Abstract
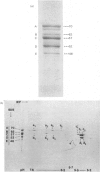
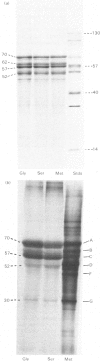
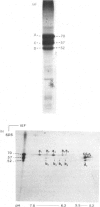
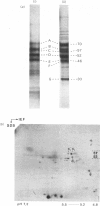
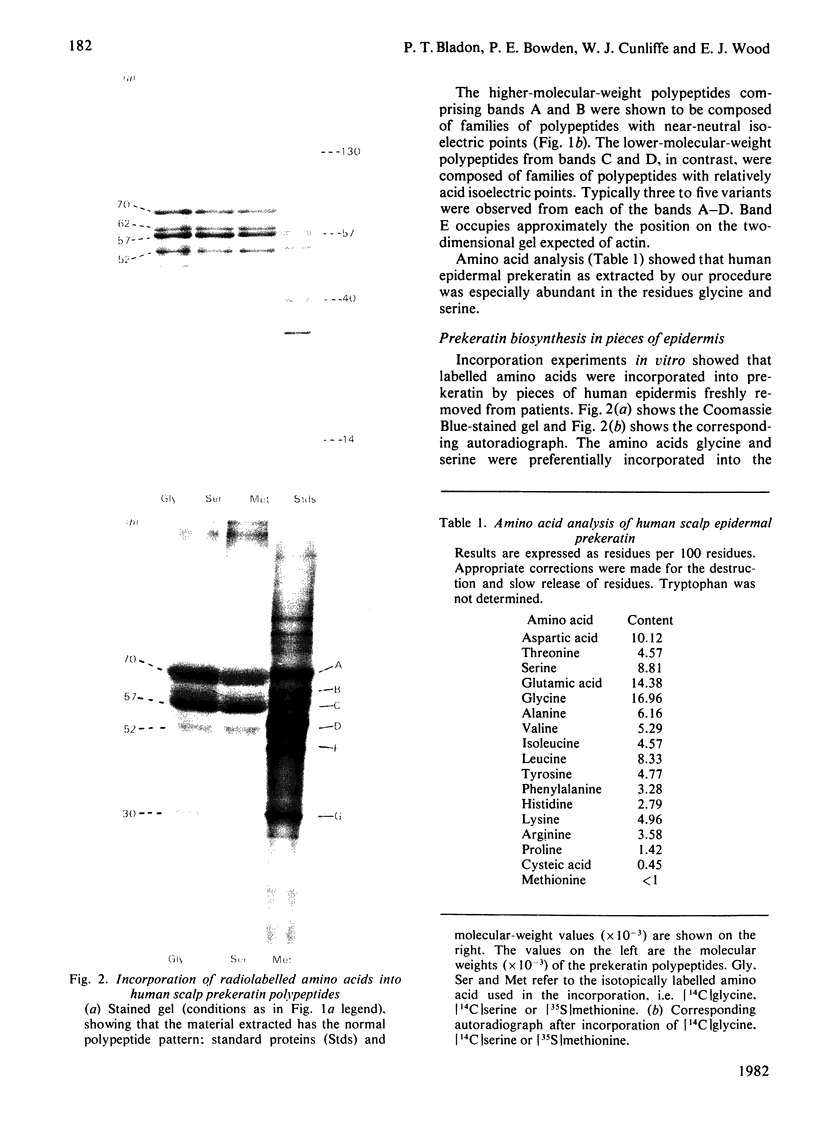
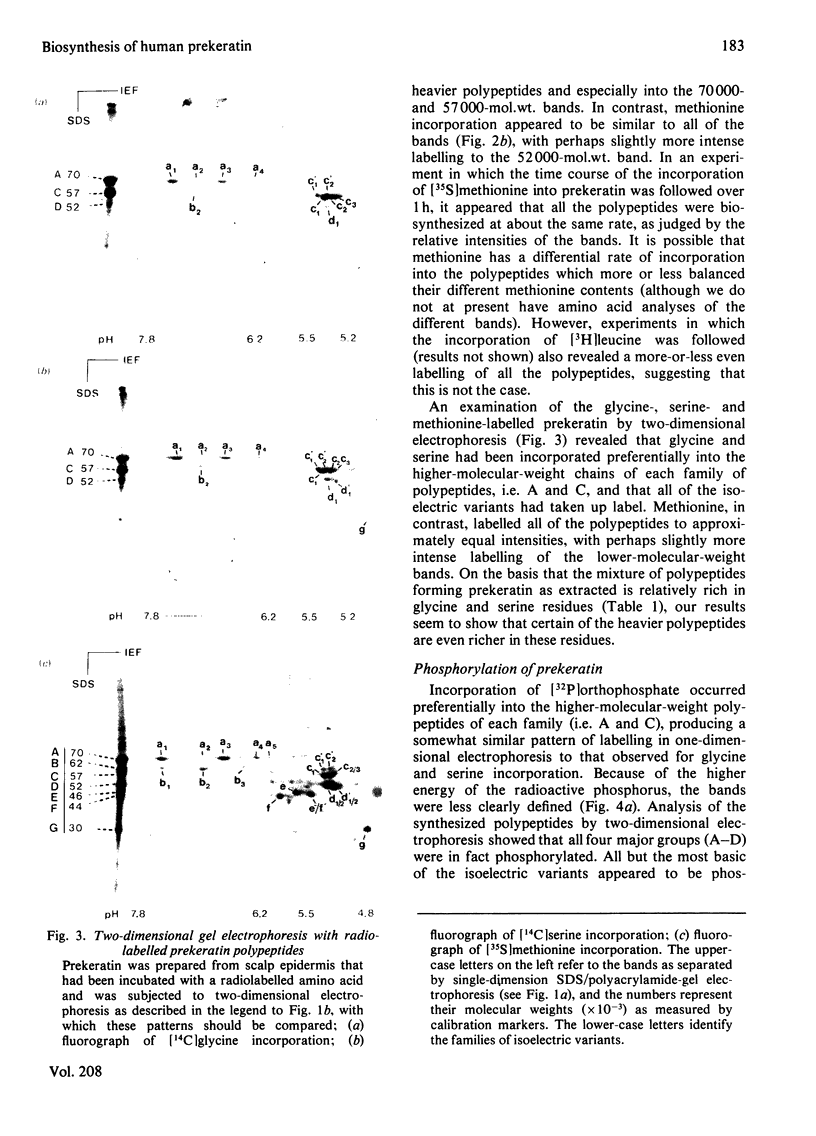
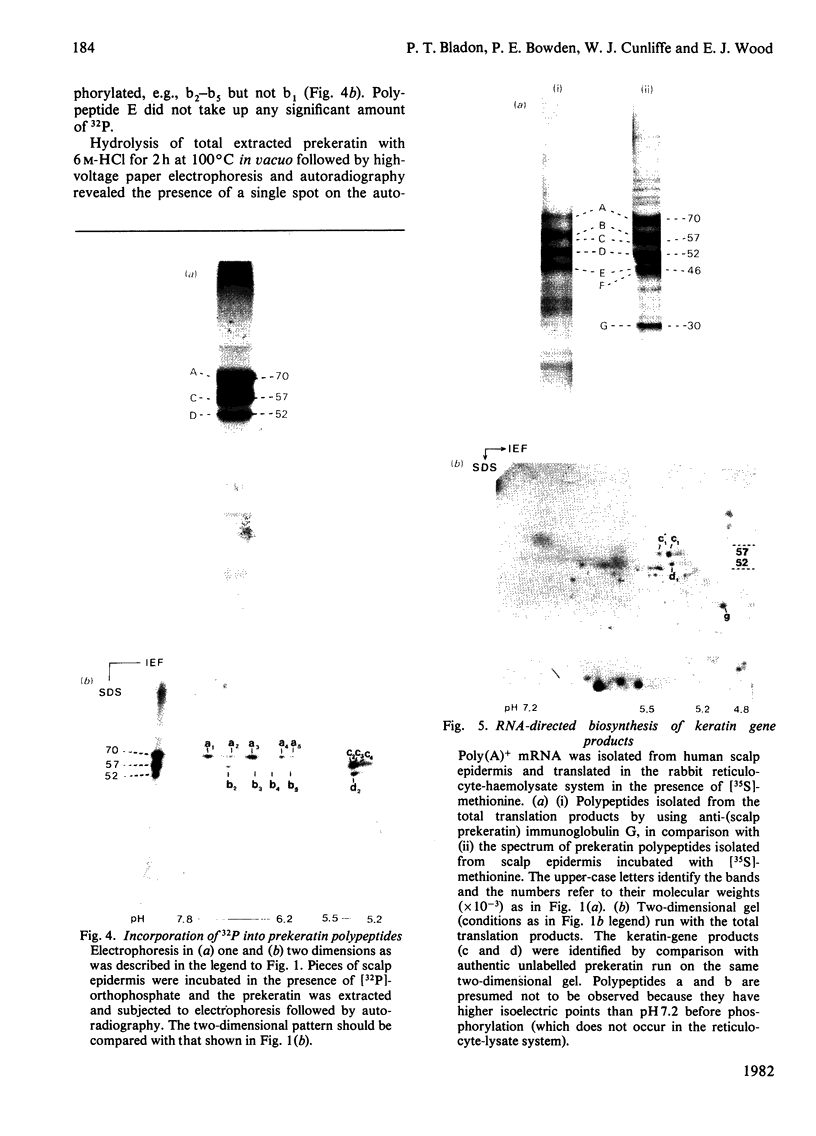
Analysis of human scalp epidermal prekeratin polypeptides by two-dimensional gel electrophoresis revealed that each of the bands observed in one-dimensional electrophoresis consisted of three to five polypeptides of the same molecular weight but differing in isoelectric points. It was possible to divide the polypeptides into two families, with isoelectric points in the ranges pH 6.0-8.0 and pH 5.0-5.5 respectively. Incorporation of radiolabelled amino acids into freshly excised pieces of scalp epidermis showed that some of the polypeptides had relatively greater contents of glycine and serine than others. Radiolabelled methionine and leucine were, in contrast, incorporated more or less uniformly into all the polypeptides. After incubation with 32P-labelled orthophosphate, relatively more intense labelling by 32P was observed in the higher molecular weight bands of each family. The most basic of the isoelectric variants in each case did not take up phosphate, implying that at least some of the variation in charge was due to different degrees of phosphorylation. Polyadenylated RNA isolated from scalp epidermis was translated in an RNA-dependent reticulocyte haemolysate system followed by immunoprecipitation and electrophoresis. The polypeptides isolated by using anti-(human scalp prekeratin) immunoglobulin G had similar electrophoretic mobilities in sodium dodecyl sulphate/polyacrylamide gels to authentic prekeratin polypeptides, but had different isoelectric properties. This suggested that the products of keratin gene expression undergo post-translational modification.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Nikaido K. Two-dimensional gel electrophoresis of membrane proteins. Biochemistry. 1976 Feb 10;15(3):616–623. doi: 10.1021/bi00648a026. [DOI] [PubMed] [Google Scholar]
- Applebaum S. W., James T. C., Wreschner D. H., Tata J. R. The preparation and characterization of locust vitellogenin messenger RNA and the synthesis of its complementary DNA. Biochem J. 1981 Jan 1;193(1):209–216. doi: 10.1042/bj1930209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRENTANI M., BRENTANI R., RAW I. ROLE OF NUCLEOLAR RIBONUCLEIC ACID IN INCORPORATION OF RIBOSOMAL AMINO ACID. Nature. 1964 Mar 14;201:1130–1130. doi: 10.1038/2011130a0. [DOI] [PubMed] [Google Scholar]
- Baynes J., Levine M., McLeod A., Wilkinson A. Precursor keratin protein from human epidermis. Br J Dermatol. 1978 Feb;98(2):165–173. doi: 10.1111/j.1365-2133.1978.tb01618.x. [DOI] [PubMed] [Google Scholar]
- Bernstein I. A., Chakrabarti S. G., Kumaroo K. K., Sibrack L. A. Synthesis of protein in the mammalian epidermis. J Invest Dermatol. 1970 Nov;55(5):291–302. doi: 10.1111/1523-1747.ep12260109. [DOI] [PubMed] [Google Scholar]
- Bowden P. E., Cunliffe W. J. Modification of human prekeratin during epidermal differentiation. Biochem J. 1981 Oct 1;199(1):145–154. doi: 10.1042/bj1990145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. V., Coppock S. M., Green H., Cleveland D. W. Two distinct classes of keratin genes and their evolutionary significance. Cell. 1981 Nov;27(1 Pt 2):75–84. doi: 10.1016/0092-8674(81)90362-7. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980 Apr;19(4):1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Multiple keratins of cultured human epidermal cells are translated from different mRNA molecules. Cell. 1979 Jul;17(3):573–582. doi: 10.1016/0092-8674(79)90265-4. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. The expression of keratin genes in epidermis and cultured epidermal cells. Cell. 1978 Nov;15(3):887–897. doi: 10.1016/0092-8674(78)90273-8. [DOI] [PubMed] [Google Scholar]
- Gibbs P. E., Freedberg I. M. Epidermal keratin messenger RNAs: a heterogeneous family. Biochim Biophys Acta. 1982 Feb 26;696(2):124–133. doi: 10.1016/0167-4781(82)90019-7. [DOI] [PubMed] [Google Scholar]
- Gibbs P. E., Freedberg I. M. Mammalian epidermal messenger RNA: identification and characterization of the keratin messengers. J Invest Dermatol. 1980 Jun;74(6):382–388. doi: 10.1111/1523-1747.ep12544461. [DOI] [PubMed] [Google Scholar]
- Gilmartin M. E., Culbertson V. B., Freedberg I. M. Phosphorylation of epidermal keratins. J Invest Dermatol. 1980 Sep;75(3):211–216. doi: 10.1111/1523-1747.ep12522887. [DOI] [PubMed] [Google Scholar]
- Hunter L., Skerrow D. The proteins of living psoriatic epidermis. Biochim Biophys Acta. 1982 Jan 12;714(1):164–169. doi: 10.1016/0304-4165(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Schweizer J., Goerttler K. Synthesis in vitro of keratin polypeptides directed by mRNA isolated from newborn and adult mouse epidermis. Eur J Biochem. 1980 Nov;112(2):243–249. doi: 10.1111/j.1432-1033.1980.tb07200.x. [DOI] [PubMed] [Google Scholar]
- Scott I. R., Harding C. R. Studies on the synthesis and degradation of a high molecular weight, histidine-rich phosphoprotein from mammalian epidermis. Biochim Biophys Acta. 1981 Jun 29;669(1):65–78. doi: 10.1016/0005-2795(81)90224-5. [DOI] [PubMed] [Google Scholar]
- Skerrow D., Hunter I. Protein modifications during the keratinization of normal and psoriatic human epidermis. Biochim Biophys Acta. 1978 Dec 20;537(2):474–484. doi: 10.1016/0005-2795(78)90532-9. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W. Postsynthetic modifications of mammalian epidermal alpha-keratin. Biochemistry. 1979 Dec 11;18(25):5664–5669. doi: 10.1021/bi00592a022. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W. The polypeptide composition of bovine epidermal alpha-keratin. Biochem J. 1975 Dec;151(3):603–614. doi: 10.1042/bj1510603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Wantz M. L. Characterization of the keratin filament subunits unique to bovine snout epidermis. Biochem J. 1980 Jun 1;187(3):913–916. doi: 10.1042/bj1870913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Wantz M. L., Idler W. W. O-phosphoserine content of intermediate filament subunits. Biochemistry. 1982 Jan 5;21(1):177–183. doi: 10.1021/bi00530a030. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Keratin filaments of cultured human epidermal cells. Formation of intermolecular disulfide bonds during terminal differentiation. J Biol Chem. 1978 Mar 25;253(6):2053–2060. [PubMed] [Google Scholar]
- Wood E. J., Bonaventura J. Identification of Limulus polyphemus haemocyanin messenger RNA. Biochem J. 1981 May 15;196(2):653–656. doi: 10.1042/bj1960653. [DOI] [PMC free article] [PubMed] [Google Scholar]