Abstract
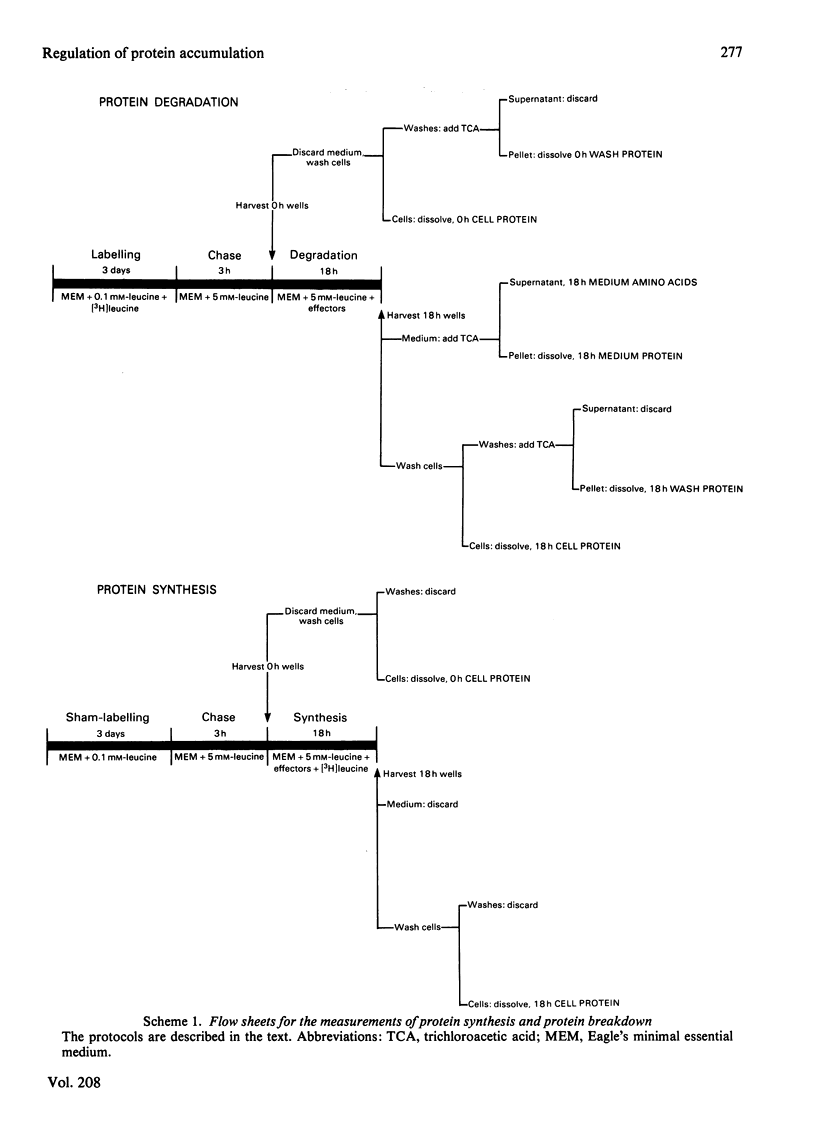
1. A technique is described whereby protein synthesis, protein breakdown and net protein accumulation are measured separately in monolayer cultures of mammalian cells. All rates are expressed as microgram of protein per 18 h incubation. 2. Under most incubation conditions with either L6 rat myoblasts or T47D human breast carcinoma cells the rates of protein accumulation, determined directly, agreed with the rates obtained by subtracting protein breakdown from protein synthesis. 3. Foetal calf serum, human and bovine colostrum, human milk and insulin increased protein accumulation in both cell lines, mainly as a consequence of effects on protein synthesis. 4. NH4Cl, in addition to inhibiting protein breakdown in both cell lines in the presence and in the absence of serum, stimulated protein synthesis in L6 myoblasts. 5. Leupeptin slightly inhibited protein breakdown without affecting protein-synthesis rates. 6. Cycloheximide almost completely inhibited protein synthesis, but restricted the net loss of cell proteins under most conditions because protein-breakdown rates were also decreased. 7. The assumptions, limitations and potential application of this technique for evaluating changes in protein turnover are described.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amenta J. S., Brocher S. C. Mechanisms of protein turnover in cultured cells. Life Sci. 1981 Mar 16;28(11):1195–1208. doi: 10.1016/0024-3205(81)90444-6. [DOI] [PubMed] [Google Scholar]
- Amenta J. S., Brocher S. C. Mechanisms of protein turnover in cultured fibroblasts. Differential inhibition of two lysosomal mechanisms with insulin and NH4Cl. Exp Cell Res. 1980 Mar;126(1):167–174. doi: 10.1016/0014-4827(80)90482-6. [DOI] [PubMed] [Google Scholar]
- Ballard F. J. Intracellular protein degradation. Essays Biochem. 1977;13:1–37. [PubMed] [Google Scholar]
- Ballard F. J., Nield M. K., Francis G. L., Dahlenburg G. W., Wallace J. C. The relationship between the insulin content and inhibitory effects of bovine colostrum on protein breakdown in cultured cells. J Cell Physiol. 1982 Mar;110(3):249–254. doi: 10.1002/jcp.1041100305. [DOI] [PubMed] [Google Scholar]
- Ballard F. J., Wong S. S., Knowles S. E., Partridge N. C., Martin T. J., Wood C. M., Gunn J. M. Insulin inhibition of protein degradation in cell monolayers. J Cell Physiol. 1980 Nov;105(2):335–346. doi: 10.1002/jcp.1041050216. [DOI] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Fry D. M., Morales M. F. A reexamination of the effects of creatine on muscle protein synthesis in tissue culture. J Cell Biol. 1980 Feb;84(2):294–297. doi: 10.1083/jcb.84.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Grinde B., Seglen P. O. Differential effects of proteinase inhibitors and amines on the lysosomal and non-lysosomal pathways of protein degradation in isolated rat hepatocytes. Biochim Biophys Acta. 1980 Sep 17;632(1):73–86. doi: 10.1016/0304-4165(80)90250-0. [DOI] [PubMed] [Google Scholar]
- Gunn J. M., Clark M. G., Knowles S. E., Hopgood M. F., Ballard F. J. Reduced rates of proteolysis in transformed cells. Nature. 1977 Mar 3;266(5597):58–60. doi: 10.1038/266058a0. [DOI] [PubMed] [Google Scholar]
- Hildebran J. N., Airhart J., Stirewalt W. S., Low R. B. Prolyl-tRNA-based rates of protein and collagen synthesis in human lung fibroblasts. Biochem J. 1981 Aug 15;198(2):249–258. doi: 10.1042/bj1980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopgood M. F., Clark M. G., Ballard F. J. Stimulation by glucocorticoids of protein degradation in hepatocyte monolayers. Biochem J. 1981 Apr 15;196(1):33–40. doi: 10.1042/bj1960033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingwall J. S., Morales M. F., Stockdale F. E. Creatine and the control of myosin synthesis in differentiating skeletal muscle. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2250–2253. doi: 10.1073/pnas.69.8.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley D. S., Becker J. E., Potter V. R. Effect of insulin, dexamethasone, and glucagon on the amino acid transport ability of four rat hepatoma cell lines and rat hepatocytes in culture. Cancer Res. 1978 Dec;38(12):4591–4600. [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J., Livesey G., Williams K. E. Effects of microbial proteinase inhibitors on the degradation of endogenous and internalized proteins by rat yolk sacs. Biochem J. 1981 Apr 15;196(1):41–48. doi: 10.1042/bj1960041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Goldberg A. L. Leupeptin, a protease inhibitor, decreases protein degradation in normal and diseased muscles. Science. 1978 Feb 3;199(4328):534–536. doi: 10.1126/science.622552. [DOI] [PubMed] [Google Scholar]
- Rønning O. W., Lindmo T., Pettersen E. O., Seglen P. O. Effect of serum step-down on protein metabolism and proliferation kinetics of NHIK 3025 cells. J Cell Physiol. 1981 Apr;107(1):47–57. doi: 10.1002/jcp.1041070107. [DOI] [PubMed] [Google Scholar]
- Schneible P. A., Airhart J., Low R. B. Differential compartmentation of leucine for oxidation and for protein synthesis in cultured skeletal muscle. J Biol Chem. 1981 May 25;256(10):4888–4894. [PubMed] [Google Scholar]
- Seglen P. O. Effects of amino acids, ammonia and leupeptin on protein synthesis and degradation in isolated rat hepatocytes. Biochem J. 1978 Aug 15;174(2):469–474. doi: 10.1042/bj1740469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Protein degradation in isolated rat hepatocytes is inhibited by ammonia. Biochem Biophys Res Commun. 1975 Sep 2;66(1):44–52. doi: 10.1016/s0006-291x(75)80292-0. [DOI] [PubMed] [Google Scholar]
- Tischler M. E., Desautels M., Goldberg A. L. Does leucine, leucyl-tRNA, or some metabolite of leucine regulate protein synthesis and degradation in skeletal and cardiac muscle? J Biol Chem. 1982 Feb 25;257(4):1613–1621. [PubMed] [Google Scholar]
- Wibo M., Poole B. Protein degradation in cultured cells. II. The uptake of chloroquine by rat fibroblasts and the inhibition of cellular protein degradation and cathepsin B1. J Cell Biol. 1974 Nov;63(2 Pt 1):430–440. doi: 10.1083/jcb.63.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]


