Abstract
Polycystic ovary syndrome (PCOS) is defined as a chronic low-grade inflammatory reproductive endocrine disorder. PCOS can induce various metabolic disorders, which are associated with a state of mild and slow-acting inflammation. Nevertheless, the causal relationship between polycystic ovary syndrome and inflammatory factors is uncertain. The causality between inflammatory cytokines and PCOS was analyzed by bidirectional Mendelian randomization (MR) in this current probe. We performed an interactive MR study to assess the causal relationships between 91 inflammatory cytokines and PCOS using Genome Wide Association Study (GWAS) data. We underwent dual-sample MR analysis with inverse variance weights (IVW) as the predominant MR methodology with multiple validity and heterogeneity analyses. MR-Egger, weighted median, simple mode, weighted mode and MR-PRESSO were analyzed as multiple likelihood sensitivity analyses to enhance the final results.The results came out interleukin-1-alpha (IL-1 A) levels (odds ratio [OR] = 1.051, 95% fiducial interval [95% CI] = 1.009–1.095, P = 0.02) and oncostatin-M (OSM) levels ( [OR] = 1.041, [95% CI] = 1.001–1.082, P = 0.04) were positively associated with the development of PCOS. Moreover, interleukin-7 (IL-7) levels ([OR] = 0.935, [95% CI] = 0.884–0.989, P = 0.02); interleukin-15 receptor subunit alpha (IL15RA) levels ([OR] = 0.959, [95% CI] = 0.929–0.99, P = 0.01); and C-X-C motif chemokine 11 (CXCL11) levels ([OR] = 0.959, [95% CI] = 0.922–0.996. P = 0.03) were strongly negatively associated with PCOS. However, we did not find any strong positive results in the reverse analysis, suggesting that although inflammatory factors contribute to the pathogenesis of PCOS, PCOS itself does not trigger inflammatory factor production.Our study provides genetic evidence for the connection between systemic inflammatory regulators and PCOS. Treatments targeting specific inflammatory factors may help to mitigate the risk of PCOS. The levels of five of the 91 inflammatory factors included in this study, namely, IL1A and OSM, were associated with PCOS. IL1A and OSM contribute to the progression of PCOS while IL-7, IL15RA, and CXCL11 levels are negatively correlated with the development of PCOS.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13048-024-01525-x.
Keywords: Inflammatory cytokines, Mendelian randomization, Polycystic ovary syndrome, Relationship, Reverse
Introduction
Polycystic ovary syndrome (PCOS) become a usual suspect, whose morbidity fall in between 5 and 15% among women of childbearing age [1]. Its clinical manifestations include menstrual irregularities, hyperandrogenemia, and polycystic ovarian morphology. The etiology of PCOS is complex and its risk factors include genetic factors, excess androgens and insulin resistance (IR), obesity and low-grade inflammation [2, 3]. Previous studies found that mild inflammation takes a significant effect on nosogenesis of polycystic ovary syndrome [4]. One study demonstrated that the dysregulation of glucose metabolism and lipid metabolism caused by PCOS triggers low-grade inflammation in the endothelium, which also affects the ovarian tissue. Moreover, the presence of chronic inflammation may contribute to the development of insulin resistance (IR), which in turn is intensified by the release of androgens and proinflammatory cytokines from adipose tissue [5]. Furthermore, hirsutism, androgenetic alopecia, and acne are including [6].
In recent years, more and more studies have gradually focused on the role of cytokines in the pathogenesis of PCOS. A large body of evidence suggests that inflammation-related genes encoding tumor necrosis factor-α (TNF-α), TNF receptor 2 (TNFR2), interleukin 6 (IL-6), and interleukin-17 (IL-17) are associated with the development of PCOS [7, 8]. In addition, some studies have linked the development of PCOS to growth factors such as vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF) [9]. In addition, the symptoms of PCOS have been found to be relieved by applying anti-inflammatory therapies to patients [10]. However, the above findings are mainly derived from observational studies, which are susceptible to confounding factors, too small sample sizes, and so on, biasing the results. While the effects of several cytokines on PCOS have been discussed, in these findings the remaining cytokines have not been reported. Thus, the involvement for polycystic ovary syndrome with inflammatory factors still appears to be unknown. Further exploration of the underlying mechanism of PCOS is warranted for the development of novel and effective clinical interventions.
Mendelian randomization (MR) utilizes genetic variants that are closely associated with the underlying trait as instrumental variables (IVs) to identify causal associations between exposures and outcomes [11]. Compared to traditional epidemiological methods, MR analysis reduces the bias introduced by confounding factors to the results [12]. In addition, MR is an efficient and cost-effective method because published data are widely available to screen for suitable genetic variants [13].
In this study, we investigated the causal associations between inflammatory factors and PCOS by two-sample MR, assessed whether certain inflammatory factors may serve as potential risk factors for the pathogenesis of PCOS, and provided clues for the prevention of PCOS as well as the search for therapeutic targets.
Materials and methods
Study design and data sources
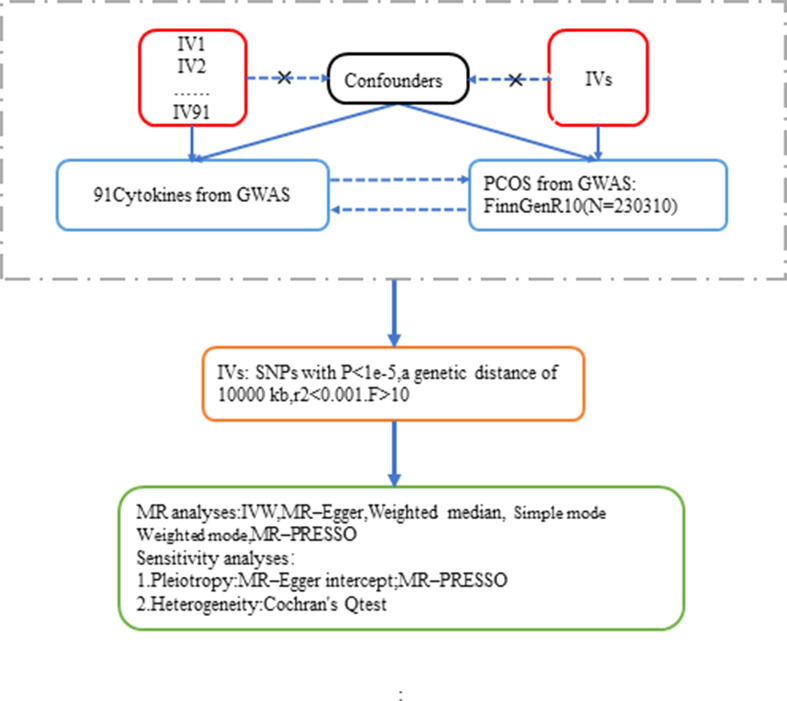
In order to obtain reliable results, MR analyses must fulfill the following three assumptions: (i) screened IVs must be strongly associated with exposure; (ii) IVs are not subject to other confounding factors; and (iii) IVs affect outcomes only through exposure and not through other pathways [14]. The genetic data on the 91 inflammatory factors used in this study were derived from the most recent Genome-Wide Association Study (GWAS) pooled study, as determined by genome-wide pQTL analysis [15]. The GWAS data for PCOS were obtained from the FinnGen database, which includes 34,388 patients with polycystic ovary syndrome and 195,922 controls of European descent. The data are publicly available. The screening process is shown in Fig. 1.
Fig. 1.
This flowchart examines the bidirectional causality of 91 selected IVs for inflammatory cytokines and PCOS. Three basic assumptions of MR analysis, namely, correlation, independence and exclusion restrictions, are shown
The selection of IVs
We selected SNPs that were strongly associated with PCOS and inflammatory cytokines using a threshold of P < 1e-5. To avoid potential pleiotropy, the screening process was based on the coefficient of linkage disequilibrium (kb = 10,000, r2 = 0.001) to remove high linkage SNPs. Meanwhile, we calculated the F-statistic of individual SNPs, and selected those with F > 10 as instrumental variables to avoid weak instrumental bias. In addition, we excluded palindromic SNPs to avoid the ambiguity of the presence of the same allele in the underlying trait and outcome. Detailed information of the SNPs involved in this study is shown in Supplementary1 Table 1.
Statistical analysis
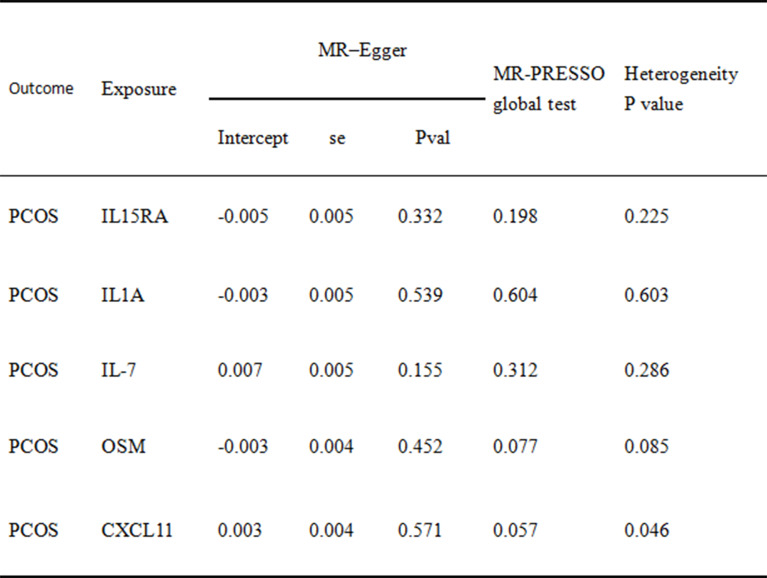
In this study, the inverse variance weighting (IVW) method was used as the primary analytical method to detect the causal association between inflammatory factors and PCOS. Various sensitivity analyses were also used to ensure the robustness of the results. The Cochran Q-test was applied to the IVW estimates to test for heterogeneity. We also used the MR-PRESSO method to further assess and correct for the presence of horizontal pleiotropy [16, 17]. In addition, MR-Egger regression was used to assess potential multiple-effects bias [18, 19]. The results of the sensitivity analysis are presented in Supplementary 1 Table 9.
All of the statistical analyses were performed using the “TwoSampleMR version 0.5.6” package and a two-tailed p-value of less than 0.05 was recognized statistically significant.
Results
Relationship between inflammatory regulatory factors and PCOS
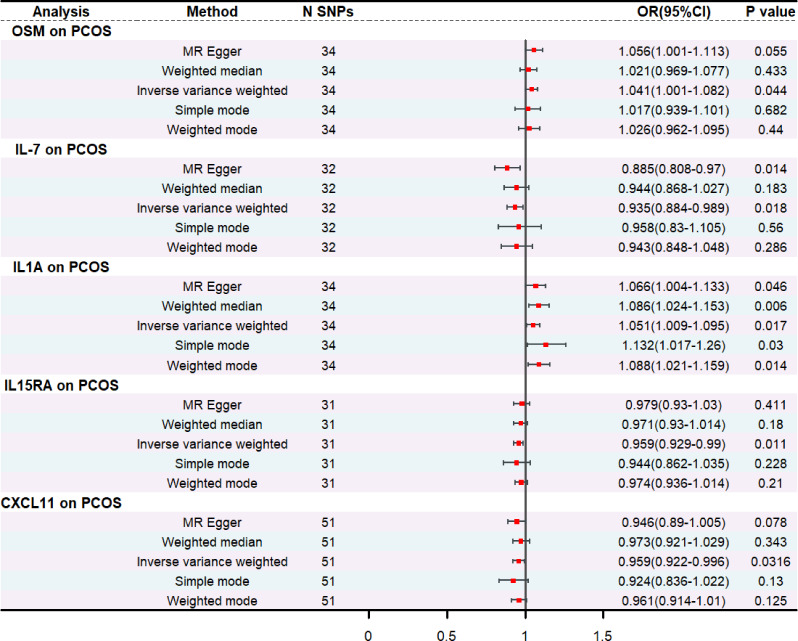
The F-statistics of the SNPs of the 91 inflammatory factors screened were all greater than 10, indicating the absence of weak IV. Two-sample MR analyses were performed using the IVW method as the primary analytical method. We found IL-1 A to be a risk factor for PCOS (OR = 1.051, 95% CI = 1.009 ~ 1.095, P = 0.02), and this association was also demonstrated in the other four methods. In addition, oncostatin- m (OSM) levels were also positively associated with the development of PCOS (OR = 1.041, 95% CI = 1.001 ~ 1.082, P = 0.04). While the expression levels of IL-7 (OR = 0.935 95% CI = 0.884–0.989, P = 0.02), IL15RA (OR = 0.959, 95% CI = 0.929–0.99, P = 0.01) and CXCL11 (OR = 0.959, 95% CI = 0.922–0.996, P = 0.03) were all positively associated with the the occurrence of PCOS showed negative correlation (Fig. 2). No heterogeneity among genetic variants was found by Cochrane’s Q test. Also by MR-Egger and MR-PRESSO, it was found that there was no horizontal pleiotropy among the SNPs used, detailed information is shown in Table 1.
Fig. 2.
Forest plot of Mendelian randomization study on inflammatory biomarkers and PCOS
Table 1.
Sensitivity analysis of 5 inflammatory factors to mendelian randomization analysis of PCOS
Relationship between PCOS and inflammatory regulatory factors
Reverse MR was performed with PCOS as the exposure and the 91 inflammatory factors identified in the present study as the outcome. The procedure included a total of 16 IVs under the screening conditions of P < 1 × 10− 5 and r2 = 0.001, kb = 10,000, but none of the subsequent MR analyses revealed significant reverse causality. The statistical consequences can be summarized in the additional documentation. (Supplementary2 Table 2).
Discussion
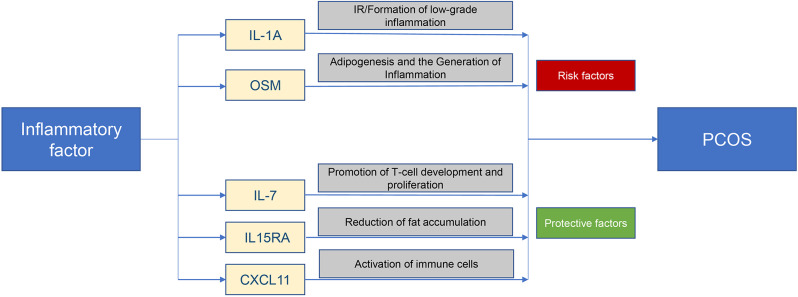
Using the GWAS database, we performed a two-sample bidirectional MR analysis to investigate the causal relationship between inflammatory cytokines and PCOS. The results showed that IL1A and OSM levels are positively associated with the development of PCOS while IL-7, IL15RA, and CXCL11 levels are negatively associated with the development of PCOS. The pathways through which the above inflammatory factors act with PCOS are shown in Fig. 3.
Fig. 3.
Summary plot of inflammatory factors and pathways of PCOS development
Interleukin-1α(IL1A) gene encodes a protein that is a pleiotropic cytokine participating in diverse immortal reactions and inflamatory procedures. Studies have shown that the presence of polymorphisms in the IL-1 gene is associated with the development of polycystic ovary syndrome and can affect serum follicle-stimulating hormone (FSH) and luteinizing hormone (LH)-FSH ratios [20]. Other studies have shown that IL-1 A and IL-6 levels are elevated in patients with polycystic ovary syndrome [21]. A meta-analysis showed that IL-1B and IL-1 A polymorphisms may influence PCOS susceptibility in Asians and Caucasians, respectively [22]. However, no association between IL-1 and PCOS susceptibility was found in the Chinese population [23]. All of the above suggests that IL1A may be an important factor in the pathogenesis of polycystic ovary syndrome, which is of great significance in elucidating the pathophysiologic mechanism, clinical diagnosis and treatment of polycystic ovary syndrome. Our study identified IL-1 A as a risk factor for PCOS, so blocking IL-1 signaling may lead to improvement of hormonal abnormalities and symptoms associated with PCOS pathogenesis, making it a potential therapeutic target.
Oncostatin M is a member of the IL-6 cytokine family of novel adipokines that stimulate JAK/STAT signaling and activate transcriptional pathways by binding to transmembrane receptors. Oncostatin M plays a role in a various biological processes, including adipogenesis/lipogenesis, hematopoiesis, osteogenesis, and inflammation. Moreover, Oncostatin M and its receptor are expressed in human oocytes and granulosa cells. In addition, oncostatin M has been reported to have a promotive influence on the number and growth of primordial gametocytes in the ovary [24]. Oncostatin M supports and promotes the development of primordial follicles by stimulating the production of more growth factors. The increase in Oncostatin M signaling after injection of human chorionic gonadotropin and subsequent ovulation suggests an important role in ovulation. OSM levels were significantly lower in PCOS patients than in controls and were positively correlated with oocyte maturation and fertilization rates [25]. The relationship between oncostatin M and polycystic ovary syndrome has been investigated in many studies. Bailey et al. [26] reported that operating system of the OSM induces adipocyte lipolysis through the p66Shc-ERK pathway and inhibits the inhibitory effect of insulin on lipolysis, and also induces phosphorylation of inhibitory IRS1 residues. Moreover, OSM promotes lipolysis in white adipocytes in vitro. However, our findings are not consistent with those of a previous case‒control study [27].
IL-7 is a cytokine that promotes the development and proliferation of T cells, which play an important role in the immune system [28]. It has been demonstrated that IL-1β levels are elevated in PCOS patients; while IL-7 levels are reduced [29]. Additionally, CXCL11, a novel chemokine associated with sex hormones in women with PCOS, recruits cells of the immune system to sites of infection or tissue damage and regulates the activation state of immune cells at various stages of the immune response. Systemic levels of chemokines may be a marker of immune activation in women with PCOS [30]. Women with PCOS have increased levels of G-CSF and IL15 in serum and follicular fluid. Moreover, BMI was negatively correlated with serum and follicular fluids levels of G-CSF and IL15 in women with PCOS [31]. Studies have shown that IL-15 is negatively correlated with obesity index, which is a risk factor for PCOS [32]. Meanwhile, the accumulation of adipocytes and macrophages may cause a chronic low-grade inflammatory state throughout the body, which affects ovarian function and promotes the development of PCOS [33]. In addition, a large body of literature suggests that the IL-15 endocrine axis reduces adiposity in mice [34]. Meanwhile, sIL- 15Rα is an important factor affecting body composition and glucagon sensitivity, and insulin resistance promotes PCOS [35]. A large body of evidence suggests that lifestyle improvement should be the mainstay of treatment for women with polycystic ovary syndrome. Our study identified IL-15 as a protective factor in PCOS, and IL-15 contributes to adipose tissue storage and skeletal muscle mitochondrial biogenesis, thus IL-15 has the potential to be a new therapeutic target for PCOS.
In this study, we comprehensively and systematically explored the causal association between inflammatory factors and PCOS by MR analysis. This method can minimize the influence of confounding factors, while the GWAS data of this study are derived from a large population, and the results are credible. However, the study still has some limitations. First, we used P < 1 × 10− 5 as the threshold to screen the required instrumental variables, which is because fewer significant SNPs were obtained when P < 5 × 10− 8 was chosen as the condition, but a lower threshold may make the data less precise. Secondly the populations used in the analyses were all European populations and the symptoms of PCOS vary between races and individuals, it is not clear how inflammatory factors are associated with PCOS in other ancestries. Finally, reverse MR analysis did not demonstrate an association between PCOS and inflammatory factors, possibly due to insufficient sample size, and in summary, the association between inflammatory factors and PCOS requires further analysis in multicenter, larger populations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the studies or consortiums referenced andincluded in the present analysis for providing communal datasets.
Author contributions
Danling Tian, The first author, Writing–original draft and editing. Jinfeng Chen, Liang Liu: Supervision, review and editing.
Funding
No funding.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Azziz R. PCOS in 2015: new insights into the genetics of polycystic ovary syndrome [J]. Nat Reviews Endocrinol. 2016;12(2):74–5. [DOI] [PubMed] [Google Scholar]
- 2.Qin L, Chen J, Tang L, Zuo T, Chen H, Gao R, Xu W. Significant Role of Dicer and miR-223 in Adipose Tissue of Polycystic Ovary Syndrome Patients [J]. BioMed Research International, 2019, 2019: 1–9. [DOI] [PMC free article] [PubMed]
- 3.Escobar-Morreale HFJNRE. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. J. 2018;14(5):270–84. [DOI] [PubMed] [Google Scholar]
- 4.González F. Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction [J]. Steroids. 2012;77(4):300–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kicińska AM, Maksym RB, Zabielska-Kaczorowska MA, Stachowska A, Babińska A. Immunological and metabolic causes of infertility in polycystic ovary syndrome [J]. Biomedicines. 2023;11(6):1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shorakae S, Teede H, De Courten B, Lambert G, Boyle J, Moran LJ. The emerging role of chronic low-grade inflammation in the pathophysiology of polycystic ovary syndrome [J]. Semin Reprod Med. 2015;33(4):257–69. [DOI] [PubMed] [Google Scholar]
- 7.Zhang M, Kuang H, Duan Y, Li D, Xu Y, Ai W, Li W, Wang Y, Liu S, Li M, Liu X, Shao M. The role of serum inflammatory cytokines and berberine in the insulin signaling pathway among women with polycystic ovary syndrome [J]. PLoS ONE, 2020, 15(8). [DOI] [PMC free article] [PubMed]
- 8.Rostamtabar M, Esmaeilzadeh S, Tourani M, Rahmani A, Baee M, Shirafkan F, Saleki K, Mirzababayi SS, Ebrahimpour S, Nouri HR. Pathophysiological roles of chronic low-grade inflammation mediators in polycystic ovary syndrome [J]. J Cell Physiol. 2020;236(2):824–38. [DOI] [PubMed] [Google Scholar]
- 9.Chen H, Zhang Y, Li S, Tao Y, Gao R, Xu W, Yang Y, Cheng K, Wang Y, Qin L. The association between genetically predicted systemic inflammatory regulators and polycystic ovary syndrome: a mendelian randomization study [J]. Front Endocrinol. 2021;12:731569. [DOI] [PMC free article] [PubMed]
- 10.González F, Mather KJ, Considine RV, Abdelhadi OA, Acton AJ. Salicylate administration suppresses the inflammatory response to nutrients and improves ovarian function in polycystic ovary syndrome [J]. Am J Physiol Endocrinol Metab. 2020;319(4):E744–52. [DOI] [PMC free article] [PubMed]
- 11.Wang Q, Shi Q, Lu J, Wang Z, Hou J. Causal relationships between inflammatory factors and multiple myeloma: a bidirectional mendelian randomization study [J]. Int J Cancer. 2022;151(10):1750–9. [DOI] [PubMed] [Google Scholar]
- 12.Richmond RC, Smith GD. Mendelian randomization: concepts and scope [J]. Cold Spring Harbor Perspect Med. 2022;12(1):a040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sekula P, Del Greco MF, Pattaro C, Köttgen A. Mendelian randomization as an approach to assess causality using observational data [J]. J Am Soc Nephrology: JASN. 2016;27(11):3253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, Vanderweele TJ, Higgins JPT, Timpson NJ, Dimou N, Langenberg C, Golub RM, Loder EW, Gallo V, Tybjaerg-Hansen A, Davey Smith G, Egger M, Richards JB. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the STROBE-MR statement [J]. JAMA. 2021;326(16):1614–21. [DOI] [PubMed] [Google Scholar]
- 15.Zhao JH, Stacey D, Eriksson N, Macdonald-Dunlop E, Hedman ÅK, Kalnapenkis A, Enroth S, Cozzetto D, Digby-Bell J, Marten J, Folkersen L, Herder C, Jonsson L, Bergen SE, Gieger C, Needham EJ, Surendran P, Metspalu A, Milani L, Mägi R, Nelis M, Hudjašov G, Paul DS, Polasek O, Thorand B, Grallert H, Roden M, Võsa U, Esko T, Hayward C, Johansson Å, Gyllensten U, Powell N, Hansson O, Mattsson-Carlgren N, Joshi PK, Danesh J, Padyukov L, Klareskog L, Landén M, Wilson JF, Siegbahn A, Wallentin L, Mälarstig A, Butterworth AS, Peters JE. Estonian Biobank Research T. Genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets [J]. Nat Immunol. 2023;24(9):1540–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greco MF, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in mendelian randomisation studies with summary data and a continuous outcome [J]. Stat Med. 2015;34(21):2926–40. [DOI] [PubMed] [Google Scholar]
- 17.Verbanck M, Chen C-Y, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases [J]. Nat Genet. 2018;50(5):693–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tin A, Köttgen A. Mendelian randomization analysis as a tool to gain insights into causes of diseases: a primer [J]. J Am Soc Nephrology: JASN. 2021;32(10):2400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression [J]. Int J Epidemiol. 2015;44(2):512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolbus A, Walch K, Nagele F, Wenzl R, Unfried G, Huber JC. Interleukin-1 alpha but not interleukin-1 beta gene polymorphism is associated with polycystic ovary syndrome [J]. J Reprod Immunol. 2007;73(2):188–93. [DOI] [PubMed] [Google Scholar]
- 21.Eser B, Taskin MI, Hismiogullari AA, Aksit H, Bodur AS. The effects of IL-1A and IL-6 genes polymorphisms on gene expressions, hormonal and biochemical parameters in polycystic ovary syndrome [J]. J Obstet Gynaecology: J Inst Obstet Gynecol. 2017;37(3):358–62. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Che L, Zhang M, He J. Common cytokine polymorphisms and predisposition to polycystic ovary syndrome: a meta-analysis [J]. Endocr J. 2020;67(5):561–7. [DOI] [PubMed] [Google Scholar]
- 23.Wang B, Zhou S, Wang J, Liu J, Ni F, Liu C, Yan J, Mu Y, Cao Y, Ma X. Lack of association between interleukin-1a gene (IL-1a) C (-889) T variant and polycystic ovary syndrome in Chinese women [J]. Endocrine. 2009;35(2):198–203. [DOI] [PubMed] [Google Scholar]
- 24.Eddie SL, Childs AJ, Jabbour HN, Anderson RA. Developmentally regulated IL6-type cytokines signal to germ cells in the human fetal ovary [J]. Mol Hum Reprod. 2012;18(2):88–95. [DOI] [PubMed] [Google Scholar]
- 25.Nikanfar S, Hamdi K, Haiaty S, Samadi N, Shahnazi V, Fattahi A, Nouri M. Oncostatin M and its receptor in women with polycystic ovary syndrome and association with assisted reproductive technology outcomes [J]. Reprod Biol. 2022;22(2):100633. [DOI] [PubMed] [Google Scholar]
- 26.Bailey JL, Hang H, Boudreau A, Elks CM. Oncostatin M induces lipolysis and suppresses insulin response in 3T3-L1 adipocytes [J]. Int J Mol Sci. 2022;23(9):4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camili FE, Akis M, Adali E, Hismiogullari AA, Taskin MI, Guney G, Afsar S. Oncostatin M is related to polycystic ovary syndrome-case control study [J]. Biomedicines. 2024;12(2):355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawa Y, Arima Y, Ogura H, Kitabayashi C, Jiang JJ, Fukushima T, Kamimura D, Hirano T, Murakami M. Hepatic interleukin-7 expression regulates T cell responses [J]. Immunity. 2009;30(3):447–57. [DOI] [PubMed] [Google Scholar]
- 29.Knebel B, Janssen OE, Hahn S, Jacob S, Gleich J, Kotzka J, Muller-Wieland D. Increased low grade inflammatory serum markers in patients with polycystic ovary syndrome (PCOS) and their relationship to PPARgamma gene variants [J]. Experimental and clinical endocrinology & diabetes: official journal. German Soc Endocrinol [and] German Diabetes Association. 2008;116(8):481–6. [DOI] [PubMed] [Google Scholar]
- 30.Hatziagelaki E, Pergialiotis V, Kannenberg JM, Trakakis E, Tsiavou A, Markgraf DF, Carstensen-Kirberg M, Pacini G, Roden M, Dimitriadis G, Herder C. Association between biomarkers of low-grade inflammation and sex hormones in women with polycystic ovary syndrome [J]. Experimental and clinical endocrinology & diabetes: official journal. Volume 128. German Society of Endocrinology [and] German Diabetes Association; 2020. pp. 723–30. 11. [DOI] [PubMed]
- 31.Reina-Campos M, Scharping NE, Goldrath AW. CD8(+) T cell metabolism in infection and cancer [J]. Nat Rev Immunol. 2021;21(11):718–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hingorjo MR, Zehra S, Saleem S, Qureshi MA. Serum interleukin-15 and its relationship with adiposity indices before and after short-term endurance exercise [J]. Pakistan J Med Sci. 2018;34(5):1125–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weyer C, Yudkin JS, Stehouwer CD, Schalkwijk CG, Pratley RE, Tataranni PA. Humoral markers of inflammation and endothelial dysfunction in relation to adiposity and in vivo insulin action in Pima indians [J]. Atherosclerosis. 2002;161(1):233–42. [DOI] [PubMed] [Google Scholar]
- 34.Pierce JR, Maples JM, Hickner RC. IL-15 concentrations in skeletal muscle and subcutaneous adipose tissue in lean and obese humans: local effects of IL-15 on adipose tissue lipolysis [J]. Am J Physiol Endocrinol Metabolism. 2015;308(12):E1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park K, Gross M, Lee D-H, Holvoet P, Himes JH, Shikany JM, Jacobs DR. Oxidative stress and insulin resistance [J]. Diabetes Care. 2009;32(7):1302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.