ranging from 0% to 14.3% (Table 3). The sample with 14.3% positive tumor cells was analyzed by RNA-seq and revealed no fusion transcript. The mean and median percentages of FISH-positive cells in the RET-FISH-positive samples were 55.3% and 57.7%, respectively, ranging from 17.7% to 94% (Table 3).
Targeted RNA-seq Results
All 32 samples showing a RET-FISH positive result were analyzed by RNA-seq using targeted panels from Thermo Fisher Scientific or ArcherDx or both. As shown in Figure 3, a RET fusion transcript was detected in 22 (69%) samples. In three (14%) cases, a CCDC6::RET(exon 12) transcript was found (CCDC6 exon 1 in two cases, exon 8 in one case), and in all other positive cases (19, 86%), a KIF5B::RET fusion transcript was found. All KIF5B::RET fusions were between exon 15 of KIF5B and exon 12 of RET, except for one sample for which the fusion was within intron 11 of RET.
Comparison Between FISH And RNA-seq Results For RET-FISH Positive Samples
When analyzed in detail, the FISH patterns between the 22 RET-FISH positive and RNA-seq positive samples and the 10 RET-FISH positive and RNA-seq negative samples appeared to be somewhat different (Table 4). Indeed, in the FISH and RNA-seq discordant samples, the isolated 3′ signal pattern was more represented (median of 86% of positive nuclei) than in the FISH and RNA-seq concordant samples (37%). Conversely, the split pattern was more represented in the FISH and RNA-seq concordant samples (median of 63%) than in the FISH and RNA-seq discordant samples (14%).
Table 4.
Concordance Between RET-FISH and RET-RNA-seq in the 32 RET-FISH-Positive NSCLC Samples
|
N = 32 |
RET-FISH Positive/RNA-seq Positive Samples n = 22 | RET-FISH Positive/RNA-seq Negative Samples n = 10 |
|---|---|---|
| % of positive cells showing a split pattern, median | 62.9 | 13.7 |
| % of positive cells showing an isolated 3′ signal pattern, median | 37.1 | 86.3 |
FISH, fluorescence in situ hybridization; RNA-seq, RNA-sequencing.
The 10 RET-FISH positive and RNA-seq negative samples were checked twice, either by two different RNA-seq panels (ThermoFisher and Archer Dx) or by RNA-seq and real-time RT-PCR for KIF5B::RET and CCDC6::RET fusions, if not enough material was available for another round of RNA-seq. All 10 cases were negative by all these RNA-based techniques, pointing toward a false-positivity of the RET-FISH analysis of 31%.
Discussion
Since the approval of RET inhibitors by the United States Food and Drug Administration for the treatment of RET fusion-positive tumors, the accurate identification of RET rearrangements has gained predictive significance. Optimizing diagnostic tools for sensitivity and specificity is therefore essential but these techniques must also be adapted to the low prevalence of RET fusions in NSCLC. Historically, FISH has been the reference technique used for rearrangements and fusions detection in solid tumors, but several other diagnostic tools have been developed, such as IHC, RT-PCR, and NGS techniques. Nevertheless, even if RET antibodies are available, they do not have the sensitivity/specificity to be clinically relevant for the detection of RET fusion proteins, and International Association for the Study of Lung Cancer/College of American Pathologists/Association for Molecular Pathology Guidelines recommend against using IHC to test for RET fusions in patients with lung cancer.58 PCR-based methods, such as RT-PCR and amplicon-based NGS can be limited by the set of primers available for each assay, potentially missing novel fusion partners or atypical breakpoints, and NGS techniques on the basis of hybrid capture or multiplex anchored PCR can detect RET fusions regardless of the fusion partner, but are not necessarily available in every laboratory. Therefore, FISH continues to be used as a screening method in some instances, for example when testing RET in a sequential algorithm, especially in EGFR-, KRAS-, ALK- and ROS1-negative NSCLC samples and/or when NGS or RT-PCR are not available or technically feasible. FISH turn-around time is short (1–2 d) and requires small amounts of tissue. Among the various RET-FISH probes available, break-apart probes are more suited to the detection of RET rearrangements compared with fusion probes since nearly 50 RET fusion partners have been identified in NSCLC.59
In the present report, we aimed to compare our data with the available published data from 2000 to 2022 regarding RET-FISH testing to understand the testing environment and to analyze the advantages and drawbacks of RET-FISH compared with other molecular testing methods.
In the absence of validated interpretation criteria for the detection of RET rearrangements by break-apart FISH, we applied criteria extrapolated from ALK FISH testing60,61 and found them comparable to those used by most of the published studies. Indeed, out of the 52 publications reporting the use of FISH for RET-rearrangement detection in lung cancer, when mentioned, the most often used positivity threshold (cutoff value) was 15%, and the FISH patterns considered positive in most studies were split signals (one to two signal diameters apart) and isolated 3′ signals. With respect to the FISH probes used, we found an impressive variety (>10) of commercial probes, but the most largely used was the ZytoVision RET break-apart probe.
Regarding the positivity thresholds applied, in the study by Michels et al.,62 two different positivity thresholds (either 15% or 20%) were used by the two centers participating in the study, and no differences were found, as the 22 RET-FISH positive cases were all found to have more than 20% tumor cells with the ZytoVision RET break-apart probe, and the mean percentage of positive cells (fraction of RET rearranged cells) in the positive cases was 47.9%, largely above the two positivity thresholds. Unfortunately, in this study, the authors did not compare the FISH results with another technique. In another study by Baker et al.,31 in which various positivity thresholds were tested, by using training and validation sets of both NSCLC and non-medullary thyroid cancers, the authors proposed for Abbott’s Vysis RET break-apart probe a three-tiered scoring system aiming at maximizing sensitivity given the small number of RET fusion-positive cases. Samples with less than 13% interpretable tumor nuclei with abnormal signal patterns were considered negative, samples harboring 13% to 18% abnormal signal patterns were equivocal, needing to be checked by another technique, and samples with 19% or more nuclei with an abnormal signal pattern were considered positive. This 3-tiered system led to 100% sensitivity and 96% specificity of RET break-apart FISH on a validation set consisting of 96 samples, out of which were 14 NSCLC RET fusion-positive samples confirmed by NGS. Notably, two samples that were equivocal or negative by FISH (13% and 18% of rearranged tumor nuclei) were found to be positive by NGS, with KIF5B as the fusion partner, pointing toward the importance of checking both borderline positive and borderline negative samples.
When studying the articles evaluating the concordance between RET-FISH and at least one other molecular diagnostic technique (excluding RET IHC and flow cytometry) on eight or more RET-FISH positive samples, we found highly variable concordance rates, ranging from 5.9% to 66.7%. Many reasons can explain this variability in concordance rates, especially the wide variety of FISH probes, interpretation criteria, and the comparison methods used (various DNA-based and RNA-based NGS panels, (RT)-PCR, NanoString, Agena, among others). In our laboratory, by using RET-FISH as a screening method for RET-rearrangement detection on 784 EGFR-, KRAS-, ALK- and ROS1-negative NSCLC samples, we found 32 samples to be positive by FISH with the previously mentioned positivity criteria, making our study the second largest study of RET-FISH positive samples to date after the study by Kim et al.32 Nevertheless, in this latter study, a fusion transcript was identified by NanoString analysis in only three samples leading to a very low concordance (6%) between the two techniques. In contrast, after performing RNA-seq on all 32 RET-FISH-positive samples of our cohort to check for the presence or absence of a RET fusion transcript, we found a RET fusion transcript in 22 samples, showing a concordance of 69% between FISH and RNA-seq. Therefore, if we compare our concordance rate to those of the 6 previous studies we found in the literature which used RET-FISH and then confirmed the results on more than 8 samples, our concordance rate is the highest.
In addition, similar to the findings by Michels et al.,62 and by using the same commercial probe, the mean percentage of positive cells by FISH in RET rearranged samples in our cohort (55%) was largely above the most often used 15% positivity threshold.
Nevertheless, as shown by our results (discordance rate of 31% between FISH and RNA-seq) and those found in the literature, RET-FISH can yield false-positive results.23,29, 30, 31 Indeed, all rearrangements in the RET locus are detected by FISH, independent of whether they result in a transcribed oncogenic fusion or not (in-frame versus out-of-frame rearrangements). As reported in the literature, other reasons for false-positivity using FISH can include statistical sampling effects in borderline samples (percent of rearranged cells close to the positivity threshold) and the presence of multiple copies (gain or amplification) of the target gene.63,64 Nevertheless, in our cohort, out of the 10 false-positive samples (RET-FISH positive and RNA-seq negative), only one revealed a borderline positivity (18% of rearranged cells), and it also revealed multiple copies of the RET locus. No other RNA-seq negative sample was borderline positive (between 15% and 20% of rearranged nuclei) or presented an augmented number of copies of the RET locus. The mean number of positive nuclei in the discordant (FISH false-positive) samples was 47%, which is far away from the positivity threshold. Therefore, the main reason for false positivity we could find in our cohort was the presence of non-transcribed DNA rearrangement of the RET locus. Interestingly, a higher rate of samples revealed isolated 3′ signals in the 10 FISH positive and RNA-seq negative samples compared with the rest of our cohort, with the limit of the relatively low number of cases analyzed. Therefore, when using FISH for the detection of RET rearrangements, the use of an orthogonal technique able to detect all transcribed RET fusions (regardless of the fusion partners) to confirm all RET-FISH positive cases is essential to not treat patients who are not likely to benefit from a RET-targeted therapy.
As previously noted, false-negative results by FISH have also been reported in cohorts where samples were tested by both FISH and NGS.29,31,49,50,65 Limited reliability of break-apart FISH because of statistical sampling effects in borderline samples has also been reported as one of the reasons leading to false-positivity using FISH.63 A systematic selection of the areas analyzed by FISH should also always be performed by a pathologist to avoid the counting of benign nuclei. Nevertheless, it must be noted that all the cases we found in our literature review that were initially detected using an alternative molecular method (mostly RNA-seq) were found positive by RET-FISH, suggesting a good sensitivity of RET-FISH.
In view of the limits of the FISH technique for the detection of targetable fusions, RNA-based NGS (RNA-seq) has become the preferred molecular testing option for RET fusions, together with other biomarkers, because of its increasingly feasible, multigene testing capability and cost-effectiveness, and has replaced FISH as the technique used for RET-rearrangement testing in our laboratory since 2021.
In summary, FISH turn-around time is short (1–2 d) and requires small amounts of tissue, therefore can be used as a screening method for the detection of RET rearrangements in the absence of a clinically validated antibody. Nevertheless, FISH-positive and equivocal findings have to be validated by an orthogonal technique, such as RNA-seq, to ensure the detected fusion is indeed a functional oncogenic aberration, to select patients who might benefit from RET-targeted therapy.
CRediT Authorship Contribution Statement
AnneMc Leer: Conceptualization, Methodology, Validation, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Supervision, Project administration.
Julie Mondet: Validation, Investigation, Writing - review & editing.
Nelly Magnat: Validation, Investigation.
Mailys Mersch: Investigation.
Diane Giovannini: Investigation, Resources, Writing - review & editing.
Camille Emprou: Investigation, Resources, Writing - review & editing.
Anne-Claire Toffart: Investigation, Resources, Writing - review & editing.
Nathalie Sturm: Investigation, Resources, Writing - review & editing.
Sylvie Lantuéjoul: Investigation, Resources, Writing - review & editing.
David Benito: Conceptualization, Methodology, Validation, Resources, Data curation, Writing - original draft, Writing - review & editing, Supervision.
Disclosure
Dr. Mc Leer has declared consulting fees from Janssen-Cilag, Eli Lilly, Takeda, JFR Access, Pfizer, payment for presentations from Amgen, AstraZeneca, Cancerodigest, Edimark, Janssen-Cilag and support for attending meetings from Amgen, AstraZeneca, Janssen-Cilag, Pfizer. Dr. Toffart has declared consulting fees from AstraZeneca, Bristol-Myers Squibb, Amgen, Ipsen, Janssen, Merck Sharp & Dohme, Pfizer, Roche, Sanofi, Takeda, payment for presentations from AstraZeneca, Bristol-Myers Squibb, Amgen, Ipsen, Janssen, Merck Sharp & Dohme, Pfizer, Roche, Sanofi, Takeda, payment for expert testimony from AstraZeneca, Bristol-Myers Squibb, Amgen, Ipsen, Janssen, Merck Sharp & Dohme, Pfizer, Roche, Sanofi, Takeda and support for attending meetings from AstraZeneca, Pfizer, Roche, Takeda. Dr. Benito is a full-time employee at Eli Lilly Co. The remaining authors declare no conflict of interest.
Footnotes
Cite this article as: Mc Leer A, Mondet J, Magnat N, et al. RET-rearrangement detection by fluorescence in situ hybridization compared with other techniques in NSCLC. JTO Clin Res Rep. 2024;5:100714.
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology at www.jto.org and at https://doi.org/10.1016/j.jtocrr.2024.100714.
Supplementary Data
References
- 1.Delattre O., Zucman J., Plougastel B., et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359:162–165. doi: 10.1038/359162a0. [DOI] [PubMed] [Google Scholar]
- 2.Nowell P.C., Hungerford D.A. Chromosome studies on normal and leukemic human leukocytes. J Natl Cancer Inst. 1960;25:85–109. [PubMed] [Google Scholar]
- 3.Rowley J.D. The critical role of chromosome translocations in human leukemias. Annu Rev Genet. 1998;32:495–519. doi: 10.1146/annurev.genet.32.1.495. [DOI] [PubMed] [Google Scholar]
- 4.Meenakshi C., Venkatraman B. Correlation between cytogenetic biomarkers obtained from DC and CBMN assays caused by low dose radon exposure in smokers. Int J Radiat Biol. 2019;95:1268–1275. doi: 10.1080/09553002.2019.1625494. [DOI] [PubMed] [Google Scholar]
- 5.Mahato A.K., Sidorova Y.A. RET receptor tyrosine kinase: role in neurodegeneration, obesity, and cancer. Int J Mol Sci. 2020;21:7108. doi: 10.3390/ijms21197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drilon A., Hu Z.I., Lai G.G.Y., Tan D.S.W. Targeting RET-driven cancers: lessons from evolving preclinical and clinical landscapes. Nat Rev Clin Oncol. 2018;15:151–167. doi: 10.1038/nrclinonc.2017.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee M.Y., Ku B.M., Kim H.S., et al. Genetic alterations and their clinical implications in high-recurrence risk papillary thyroid cancer. Cancer Res Treat. 2017;49:906–914. doi: 10.4143/crt.2016.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prescott J.D., Zeiger M.A. The RET oncogene in papillary thyroid carcinoma. Cancer. 2015;121:2137–2146. doi: 10.1002/cncr.29044. [DOI] [PubMed] [Google Scholar]
- 9.Liu Z., Hou P., Ji M., et al. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab. 2008;93:3106–3116. doi: 10.1210/jc.2008-0273. [DOI] [PubMed] [Google Scholar]
- 10.Ju Y.S., Lee W.C., Shin J.Y., et al. A transforming KIF5B and RET gene fusion in lung adenocarcinoma revealed from whole-genome and transcriptome sequencing. Genome Res. 2012;22:436–445. doi: 10.1101/gr.133645.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohno T., Ichikawa H., Totoki Y., et al. KIF5B-RET fusions in lung adenocarcinoma. Nat Med. 2012;18:375–377. doi: 10.1038/nm.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li F., Feng Y., Fang R., et al. Identification of RET gene fusion by exon array analyses in “pan-negative” lung cancer from never smokers. Cell Res. 2012;22:928–931. doi: 10.1038/cr.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipson D., Capelletti M., Yelensky R., et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nat Med. 2012;18:382–384. doi: 10.1038/nm.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang R., Hu H., Pan Y., et al. RET fusions define a unique molecular and clinicopathologic subtype of non-small-cell lung cancer. J Clin Oncol. 2012;30:4352–4359. doi: 10.1200/JCO.2012.44.1477. [DOI] [PubMed] [Google Scholar]
- 15.Choudhury N.J., Drilon A. Decade in review: a new era for RET-rearranged lung cancers. Transl Lung Cancer Res. 2020;9:2571–2580. doi: 10.21037/tlcr-20-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters S., Camidge D.R., Shaw A.T., et al. Alectinib versus crizotinib in untreated ALK-positive non–small-cell lung cancer. N Engl J Med. 2017;377:829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 17.Drilon A., Siena S., Dziadziuszko R., et al. Entrectinib in ROS1 fusion-positive non-small-cell lung cancer: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21:261–270. doi: 10.1016/S1470-2045(19)30690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drilon A., Subbiah V., Gautschi O., et al. Selpercatinib in patients with RET fusion–positive non–small-cell lung cancer: updated safety and efficacy from the registrational LIBRETTO-001 Phase I/II trial. J Clin Oncol. 2023;41:385–394. doi: 10.1200/JCO.22.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griesinger F., Curigliano G., Thomas M., et al. Safety and efficacy of pralsetinib in RET fusion–positive non-small-cell lung cancer including as first-line therapy: update from the ARROW trial. Ann Oncol. 2022;33:1168–1178. doi: 10.1016/j.annonc.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Schoffski P., Cho B.C., Italiano A., et al. BOS172738, a highly potent and selective RET inhibitor, for the treatment of RET-altered tumors including RET-fusion+ NSCLC and RET-mutant MTC: Phase 1 study results. J Clin Oncol. 2021;39(suppl 15) 3008–3008. [Google Scholar]
- 21.Klempner S.J., Bazhenova L.A., Braiteh F.S., et al. Emergence of RET rearrangement co-existing with activated EGFR mutation in EGFR-mutated NSCLC patients who had progressed on first- or second-generation EGFR TKI. Lung Cancer. 2015;89:357–359. doi: 10.1016/j.lungcan.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Lee S.E., Lee B., Hong M., et al. Comprehensive analysis of RET and ROS1 rearrangement in lung adenocarcinoma. Mod Pathol. 2015;28:468–479. doi: 10.1038/modpathol.2014.107. [DOI] [PubMed] [Google Scholar]
- 23.Yang S.R., Aypar U., Rosen E.Y., et al. A performance comparison of commonly used assays to detect RET fusions. Clin Cancer Res. 2021;27:1316–1328. doi: 10.1158/1078-0432.CCR-20-3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tachon G., Cortes U., Richard S., et al. Targeted RNA-sequencing assays: a step forward compared to FISH and IHC techniques? Cancer Med. 2019;8:7556–7566. doi: 10.1002/cam4.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Camidge D.R., Kono S.A., Flacco A., et al. Optimizing the detection of lung cancer patients harboring anaplastic lymphoma kinase (ALK) gene rearrangements potentially suitable for ALK inhibitor treatment. Clin Cancer Res. 2010;16:5581–5590. doi: 10.1158/1078-0432.CCR-10-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takeuchi K., Soda M., Togashi Y., et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18:378–381. doi: 10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 27.Tsuta K., Kohno T., Yoshida A., et al. RET-rearranged non-small-cell lung carcinoma: a clinicopathological and molecular analysis. Br J Cancer. 2014;110:1571–1578. doi: 10.1038/bjc.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rogers T.M., Arnau G.M., Ryland G.L., et al. Multiplexed transcriptome analysis to detect ALK, ROS1 and RET rearrangements in lung cancer. Sci Rep. 2017;7 doi: 10.1038/srep42259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan A.C., Seet A.O.L., Lai G.G.Y., et al. Molecular characterization and clinical outcomes in RET-rearranged NSCLC. J Thorac Oncol. 2020;15:1928–1934. doi: 10.1016/j.jtho.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Radonic T., Geurts-Giele W.R.R., Samsom K.G., et al. RET fluorescence in situ hybridization analysis is a sensitive but highly unspecific screening method for RET fusions in lung cancer. J Thorac Oncol. 2021;16:798–806. doi: 10.1016/j.jtho.2021.01.1619. [DOI] [PubMed] [Google Scholar]
- 31.Baker J.A., Sireci A.N., Marella N., et al. Analytical accuracy of RET fusion detection by break-apart fluorescence in situ hybridization. Arch Pathol Lab Med. 2022;146:351–359. doi: 10.5858/arpa.2020-0376-OA. [DOI] [PubMed] [Google Scholar]
- 32.Kim J.O., Shin J.Y., Kim M.Y., et al. Detection of RET (rearranged during transfection) variants and their downstream signal molecules in RET rearranged lung adenocarcinoma patients. Surg Oncol. 2018;27:106–113. doi: 10.1016/j.suronc.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 33.Go H., Jung Y.J., Kang H.W., et al. Diagnostic method for the detection of KIF5B-RET transformation in lung adenocarcinoma. Lung Cancer. 2013;82:44–50. doi: 10.1016/j.lungcan.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Piton N., Ruminy P., Gravet C., et al. Ligation-dependent RT-PCR: a new specific and low-cost technique to detect ALK, ROS, and RET rearrangements in lung adenocarcinoma. Lab Invest. 2018;98:371–379. doi: 10.1038/labinvest.2017.124. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki H., Shimizu S., Tani Y., et al. RET expression and detection of KIF5B/RET gene rearrangements in Japanese lung cancer. Cancer Med. 2012;1:68–75. doi: 10.1002/cam4.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeuchi S., Yanagitani N., Seto T., et al. Phase 1/2 study of alectinib in RET-rearranged previously-treated non-small cell lung cancer (ALL-RET) Transl Lung Cancer Res. 2021;10:314–325. doi: 10.21037/tlcr-20-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song Z., Yu X., Zhang Y. Clinicopathologic characteristics, genetic variability and therapeutic options of RET rearrangements patients in lung adenocarcinoma. Lung Cancer. 2016;101:16–21. doi: 10.1016/j.lungcan.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Borrelli N., Giannini R., Proietti A., et al. KIF5B/RET fusion gene analysis in a selected series of cytological specimens of EGFR, KRAS and EML4-ALK wild-type adenocarcinomas of the lung. Lung Cancer. 2013;81:377–381. doi: 10.1016/j.lungcan.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 39.Pan Y., Zhang Y., Li Y., et al. ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: a comprehensive study of common and fusion pattern-specific clinicopathologic, histologic and cytologic features. Lung Cancer. 2014;84:121–126. doi: 10.1016/j.lungcan.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Kim J.O., Lee J., Shin J.Y., et al. KIF5B-RET Fusion gene may coincide oncogenic mutations of EGFR or KRAS gene in lung adenocarcinomas. Diagn Pathol. 2015;10:143. doi: 10.1186/s13000-015-0368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song Z., Yu X., Zhang Y. Clinicopathological characteristics and survival of ALK, ROS1 and RET rearrangements in non-adenocarcinoma non-small cell lung cancer patients. Cancer Biol Ther. 2017;18:883–887. doi: 10.1080/15384047.2016.1235660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reguart N., Teixidó C., Giménez-Capitán A., et al. Identification of ALK, ROS1, and RET fusions by a multiplexed mRNA-based assay in formalin-fixed, paraffin-embedded samples from advanced non–small-cell lung cancer patients. Clin Chem. 2017;63:751–760. doi: 10.1373/clinchem.2016.265314. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka K., Hida T., Oya Y., et al. Unique prevalence of oncogenic genetic alterations in young patients with lung adenocarcinoma. Cancer. 2017;123:1731–1740. doi: 10.1002/cncr.30539. [DOI] [PubMed] [Google Scholar]
- 44.Suehara Y., Arcila M., Wang L., et al. Identification of KIF5B-RET and GOPC-ROS1 fusions in lung adenocarcinomas through a comprehensive mRNA-based screen for tyrosine kinase fusions. Clin Cancer Res. 2012;18:6599–6608. doi: 10.1158/1078-0432.CCR-12-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lira M.E., Choi Y.L., Lim S.M., et al. A single-tube multiplexed assay for detecting ALK, ROS1, and RET fusions in lung cancer. J Mol Diagn. 2014;16:229–243. doi: 10.1016/j.jmoldx.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Velizheva N.P., Rechsteiner M.P., Valtcheva N., et al. Targeted next-generation-sequencing for reliable detection of targetable rearrangements in lung adenocarcinoma-a single center retrospective study. Pathol Res Pract. 2018;214:572–578. doi: 10.1016/j.prp.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shang G., Jin Y., Zheng Q., et al. Histology and oncogenic driver alterations of lung adenocarcinoma in Chinese. Am J Cancer Res. 2019;9:1212–1223. [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L., Chen M., Lin J., et al. Identifying a wide range of actionable variants using capture-based ultra-deep targeted sequencing in treatment-naive patients with primary lung adenocarcinoma. Int J Clin Exp Pathol. 2020;13:525–535. [PMC free article] [PubMed] [Google Scholar]
- 49.Feng J., Li Y., Wei B., et al. Clinicopathologic characteristics and diagnostic methods of RET rearrangement in Chinese non-small cell lung cancer patients. Transl Lung Cancer Res. 2022;11:617–631. doi: 10.21037/tlcr-22-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ambrosini-Spaltro A., Farnedi A., Calistri D., et al. The role of next-generation sequencing in detecting gene fusions with known and unknown partners: a single-center experience with methodologies’ integration. Hum Pathol. 2022;123:20–30. doi: 10.1016/j.humpath.2022.02.005. [DOI] [PubMed] [Google Scholar]
- 51.Yoh K., Seto T., Satouchi M., et al. Vandetanib in patients with previously treated RET-rearranged advanced non-small-cell lung cancer (LURET): an open-label, multicentre phase 2 trial. Lancet Respir Med. 2017;5:42–50. doi: 10.1016/S2213-2600(16)30322-8. [DOI] [PubMed] [Google Scholar]
- 52.Sokolova I.A., Bedroske P., Grushko T.A., et al. Multiplex fast FISH assay for detecting ROS1, RET and MET aberrations in FFPE specimens using BioView image analysis. Cancer Res. 2020;80(suppl 16):4256. [Google Scholar]
- 53.Tan A.C., Lai G.G.Y., Tan G.S., et al. Utility of incorporating next-generation sequencing (NGS) in an Asian non-small cell lung cancer (NSCLC) population: incremental yield of actionable alterations and cost-effectiveness analysis. Lung Cancer. 2020;139:207–215. doi: 10.1016/j.lungcan.2019.11.022. [DOI] [PubMed] [Google Scholar]
- 54.Novaes L.A.C., Sussuchi da Silva L., De Marchi P., et al. Simultaneous analysis of ALK, RET, and ROS1 gene fusions by NanoString in Brazilian lung adenocarcinoma patients. Transl Lung Cancer Res. 2021;10:292–303. doi: 10.21037/tlcr-20-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y., Wu S., Zhou L., Guo Y., Zeng X. Pitfalls in RET fusion detection using break-apart FISH probes in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2021;106:1129–1138. doi: 10.1210/clinem/dgaa913. [DOI] [PubMed] [Google Scholar]
- 56.Skalova A., Vanecek T., Martinek P., et al. Molecular profiling of mammary analog secretory carcinoma revealed a subset of tumors harboring a novel ETV6-RET translocation: report of 10 cases. Am J Surg Pathol. 2018;42:234–246. doi: 10.1097/PAS.0000000000000972. [DOI] [PubMed] [Google Scholar]
- 57.Colato C., Vicentini C., Cantara S., et al. Break–apart interphase fluorescence in situ hybridization assay in papillary thyroid carcinoma: on the road to optimizing the cut-off level for RET/PTC rearrangements. Eur J Endocrinol. 2015;172:571–582. doi: 10.1530/EJE-14-0930. [DOI] [PubMed] [Google Scholar]
- 58.Belli C., Penault-Llorca F., Ladanyi M., et al. ESMO recommendations on the standard methods to detect RET fusions and mutations in daily practice and clinical research. Ann Oncol. 2021;32:337–350. doi: 10.1016/j.annonc.2020.11.021. [DOI] [PubMed] [Google Scholar]
- 59.Ou S.I., Zhu V.W. Catalog of 5’ fusion partners in RET+ NSCLC Circa 2020. JTO Clin Res Rep. 2020;1 doi: 10.1016/j.jtocrr.2020.100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McLeer-Florin A., Moro-Sibilot D., Melis A., et al. Dual IHC and FISH testing for ALK gene rearrangement in lung adenocarcinomas in a routine practice: a French study. J Thorac Oncol. 2012;7:348–354. doi: 10.1097/JTO.0b013e3182381535. [DOI] [PubMed] [Google Scholar]
- 61.Lantuejoul S., Rouquette I., Blons H., et al. French multicentric validation of ALK rearrangement diagnostic in 547 lung adenocarcinomas. Eur Respir J. 2015;46:207–218. doi: 10.1183/09031936.00119914. [DOI] [PubMed] [Google Scholar]
- 62.Michels S., Scheel A.H., Scheffler M., et al. Clinicopathological characteristics of RET rearranged lung cancer in European patients. J Thorac Oncol. 2016;11:122–127. doi: 10.1016/j.jtho.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 63.von Laffert M., Stenzinger A., Hummel M., et al. ALK-FISH borderline cases in non-small cell lung cancer: implications for diagnostics and clinical decision making. Lung Cancer. 2015;90:465–471. doi: 10.1016/j.lungcan.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 64.van Gulik A.L., Sluydts E., Vervoort L., et al. False positivity in break apart fluorescence in-situ hybridization due to polyploidy. Transl Lung Cancer Res. 2023;12:676–688. doi: 10.21037/tlcr-22-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drilon A., Wang L., Arcila M.E., et al. Broad, hybrid capture-based next-generation sequencing identifies actionable genomic alterations in lung adenocarcinomas otherwise negative for such alterations by other genomic testing approaches. Clin Cancer Res. 2015;21:3631–3639. doi: 10.1158/1078-0432.CCR-14-2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.