Abstract
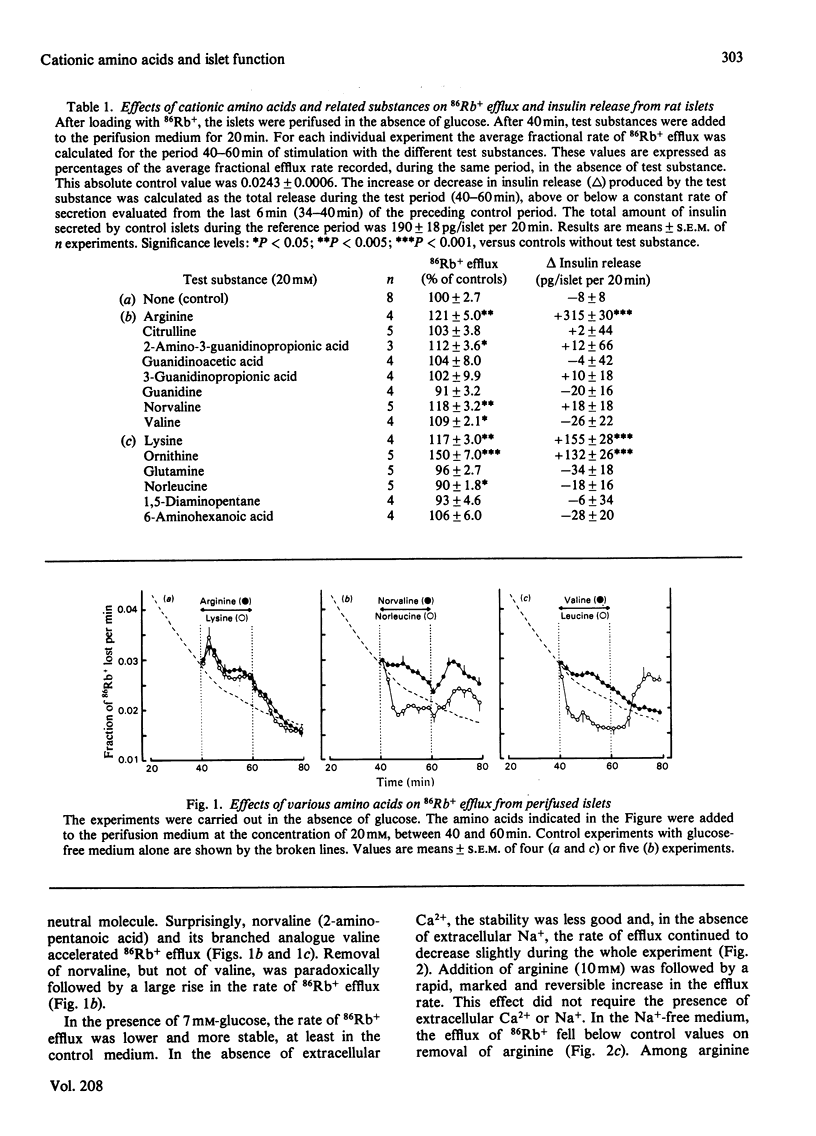
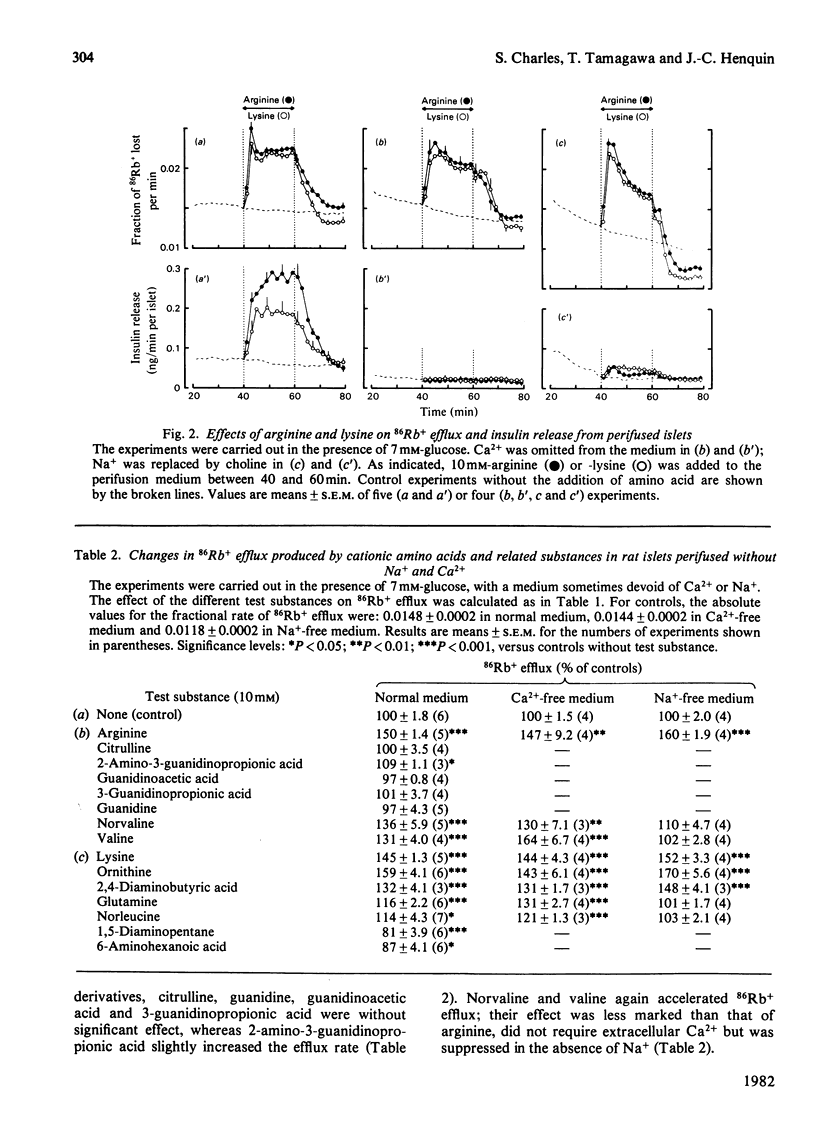
The mechanisms by which cationic amino acids influence pancreatic B-cell function have been studied by monitoring simultaneously 86Rb+ efflux and insulin release from perifused rat islets. The effects of two reference amino acids arginine and lysine were compared with those of closely related substances to define the structural requirements for recognition of these molecules as secretagogues. Arginine accelerated 86Rb+ efflux and increased insulin release in the absence or in the presence of 7mm-glucose. Its effects on efflux did not require the presence of extracellular Ca2+ or Na+, but its insulinotropic effects were suppressed in a Ca2+-free medium and inhibited in an Na+-free medium. Among arginine derivatives, only 2-amino-3-guanidinopropionic acid mimicked its effects on 86Rb+ efflux and insulin release; citrulline, guanidinoacetic acid, 3-guanidinopropionic acid and guanidine were inactive. Norvaline and valine also increased 86Rb+ efflux, but their effect required the presence of extracellular Na+; they did not stimulate insulin release. Lysine as well as the shorter-chain cationic amino acids ornithine and 2,4-diaminobutyric acid accelerated 86Rb+ efflux in a Ca2+- and Na+-independent manner. Their stimulation of insulin release was suppressed by Ca2+ omission, but only partially inhibited in an Na+-free medium. The uncharged glutamine and norleucine increased the rate of 86Rb+ efflux in the presence of glucose, only if extracellular Na+ was present. Norleucine slightly increased release in a Ca2+- and Na+-dependent manner. The effects of lysine on efflux and release were not mimicked by other related substances such as 1,5-diaminopentane and 6-aminohexanoic acid. The results suggest that the depolarizing effect of cationic amino acids is due to accumulation of these positively charged molecules in B-cells. This causes acceleration of the efflux of K+ (86Rb+) and activation of the influx of Ca2+ (which triggers insulin release). The prerequisite for the stimulation of B-cells by this mechanism appears to be the presence of a positive charge on the side chain of the amino acid, rather than a specific group.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alsever R. N., Georg R. H., Sussman K. E. Stimulation of insulin secretion by guanidinoacetic acid and other guanidine derivatives. Endocrinology. 1970 Feb;86(2):332–336. doi: 10.1210/endo-86-2-332. [DOI] [PubMed] [Google Scholar]
- Arnauld J., Lachance P. A. Basic amino acid accumulation in potassium-depleted rat muscle. J Nutr. 1980 Dec;110(12):2480–2489. doi: 10.1093/jn/110.12.2480. [DOI] [PubMed] [Google Scholar]
- Assan R., Attali J. R., Ballerio G., Boillot J., Girard J. R. Glucagon secretion induced by natural and artificial amino acids in the perfused rat pancreas. Diabetes. 1977 Apr;26(4):300–307. doi: 10.2337/diab.26.4.300. [DOI] [PubMed] [Google Scholar]
- Aynsley-Green A., Alberti K. G. In vivo stimulation of insulin secretion by guanidine derivatives in the rat. Horm Metab Res. 1974 Mar;6(2):115–120. doi: 10.1055/s-0028-1093873. [DOI] [PubMed] [Google Scholar]
- Christensen H. N., Hellman B., Lernmark A., Sehlin J., Tager H. S., Täljedal I. B. In vitro stimulation of insulin release by non-metabolizable, transport-specific amino acids. Biochim Biophys Acta. 1971 Aug 13;241(2):341–348. doi: 10.1016/0005-2736(71)90034-4. [DOI] [PubMed] [Google Scholar]
- Christensen H. N. On the development of amino acid transport systems. Fed Proc. 1973 Jan;32(1):19–28. [PubMed] [Google Scholar]
- Cooperstein S. J., Lazarow A. Uptake of amino acids by islet of Langerhans and other tissues of the toadfish. Am J Physiol. 1977 Jul;233(1):E19–E27. doi: 10.1152/ajpendo.1977.233.1.E19. [DOI] [PubMed] [Google Scholar]
- DICKERMAN H. W., WALKER W. G. EFFECT OF CATIONIC AMINO ACID INFUSION ON POTASSIUM METABOLISM IN VIVO. Am J Physiol. 1964 Feb;206:403–408. doi: 10.1152/ajplegacy.1964.206.2.403. [DOI] [PubMed] [Google Scholar]
- FLOYD J. C., Jr, FAJANS S. S., KNOPF R. F., CONN J. W. EVIDENCE THAT INSULIN RELEASE IS THE MECHANISM FOR EXPERIMENTALLY INDUCED LEUCINE HYPOGLYCEMIA IN MAN. J Clin Invest. 1963 Nov;42:1714–1719. doi: 10.1172/JCI104857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajans S. S., Floyd J. C., Jr, Knopf R. F., Guntsche E. M., Rull J. A., Thiffault C. A., Conn J. W. A difference in mechanism by which leucine and other amino acids induce insulin release. J Clin Endocrinol Metab. 1967 Nov;27(11):1600–1606. doi: 10.1210/jcem-27-11-1600. [DOI] [PubMed] [Google Scholar]
- Fajans S. S., Floyd J. C., Jr, Knopf R. F., Pek S., Weissman P., Conn J. W. Amino acids and insulin release in vivo. Isr J Med Sci. 1972 Mar;8(3):233–243. [PubMed] [Google Scholar]
- Gerich J. E., Charles M. A., Grodsky G. M. Characterization of the effects of arginine and glucose on glucagon and insulin release from the perfused rat pancreas. J Clin Invest. 1974 Oct;54(4):833–841. doi: 10.1172/JCI107823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Idahl L. A., Lernmark A., Sehlin J., Täljedal I. B. The pancreatic beta-cell recognition of insulin secretagogues. Effects of calcium and sodium on glucose metabolism and insulin release. Biochem J. 1974 Jan;138(1):33–45. doi: 10.1042/bj1380033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Effects of glucose and other modifiers of insulin release on the oxidative metabolism of amino acids in micro-dissected pancreatic islets. Biochem J. 1971 Jul;123(4):513–521. doi: 10.1042/bj1230513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C. D-glucose inhibits potassium efflux from pancreatic islet cells. Nature. 1978 Jan 19;271(5642):271–273. doi: 10.1038/271271a0. [DOI] [PubMed] [Google Scholar]
- Henquin J. C., Meissner H. P. Effects of amino acids on membrane potential and 86Rb+ fluxes in pancreatic beta-cells. Am J Physiol. 1981 Mar;240(3):E245–E252. doi: 10.1152/ajpendo.1981.240.3.E245. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. Metabolic control of potassium permeability in pancreatic islet cells. Biochem J. 1980 Feb 15;186(2):541–550. doi: 10.1042/bj1860541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henquin J. C. The effect of pH on 86Rubidium efflux from pancreatic islet cells. Mol Cell Endocrinol. 1981 Feb;21(2):119–128. doi: 10.1016/0303-7207(81)90049-6. [DOI] [PubMed] [Google Scholar]
- Henquin J. C. The potassium permeability of pancreatic islet cells: mechanisms of control and influence on insulin release. Horm Metab Res Suppl. 1980;Suppl 10:66–73. [PubMed] [Google Scholar]
- Iwatsuki N., Petersen O. H. Amino acids evoke short-latency membrane conductance increase in pancreatic acinar cells. Nature. 1980 Jan 31;283(5746):492–494. doi: 10.1038/283492a0. [DOI] [PubMed] [Google Scholar]
- Komor E., Thom M., Maretzki A. Mechanism of uptake of L-arginine by sugar-cane cells. Eur J Biochem. 1981 Jun 1;116(3):527–533. doi: 10.1111/j.1432-1033.1981.tb05368.x. [DOI] [PubMed] [Google Scholar]
- LEVINSKY N. G., TYSON I., MILLER R. B., RELMAN A. S. The relation between amino acids and potassium in isolated rat muscle. J Clin Invest. 1962 Mar;41:480–487. doi: 10.1172/JCI104501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugier R., Petersen O. H. Two different types of electrogenic amino acid action on pancreatic acinar cells. Biochim Biophys Acta. 1981 Feb 20;641(1):216–221. doi: 10.1016/0005-2736(81)90585-x. [DOI] [PubMed] [Google Scholar]
- Levin S. R., Grodsky G. M., Hagura R., Smith D. F., Forsham P. H. Relationships between arginine and glucose in the induction of insulin secretion from the isolated, perfused rat pancreas. Endocrinology. 1972 Mar;90(3):624–631. doi: 10.1210/endo-90-3-624. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Hutton J. C., Carpinelli A. R., Herchuelz A., Sener A. The stimulus-secretion coupling of amino acid-induced insulin release: metabolism and cationic effects of leucine. Diabetes. 1980 Jun;29(6):431–437. doi: 10.2337/diab.29.6.431. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A., Malaisse-Lagae F. Insulin release: reconciliation of the receptor and metabolic hypotheses. Nutrient receptors in islet cells. Mol Cell Biochem. 1981 Jul;37(3):157–165. doi: 10.1007/BF02354884. [DOI] [PubMed] [Google Scholar]
- Marco J., Calle C., Hedo J. A., Villanueva M. L. Glucagon-releasing activity of guanidine compounds in mouse pancreatic islets. FEBS Lett. 1976 Apr 15;64(1):52–54. doi: 10.1016/0014-5793(76)80246-3. [DOI] [PubMed] [Google Scholar]
- Massara F., Martelli S., Ghigo E., Camanni F., Molinatti G. M. Arginine-induced hypophosphatemia and hyperkaliemia in man. Diabete Metab. 1979 Dec;5(4):297–300. [PubMed] [Google Scholar]
- Panten U., Christians J. Effect of 2-endo-aminonorbornane-2-carboxylic acid upon insulin secretion and fluorescence of reduced pyridine nucleotides of isolated perifused pancreatic islets. Naunyn Schmiedebergs Arch Pharmacol. 1973;276(1):55–62. doi: 10.1007/BF00500778. [DOI] [PubMed] [Google Scholar]
- Sener A., Somers G., Devis G., Malaisse W. J. The stimulus-secretion coupling of amino acid-induced insulin release. Biosynthetic and secretory responses of rat pancreatic islet to L-leucine and L-glutamine. Diabetologia. 1981 Aug;21(2):135–142. doi: 10.1007/BF00251281. [DOI] [PubMed] [Google Scholar]


