Abstract
Background
Weighted blanket is an emerging non-pharmacotherapy for sleep disorders, but its effect on sleep among relatively healthy adults with insomnia remains uncertain. This study aimed to evaluate whether weighted blankets could better improve sleep quality and sleep-related symptoms among adults with insomnia.
Methods
In a prospective, pilot randomized controlled trial conducted in three tertiary hospitals in China, participants with clinical insomnia were randomized (1:1) to receive weighted blanket intervention or normal blanket intervention for 1 month by random-number tables. The primary outcomes were sleep quality assessed with Pittsburgh Sleep Quality Index (PSQI) and insomnia severity assessed with Insomnia Severity Index. Subjective outcomes were measured at baseline, 1 week, and 1-month post-intervention. Sleep was also objectively monitored by actigraphy. We did analysis by intention to treat.
Results
A total of 102 participants were randomly assigned to receive weighted blanket intervention (n = 52) or normal blanket intervention (n = 50). 95 (93.1%) participants completed the follow-up, and 7 (6.9%) participants dropped out of the study. The weighted blanket group had significant improvements in sleep quality compared to the normal blanket group after 1 month of intervention (changes in the mean [SD] of PSQI score: -4.1 [4.1] vs. -2.0 [3.2], P = 0.006). Similar results were observed for daytime sleepiness, stress, anxiety, fatigue, and bodily pain (all P < 0.05). Recordings from actigraphy showed a decrease in the mean (SD) of the number of awakenings in weighted blanket group (-1.4 [9.5]) and an increase in normal blanket group (+ 1.0 [7.9]) (P = 0.280). No severe adverse events occurred.
Conclusions
Weighted blanket might be an effective, safe and promising non-pharmacotherapy tool for improving sleep-related symptoms among adults with insomnia, although validation with a larger sample size is needed.
Trial registration
Chinese Clinical Trial Registry: ChiCTR2300078011, date of registration: 11/27/2023, retrospectively registered.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-024-06218-9.
Keywords: Weighted blanket, Sleep, Insomnia, Physical therapy
Background
Sleep plays a pivotal role in sustaining everyday functioning, regulating brain activity and the stress system, with sleep quality being essential for mental health [1–3]. Indeed, sleep disorders are a global concern affecting over 40% of individuals during the COVID-19 pandemic [4]. In particular, among these disorders, insomnia stands out as a recognized risk factor and early sign for a wide range of mental disorders. It often contributes to the dysregulation of emotional, immune and neurobiological pathways [5, 6]. In this framework, effective treatments for insomnia may not only regulate the sleep process but also the accompanied symptoms including anxiety, depression, fatigue, and stress. Primary treatments for insomnia in clinical practice include cognitive behavioral therapy for insomnia (CBT-I) and pharmacotherapy, with CBT-I being the first-line treatment at present [7]. However, about 40% of patients with chronic insomnia do not sustainedly remit with CBT-I combined medication treatment [8]. In addition, pharmacotherapy for insomnia is associated with a high risk of adverse events and drug abuse/dependency. Also, the drop-out rates of CBT-I intervention ranged from 5% to 21% in recent randomized controlled trials [9–12]. Thus, there is a pressing need for safer non-pharmacotherapy alternatives.
Weighted blanket, a physical therapy of deep pressure stimulation, has been increasingly recommended by occupational therapists for individuals with sleep disorders [13]. Weighted blankets are designed with special weight-adding materials such as chains and beads, to evenly cover the body and provide widespread pressure. To date, as we recently reviewed [14], weighted blankets have been applied across various populations and mostly showed beneficial effects on improving sleep quality (e.g., shorter nighttime awakenings, shorter sleep latency, and extended sleep duration) [13, 15], neurodevelopmental disorders (e.g., attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) [16–18], negative emotions (e.g., anxiety, depression, and stress) [19, 20], and other related disorders. Meanwhile, a few studies have reported nonsignificant results [21]. More importantly, few clinical trials have investigated the efficacy of weighted blankets in improving sleep and other accompanied symptoms among relatively healthy adults with insomnia and without major psychiatric diseases using objective measurement of sleep.
Therefore, this pilot randomized clinical trial aimed to evaluate whether the weighted blankets, relative to the normal blankets, could better improve sleep quality and sleep-related symptoms (e.g., daytime sleepiness, fatigue, depression, anxiety, stress, and quality of life) in adults with insomnia using both self-reported and objective sleep measurements, providing preliminary evidence for a safer non-pharmacotherapy for insomnia.
Methods
Study design and participants
This study was reviewed and approved by the Zhejiang University School of Public Health Medical Ethics Committee (registration number ZGL202201-7) and complied with the World Medical Association Declaration of Helsinki. All participants provided written informed consent and had the option to withdraw from the study at any point without facing discrimination or unfair treatment. Participants were recruited from the outpatient sleep clinics of three hospitals in Zhejiang, China (Sir Run Run Shaw Hospital in Hangzhou, Affiliated Hospital of Shaoxing University in Shaoxing, and Shaoxing Hospital of Traditional Chinese Medicine). Clinical diagnoses of insomnia were made by physicians not affiliated with the research team, who referred eligible individuals to the study. The diagnoses referred to the Guideline for the Evaluation and Treatment of Insomnia in Chinese Adults [22], including presence of abnormal sleep symptoms and daytime symptoms related to insomnia, which cannot be solely explained by inadequate sleep duration or inappropriate sleep environment, nor can they be attributed to other types of sleep disorders. This guideline is basically equivalent to the currently recognized diagnostic criteria of ICSD-3. We followed the Consolidated Standards of Reporting Trials (CONSORT) guideline for the designing and reporting of this trial.
In this study, the inclusion criteria were people aged ≥ 18 years old, diagnosed with clinical insomnia by physicians, Pittsburgh Sleep Quality Index (PSQI) score ≥ 5 [23], living in Hangzhou and surrounding cities (such as Shaoxing city), having a stable occupation and lifestyle for the upcoming year, and having normal communication skills. A stable occupation and lifestyle indicated that the occupation, living environment, and lifestyle including smoking, drinking, exercise, and diet of participants would not change over the near future. Having normal communication skill was defined as participants could understand the purpose of the study, the guide and precautions for using actigraphy and blankets, and the content of the questionnaires after explained by physicians or investigators. Participants with active drug abuse, excessive use of sleeping pills, severe cognitive impairment, and a history of major diseases including coronary heart disease, cerebral infarction, and cancer were excluded. The definition of drug abuse and excessive use of sleeping pills were based on the substance use disorders criteria defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [24]. Cognitive impairment was screened using the Mini-Mental State Examination combined with assessment by the physician [25].
Procedures
From March 2022 to April 2023, individuals diagnosed with clinical insomnia in the outpatient sleep clinics of the three hospitals by physicians were included. The study team subsequently assessed whether these individuals met the inclusion and exclusion criteria. Those who qualified participated in a baseline survey, which covered demographics, disease history, sleep quality, daytime symptoms, negative emotions, and quality of life. After completing the baseline assessment, participants were randomly assigned to either the weighted blanket group or the normal blanket group in a 1:1 ratio by opening sealed randomization envelope. For selecting the style of the weighted blanket, first, previous studies generally suggested that the weight of a weighted blanket should be about 10% of the sleeper’s body weight, and the average weight of our participants was 56.3 kg [26]. Second, compared with blankets filled with metal chains, blankets filled with glass beads produce less noise when moving, and their denser composition making the blankets thinner and smoother. Thus, we finally selected a 5.5 kg blanket sewn with special weight-adding glass beads (silica) for participants assigned to the weighted blanket group, while those in the normal blanket group received a 3.5 kg blanket of identical shape but without glass beads. All participants were also provided with an actigraphy (a smart monitoring belt) and were instructed to place it under the bed sheet, corresponding to the chest position. Participants were asked to continuously use the blankets for 1 month and the actigraphy in the first week. Outcomes were assessed at baseline, 1 week, and 1-month post-intervention. Adverse events were actively monitored throughout the study. Participants were asked to report any discomfort to the researchers or doctors at any time during the trial. Meanwhile, the researchers kept close contact with the participants during the follow-up, and inquired about their feelings and physical conditions every day. Drop-out was considered only after completing the baseline survey. After the trial was completed, participants were allowed to keep the blankets.
Randomization and masking
We randomly assigned participants (1:1), via random-number tables, to receive either weighted blanket intervention or normal blanket intervention. The physicians not affiliated with the research team opened sealed opaque envelopes to maintain allocation concealment. We blinded the participants to the study design and randomization. Participants were only told that two blankets of different weights and identical shapes would be issued, but the specific style and weight were not informed. Therefore, most participants did not know exactly what blanket they were using, and only a few participants may confirm the blanket type by searching online or other ways. Although a small part of our outcome assessors were not masked to the group assignments, the assessment of outcomes was largely filled in by the participants themselves. Participants were instructed to fill out the questionnaire carefully according to their actual conditions, and during this process, the assessor did not interfere with the choice of participants.
Outcomes
Primary outcomes
The PSQI and Insomnia Severity Index (ISI) were used as the primary outcomes in this study. The PSQI is a self-reported scale assessing the sleep quality of patients in the last month, consisting of 7 dimensions including sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medications, and daytime dysfunction. The good internal reliability and validity of PSQI has been verified, with Cronbach’s α of 0.83 and test-retest reliability coefficient of 0.85 [27]. Based on a 4-point Likert scale, the total score ranges from 0 to 21, with a higher score indicating poorer sleep quality. According to previous studies [23], a score greater than 5 was interpreted as sleep disorder. PSQI scores were assessed at baseline and 1-month post-intervention.
ISI was used to assess the insomnia severity of participants, consisting of 7 items regarding difficulties falling or staying asleep, waking up too early, sleep satisfaction, daytime functioning, and distress about sleep difficulties. ISI has been demonstrated to have good reliability and validity, with Cronbach’s α of 0.83 and test-retest reliability coefficient of 0.79 [28]. Each item was scored from 0 to 4 points, yielding a summed score ranging from 0 to 28. ISI scores were categorized as follows: no clinically significant insomnia (0–7), sub-threshold insomnia (8–14), moderate insomnia (15–21), and severe insomnia (22–28) [29]. It was assessed at baseline, 1 week, and 1-month post-intervention.
Secondary outcomes
Secondary outcomes included daytime sleepiness assessed using the Epworth Sleepiness Scale (ESS) [30], perception of stress assessed using the Perceived Stress Scale (PSS) [31], depression assessed using the Center for Epidemiologic Studies Depression Scale-10 (CESD-10) [32], daytime fatigue assessed using the Fatigue Scale-14 (FS-14), anxiety assessed using the Self-Rating Anxiety Scale (SAS) [33], and daily quality of life assessed using the Quality of Life Scale 36-item Short-Form (SF-36) [34]. PSS and SF-36 were assessed at baseline and 1-month post-intervention. ESS, CESD-10, SAS, and FS-14 were assessed at baseline, 1 week and 1-month post-intervention.
Objective sleep evaluation
In this study, sleep quality was also monitored and recorded in real-time using actigraphy, which is a smart monitoring belt from Damian Health Tech (Hangzhou) CO., LTD. Participants were instructed to place the belt under the bed sheet, corresponding to the chest position, and needed to ensure that it was plugged in before sleep. The actigraphy was continuously used during the first week of the intervention. It monitored various nighttime sleep parameters, including sleep quality, sleep latency, sleep duration, sleep efficiency, number of awakenings, heart rate, and respiration rate. The recordings were automatically uploaded to a cloud server through WIFI/4G chip communication.
The actigraphy works based on piezoelectric PVDF film sensors; the weak body movements triggered by the human heartbeat can be captured by the highly sensitive piezoelectric PVDF film sensors placed inside the actigraphy, generating a ballistocardiogram (BCG) signal which can be transmitted to the cloud server via WIFI/4G chip communication. Based on the captured BCG signal, we constructed artificial intelligence deep learning algorithms to calculate the user’s physiological indicators including limb movement, heart rate, and respiration rate. This allowed for the deduction of the user’s nighttime sleep profile [35]. Compared with polysomnogram, this kind of actigraphy based on BCG technology demonstrates relatively satisfying performance for tracking sleep and wake, with accuracy comparable to polysomnogram in some metrics [36, 37].
Statistical analysis
The sample size was calculated based on the variation in the ISI scores in our early study in April, 2022, a preliminary analysis of 10 participants in the first stage of this study. The mean (SD) of ISI scores in the weighted blanket group was 8.7 (6.7) and in the normal blanket group was 13.3 (3.2) after a 1-month intervention. The power of statistical efficiency was set to 80%, with a two-sided significance level of 0.05. A sample size of 40 (20 per group) was needed. Considering a 10% dropout rate, the minimum sample size for this trial was 44.
Intention-to-treat method was used for data analysis. The baseline characteristics were analyzed by t-test for continuous variables and either Chi-square test or Fisher’s test for categorical variables. The normality of data was tested through graph and the Shapiro-Wilk test. For comparison between groups, generalized linear mixed models were applied for subjective variables (PSQI, ISI, ESS, CESD-10, SAS, PSS, FS-14, and SF-36). Group, time, and time by group interaction were included in the models as fixed effects, while participants were the random effects. Additionally, age and sex were included as fixed effects in all models. The conclusions about the effects of weighted blanket on sleep and other sleep-related outcomes were determined by the time by group interaction coefficients in the model. For objectively measured variables, changes between groups were compared using unpaired Student’s t-tests. The change in each variable was calculated as the difference between the first and last day of the sleep monitoring for each patient. All analyses were two-sided, and a P-value < 0.05 was considered statistically significant. All statistical analyses were conducted with SAS version 9.4.
Results
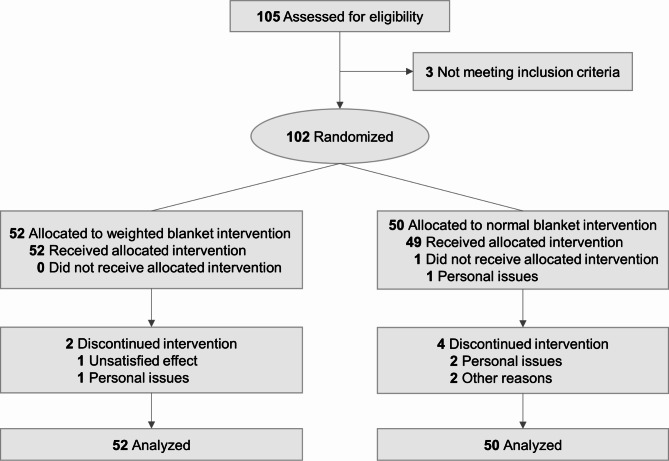
Participants were recruited between March 31, 2022, and April 16, 2023. Figure 1 shows the trial profile. A total of 105 participants were initially diagnosed with clinical insomnia by physicians in the outpatient sleep clinics. Following evaluation by the study team, 3 participants did not meet the inclusion criteria of the study. Consequently, 102 participants were enrolled in the study. 7 participants (2 in weighted blanket group, 5 in normal blanket group) discontinued the study, with one reporting feelings of cold while sleeping with the assigned blanket, two reporting feelings of uncomfortable while using the actigraphy, and four unable to use the blankets due to frequent changes in sleeping places. Thus, 95 participants finally completed the intervention (50 with weighted blankets and 45 with normal blankets). Of the 102 participants included in the baseline analyses (Table 1), the mean (SD) of age was 44.0 (13.9) years (range 19–78 years); 84 (82.4%) were women; 45 (44.1%) used sleep medicine. No significant differences were observed between the two groups in terms of age, sex, use of sleep medicine, sleep quality, or insomnia severity at baseline. There were no reports of severe adverse events associated with weighted blanket intervention. Only 3 participants (2 in weighted blanket group, 1 in normal blanket group) reported feelings of cold or uncomfortable when using the blankets or actigraphy.
Fig. 1.
Trial profile
Table 1.
Baseline characteristics of the study participants
| Variables | Total (N = 102) |
Weighted blanket (N = 52) |
Normal blanket (N = 50) |
|---|---|---|---|
| Age in years | 44.0 (13.9) | 44.3 (13.9) | 43.7 (14.1) |
| Sex, women, N (%) | 84 (82.4) | 41 (78.8) | 43 (86.0) |
| Currently married, N (%) | 68 (66.7) | 34 (65.4) | 34 (68.0) |
| Educational level, N (%) | |||
| Primary school | 12 (11.9) | 6 (11.8) | 6 (12.0) |
| Middle and High school | 29 (28.7) | 16 (31.4) | 13 (26.0) |
| College and Higher | 60 (59.4) | 29 (56.9) | 31 (62.0) |
| BMI, kg/m2 | 22.0 (4.0) | 22.0 (3.8) | 22.0 (4.3) |
| Current smoking, N (%) | 4 (3.9) | 0 (0.0) | 4 (7.7) |
| Current drinking, N (%) | 9 (8.9) | 3 (5.9) | 6 (12.0) |
| Physical activity, N (%) | |||
| Low activity | 69 (67.6) | 38 (73.1) | 31 (62.0) |
| Moderate and high activity | 33 (32.4) | 14 (26.9) | 19 (38.0) |
| History of hypertension, yes, N (%) | 8 (7.8) | 5 (9.6) | 3 (6.0) |
| History of cancer, yes, N (%) | 7 (6.9) | 4 (7.7) | 3 (6.0) |
| Use of sleep medicine, yes, N (%) | 45 (44.1) | 22 (42.3) | 23 (46.0) |
| Sleep quality (PSQI) | 12.9 (3.7) | 13.6 (3.8) | 12.3 (3.5) |
| Insomnia severity (ISI) | 15.1 (5.8) | 16.2 (6.2) | 14.0 (5.2) |
| Daytime sleepiness (Epworth) | 7.1 (5.7) | 8.2 (6.3) | 5.9 (4.9) |
| Depression (CESD-10) | 11.4 (5.9) | 11.5 (6.4) | 11.4 (5.4) |
| Stress (PSS) | 17.6 (7.0) | 17.7 (7.2) | 17.6 (6.8) |
| Anxiety (SAS) | 38.9 (8.2) | 38.8 (7.3) | 38.9 (9.2) |
| Fatigue (FS-14) | 8.1 (3.3) | 8.6 (3.1) | 7.6 (3.3) |
Data are expressed as mean (SD) unless otherwise stated
BMI, Body mass index; PSQI, Pittsburgh Sleep Quality Index; ISI, Insomnia Severity Index; CESD-10, Center for Epidemiologic Studies Depression Scale-10; PSS, Perceived Stress Scale; SAS, Self-Rating Anxiety Scale; FS-14, Fatigue Scale-14
Effects of weighted blankets on primary outcomes
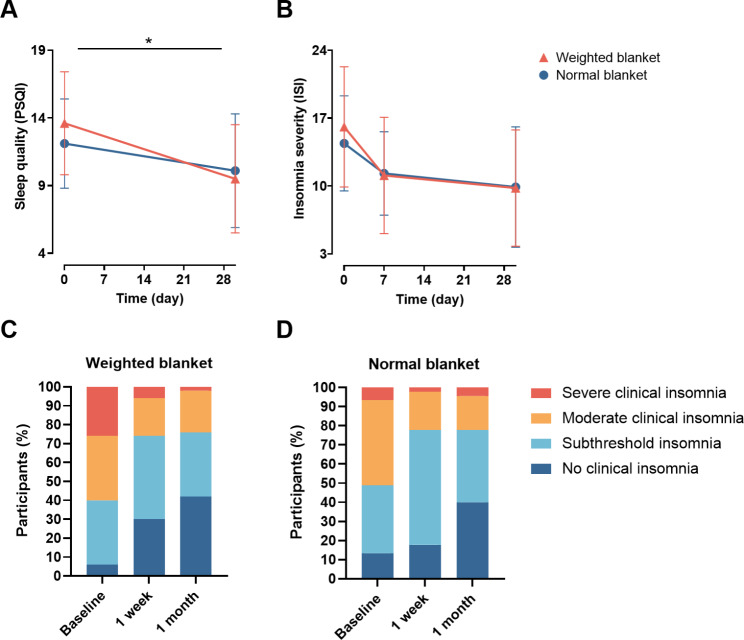
For primary outcomes, the weighted blanket intervention showed a significant beneficial effect on the PSQI. Specifically, participants using weighted blankets had a greater decrease in their PSQI score (mean [SD]: -4.1 [4.1]) compared to the normal blanket group (-2.0 [3.2]) (β: -0.072, 95% CI: -0.124, -0.021; P = 0.006) (Fig. 2A, Table S1), whereas no significant effect of weighted blankets on the ISI score was observed (β: -0.045, 95% CI: -0.120, 0.029; P = 0.233) (Fig. 2B, Table S1). The percentage of participants with different levels of insomnia (no clinical insomnia, subthreshold insomnia, moderate clinical insomnia, and severe clinical insomnia) differed between the two groups. For instance, there was a greater increase in the percentage of participants without clinical insomnia (6.0% at baseline, 30.0% at 1 week, and 42.0% at 1 month) and a greater decrease in the percentage of participants with severe clinical insomnia (26.0% at baseline, 6.0% at 1 week, and 2.0% at 1 month) in the weighted blanket group compared with normal blanket group, although the difference was not significant (β: 0.025, 95% CI: -0.011, 0.061; P = 0.181) (Fig. 2C and D).
Fig. 2.
Effects of weighted blankets on sleep quality and insomnia severity. (A) Pittsburgh sleep quality index decreased significantly in participants using weighted blankets after 1 month. (B) Using weighted blankets did not have a significant effect on ISI total score. (C) (D) Changes in the insomnia severity at baseline, 1 week, and 1 month after intervention in weighted and normal blanket group. No clinical insomnia (ISI score 0–7 points), Subthreshold insomnia (8–14 points), Moderate clinical insomnia (15–21 points), Severe clinical insomnia (22–28 points). *P < 0.05, generalized linear mixed models, adjusted for sex, age. PSQI, Pittsburgh Sleep Quality Index; ISI, Insomnia Severity Index
Effects of weighted blankets on other subjective sleep-related outcomes
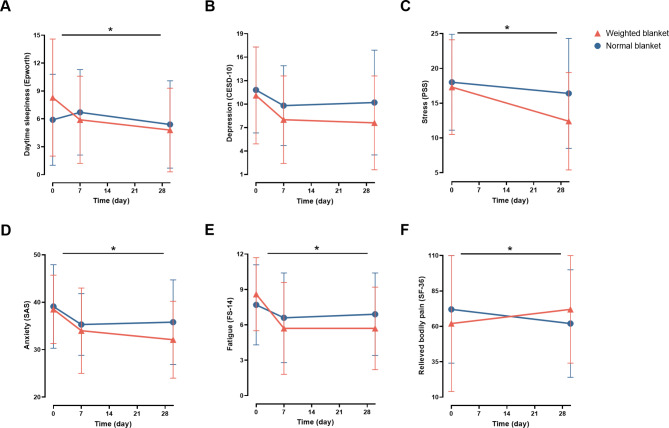
The use of weighted blankets was also beneficial for improving sleep-related negative emotions and daytime symptoms. Indeed, the weighted blanket group showed a greater improvement in daytime sleepiness after 1 month of intervention (P = 0.017) (Fig. 3A, Table S1). Significant between-group differences were also observed in stress (Fig. 3C, Table S1), anxiety (Fig. 3D, Table S1), and fatigue (Fig. 3E, Table S1) (All P < 0.05). In addition, the difference in the bodily pain dimension of SF-36 demonstrated potential benefits of weighted blankets on pain relief and improved quality of life (P = 0.010) (Fig. 3F, Table S1). However, no significant difference was observed in the CESD-10 score (P = 0.126) (Fig. 3B, Table S1).
Fig. 3.
Effects of weighted blankets on daytime sleepiness, depression, stress, anxiety, fatigue, and relieved bodily pain. (A) Daytime sleepiness score decreased significantly in participants using weighted blankets. (B) Using weighted blankets did not have a significant effect on depression. (C) Stress score decreased significantly in participants using weighted blankets. (D) Anxiety score decreased significantly in participants using weighted blankets. (E) Daytime fatigue score decreased significantly in participants using weighted blankets. (F) Bodily pain relieved significantly in participants using weighted blankets. *P < 0.05, generalized linear mixed models, adjusted for sex, age. CESD-10, Center for Epidemiologic Studies Depression Scale-10; PSS, Perceived Stress Scale; SAS, Self-Rating Anxiety Scale; FS-14, Fatigue Scale-14; SF-36, Quality of Life Scale 36-item Short-Form
Effects of weighted blankets on objectively measured number of awakenings
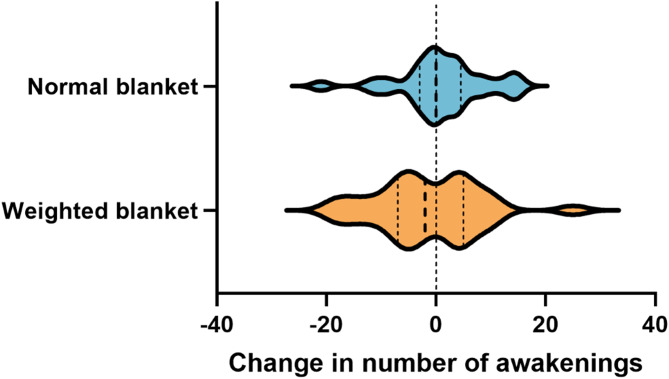
We also analyzed the objectively measured number of awakenings of the participants. Changes in number of awakenings were determined as the differences between the first day and the seventh day of actigraphy monitoring. Participants using weighted blankets experienced a decrease in the number of awakenings during the first week of intervention (mean [SD]: 16.8 [5.2] on 1st day, 15.3 [7.7] on 7th day), whereas participants in the normal blanket group experienced an increase (13.8 [5.9] on 1st day, 14.8 [6.2] on 7th day) (Fig. 4); however, the change was not significant (P = 0.280). No differences in other objective sleep parameters were observed.
Fig. 4.
Within-group distribution of change in objectively measured number of awakenings
Discussion
This pilot randomized clinical trial demonstrated a beneficial effect of weighted blankets on improving sleep quality in participants with insomnia, such that participants using weighted blankets for 1 month had a greater decrease in PSQI score. In addition, the trial revealed the significant benefits of weighted blankets on sleep-related outcomes including daytime sleepiness, fatigue, anxiety, stress, and bodily pain. Objective actigraphy recordings showed a decrease in the number of awakenings in weighted blanket group and an increase in normal blanket group, which still needs to be proved by future trials. Our preliminary findings suggested that weighted blanket might be a promising tool for sleep intervention among adults with insomnia in clinical practice.
The beneficial effect of weighted blankets on sleep quality was also observed in different populations including adults with major psychiatric disorders and co-occurring insomnia [13, 38], dementia [39–41], chronic pain [42], and children with ADHD/ASD [17, 18], CHARGE syndrome [43], and general anesthesia [44]. Notably, the efficacy of weighted blankets in individuals with ADHD/ASD and psychiatric disorders showed the strongest supporting evidence, whereas fewer studies focused on relatively healthy adults with insomnia and without major psychiatric diseases. However, this population actually accounts for a larger proportion. Thus, this study extends the evidence for the broader application of weighted blankets across more populations. Notably, this study found a significant improvement in PSQI scores, but not in ISI. The PSQI mainly reflects multiple dimensions of sleep quality; while ISI scale focuses on the severity of insomnia, the disturbance of sleep problems to life, and the satisfaction with sleep. The one-month short-term intervention did improve the sleep quality to some extent, that being said, the PSQI score was improved. However, as the improvement of sleep is a long-term process, it was hard to cure the insomnia problem of the participants in a month. Therefore, the participants felt that insomnia still had a certain impact on life, and they had not yet reached a satisfactory sleep pattern. This may result in the non-significant difference between the two groups on the ISI scale.
Significant benefits of weighted blankets on negative emotions and daytime symptoms were also observed. Regarding psychological behaviors, a few studies have showed that weighted blanket might be an effective therapeutic tool for relieving anxiety, stress, and morning mood in populations with cancer [19], ADHD/ASD [16, 17], and mental disorders [20], which was in line with our findings in adults with clinical insomnia. Given that negative emotions can be instigated and further exacerbated by sleep disturbances [45], the increased sleep quality provided by weighted blanket intervention might be an explanation for the beneficial effects on anxiety and stress. For daytime symptoms, a previous study in patients with psychiatric disorders found that weighted blanket intervention contributed to increased daytime activity level and delayed occurrence of circadian peak of activity using objective measures, which was a reflection of reduced daytime fatigue [13]. Our findings on subjective daytime sleepiness and daytime fatigue further confirmed the positive effects.
Interestingly, we found that using weighted blankets contributed to relieving self-perceived bodily pain. A previous randomized controlled trial reported that compared with lighter-weighted blankets, heavier-weighted blankets intervention produced a greater reduction in widespread chronic pain, with a stronger effect on individuals with high trait anxiety [42]. Given that chronic pain is partly determined by social and/or affective factors [46, 47], we speculate that the deep pressure provided by weighted blanket may impart a sense of physical and mental safety that could relieve anxiety and stress which were also observed in our study, and ultimately, reducing perception of bodily pain [48, 49]. In addition, the effects of deep pressure on increasing local tissue oxygenation and blood flow may also be the possible mechanism of pain relief [50, 51].
Based on objective recordings from actigraphy, we also investigated the changes in number of awakenings in the two groups. Participants using weighted blankets had a decrease in the number of awakenings whereas the normal blanket group showed an increase. However, this study was not powered to detect a significant effect, further proofs are still required. Similar results were observed in a randomized trial in patients with psychiatric disorders that responders of weighted blanket intervention had a significant decrease in objective total time awake after sleep onset and an improvement in subjective sleep maintenance compared to non-responders [13]. The positive effects on reduced nighttime awakenings were also found in children with ADHD/ASD [18, 52]. These findings suggested that weighted blanket may play a sleep-improving role primarily in reducing difficulties maintaining asleep. A possible mechanism is that the deep pressure provided by weighted blanket can stimulate the parasympathetic nervous system to release serotonin and dopamine and produce endorphins, resulting in relaxed muscles, reduced heart rate, and steady breath, and consequently, conducing to staying asleep [14, 53].
In this study, three participants reported feelings of cold or uncomfortable when using the blankets or actigraphy, and no severe adverse events occurred. This result provided evidence that weighted blanket intervention might be a safer alternative to pharmacotherapy for insomnia. However, given the limited available evidence, the safety issues in clinical application still need special caution. It would be important for clinicians to systematically monitor and record any potential adverse effects, such as increased discomfort, restricted movement, or exacerbation of other health conditions, that may arise from the use of weighted blankets. In particular, special attention should be paid to children and the elderly. To a few children, the weight of weighted blankets may cause anxiety, panic, pain, and even risk of suffocation or entrapment [54, 55]. Thus, it is advised for parents to closely supervise and avoid covering the face with a blanket while their children using a weighted blanket. For the elderly, if they have limited physical capacity and mobility, frailty, or severe dementia, and fail to remove the blanket from their head to avoid the risk of suffocation, they must use the lightest blanket possible after consulting a clinician [41].
Strengths and limitations
There are several strengths in our study. First, we investigated the efficacy of weighted blankets in adults without major diseases and with clinical insomnia, providing more evidence for the application of weighted blankets in relatively healthy individuals with insomnia. Second, this study was conducted in China, while the predominance of similar research was conducted in Europe and the United States, which provides values for the application of weighted blankets in China. Besides, the study design, including the randomization and the setting of normal blanket group, is a notable strength.
A limitation of our study was that the sleep monitoring by actigraphy only lasted for one week; and thus, the objective long-term changes in sleep could not be observed. It is possible that differences of other sleep parameters between the two groups would emerge under longer duration of sleep monitoring. Second, several assessors were not blind to treatment allocation, but the outcome evaluations were mainly completed by participants’ self-administered questionnaires without intervention from the assessors, which minimized any bias in outcome evaluations. Besides, we cannot control for the effect resulted from the suspicion of participants regarding their group allocation. Third, differences in sleep position during nighttime sleep, such as frequent turning over, may cause the move of the actigraphy and thus affect the monitoring effect. Forth, the sample size was limited as well, indicating the need for larger and high quality randomized controlled trails to further explore the long-term efficacy and mechanisms of weighted blankets. Fifth, the proportion of participants with severe and moderate clinical insomnia had a slight imbalance between the two groups at baseline, which was considered in the mixed models. Additionally, we only documented information about the participants’ marriages, but not their bed partners, whose sleep may disrupt the other’s sleep. The study was retrospectively registered; however, we strictly adhered the initial protocols without selective reporting.
Future perspectives
This study reflects several potential future directions. First, this study was conducted in three specific hospitals in China. It would be helpful to replicate in different populations and settings to assess the generalizability of the findings. Second, we did not explore the underlying mechanisms through which weighted blankets improve sleep quality. Investigating the physiological, psychological, or neurobiological mechanisms by which weighted blankets exert their effects would enhance our understanding of their therapeutic potential. Third, the control group in the study received normal blankets, it would be beneficial for future studies to include an additional control group that receives no intervention or a different type of non-weighted intervention. Forth, this study implemented a 1-month intervention period. It would be valuable for future studies to conduct follow-up assessments beyond the 1-month period to evaluate the sustainability of the observed improvements. Fifth, this study was not powered to detect significant effects on ISI and objective measures, and it remains to be proven whether benefits are maintained across insomnia severity and objective sleep measures. In this aspect, this study provided promising preliminary evidence that weighted blankets may improve sleep and related symptoms. However. larger and high quality randomized controlled trails are required on this crucial topic.
Conclusion
In conclusion, our study showed positive effects of weighted blankets on sleep quality and a range of sleep-related negative emotions and daytime symptoms. Our findings provide promising preliminary evidence for the possible efficacy and safety of weighted blankets in sleep intervention, offering an easy-to-use non-pharmacotherapy option for adults with insomnia. More high-quality and large-scale randomized controlled trials are required to further validate the long-term efficacy of weighted blankets and explore its potential mechanisms.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
We appreciate all participants who attended this study.
Abbreviations
- PSQI
Pittsburgh Sleep Quality Index
- ISI
Insomnia Severity Index
- CBT-I
Cognitive behavioral therapy for insomnia
- ADHD
Attention deficit hyperactivity disorder
- ASD
Autism spectrum disorder
- ESS
Epworth Sleepiness Scale
- PSS
Perceived Stress Scale
- CESD-10
Center for Epidemiologic Studies Depression Scale-10
- FS-14
Fatigue Scale-14
- SAS
Self-Rating Anxiety Scale
- SF-36
Quality of Life Scale 36-item Short-Form
- BCG
Ballistocardiogram
Author contributions
Conceptualization: Z.L., Y.Z.; Methodology: Z.L., J.Y., Z.Y.; Formal analysis and investigation: J.Y., J.D., Z.Y., W.C., S.S., L.M.Z., K.S., J.X., Q.X., J.K., L.S.Z., Y.Z.; Data curation: J.Y., J.D., M.G., Y.C.; Software: J.Y., J.D.; Visualization: J.Y., J.D.; Writing – original draft preparation: J.Y., J.D., M.G., Y.C.; Writing – review and editing: All authors; Funding acquisition: Z.L.; Supervision: Z.L., L.S.Z., Y.Z.; Project administration: Z.L. All authors agreed with the content and gave explicit consent to submit.
Funding
This study was supported by funding from Damian Health Tech (Hangzhou) CO., LTD (Kheng-20220141, to ZL), Zhejiang Key Laboratory of Intelligent Preventive Medicine (2020E10004), and Zhejiang University School of Public Health Interdisciplinary Research Innovation Team Development Project. The funders had no role in the study design; data collection, analysis, or interpretation; in the writing of the report; or in the decision to submit the article for publication.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. Researchers with a specified purpose should send request email to Professor Zuyun Liu: Zuyun.liu@outlook.com.
Declarations
Ethics approval and consent to participate
This study was reviewed and approved by the Zhejiang University School of Public Health Medical Ethics Committee (registration number ZGL202201-7) and complied with the World Medical Association Declaration of Helsinki. All participants provided written informed consent and had the option to withdraw from the study at any point without facing discrimination or unfair treatment.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lisan Zhang, Email: zls09@zju.edu.cn.
Yubo Zhu, Email: yishanmama131204@163.com.
Zuyun Liu, Email: Zuyun.liu@outlook.com, Email: zuyunliu@zju.edu.cn.
References
- 1.Lo Martire V, Caruso D, Palagini L, Zoccoli G, Bastianini S. Stress & sleep: a relationship lasting a lifetime. Neurosci Biobehav Rev. 2020;117:65–77. [DOI] [PubMed] [Google Scholar]
- 2.Palagini L, Hertenstein E, Riemann D, Nissen C. Sleep, insomnia and mental health. J Sleep Res. 2022;31(4):e13628. [DOI] [PubMed] [Google Scholar]
- 3.Maier JG, Nissen C. Sleep and memory: mechanisms and implications for psychiatry. Curr Opin Psychiatry. 2017;30(6):480–4. [DOI] [PubMed] [Google Scholar]
- 4.Jahrami HA, Alhaj OA, Humood AM, Alenezi AF, Fekih-Romdhane F, AlRasheed MM, et al. Sleep disturbances during the COVID-19 pandemic: A systematic review, meta-analysis, and meta-regression. Sleep Med Rev. 2022;62:101591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palagini L, Bastien CH, Marazziti D, Ellis JG, Riemann D. The key role of insomnia and sleep loss in the dysregulation of multiple systems involved in mood disorders: A proposed model. J Sleep Res. 2019;28(6):e12841. [DOI] [PubMed] [Google Scholar]
- 6.Riemann D, Nissen C, Palagini L, Otte A, Perlis ML, Spiegelhalder K. The neurobiology, investigation, and treatment of chronic insomnia. Lancet Neurol. 2015;14(5):547–58. [DOI] [PubMed] [Google Scholar]
- 7.Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia:update of the recent evidence (1998–2004). Sleep. 2006;29(11):1398–414. [DOI] [PubMed] [Google Scholar]
- 8.Morin CM, Vallières A, Guay B, Ivers H, Savard J, Mérette C, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301(19):2005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajabi Majd N, Broström A, Ulander M, Lin CY, Griffiths MD, Imani V, et al. Efficacy of a Theory-Based Cognitive Behavioral Technique App-Based Intervention for Patients With Insomnia: Randomized Controlled Trial. J Med Internet Res. 2020;22(4):e15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Irwin MR, Carrillo C, Sadeghi N, Bjurstrom MF, Breen EC, Olmstead R. Prevention of Incident and Recurrent Major Depression in Older Adults With Insomnia: A Randomized Clinical Trial. JAMA Psychiatry. 2022;79(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garland SN, Xie SX, DuHamel K, Bao T, Li Q, Barg FK, et al. Acupuncture Versus Cognitive Behavioral Therapy for Insomnia in Cancer Survivors: A Randomized Clinical Trial. J Natl Cancer Inst. 2019;111(12):1323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felder JN, Epel ES, Neuhaus J, Krystal AD, Prather AA. Efficacy of Digital Cognitive Behavioral Therapy for the Treatment of Insomnia Symptoms Among Pregnant Women: A Randomized Clinical Trial. JAMA Psychiatry. 2020;77(5):484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ekholm B, Spulber S, Adler M. A randomized controlled study of weighted chain blankets for insomnia in psychiatric disorders. J Clin Sleep Med. 2020;16(9):1567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, Yang Z, Sun S, Sun K, Chen W, Zhang L et al. The effect of weighted blankets on sleep and related disorders: a brief review. Front Psychiatry. 2024;15. [DOI] [PMC free article] [PubMed]
- 15.Danoff-Burg S, Rus HM, Cruz Martir L, Raymann RJ. 1203 Worth The Weight: Weighted Blanket Improves Sleep And Increases Relaxation. Sleep. 2020;43(SUPPL 1):A460. [Google Scholar]
- 16.Gee B, McOmber T, Sutton J, Lloyd K. Efficacy of Weighted Blankets for Children With Autism Spectrum Disorder, Sensory Overresponsivity, and Sleep Disturbance. Am J Occup Ther. 2017;71(4Supplement1):7111515242p1. [Google Scholar]
- 17.Bolic Baric V, Skuthalla S, Pettersson M, Gustafsson PA, Kjellberg A. The effectiveness of weighted blankets on sleep and everyday activities - A retrospective follow-up study of children and adults with attention deficit hyperactivity disorder and/or autism spectrum disorder. Scand J Occup Ther. 2021:1–11. [DOI] [PubMed]
- 18.Hvolby A. The Application of Ball Blankets in the Treatment of Sleeping Difficulties in Children with Attention Deficit or Hyperactivity Disorder. Effect on Quality of Life and Daily Functioning. J Sleep Med Disord. 2020;6(1):1100. [Google Scholar]
- 19.Vinson J, Powers J, Mosesso K. Weighted Blankets: Anxiety Reduction in Adult Patients Receiving Chemotherapy. Clin J Oncol Nurs. 2020;24(4):360–8. [DOI] [PubMed] [Google Scholar]
- 20.Becklund AL, Rapp-McCall L, Nudo J. Using weighted blankets in an inpatient mental health hospital to decrease anxiety. J Integr Med. 2021;19(2):129–34. [DOI] [PubMed] [Google Scholar]
- 21.Eron K, Kohnert L, Watters A, Logan C, Weisner-Rose M, Mehler PS. Weighted Blanket Use: A Systematic Review. Am J Occup Ther. 2020;74(2):p74022050101–14. [DOI] [PubMed] [Google Scholar]
- 22.Han F, Tang XD, Zhang B. The guidelines for the diagnosis and treatment of insomnia in China. Chin Med J. 2017(24):1844–56.
- 23.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 24.Diagnostic and statistical manual of mental disorders: DSM-5™, 5th ed. Arlington, VA, US: American Psychiatric Publishing, Inc. 2013:947–xliv. [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. [DOI] [PubMed] [Google Scholar]
- 26.Gee BM, Lloyd K, Sutton J, McOmber T. Weighted Blankets and Sleep Quality in Children with Autism Spectrum Disorders: A Single-Subject Design. Child (Basel). 2020;8(1). [DOI] [PMC free article] [PubMed]
- 27.Smyth C. The Pittsburgh Sleep Quality Index (PSQI). Insight. 2000;25(3):97–8. [DOI] [PubMed] [Google Scholar]
- 28.Chung KF, Kan KK, Yeung WF. Assessing insomnia in adolescents: comparison of Insomnia Severity Index, Athens Insomnia Scale and Sleep Quality Index. Sleep Med. 2011;12(5):463–70. [DOI] [PubMed] [Google Scholar]
- 29.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 30.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5. [DOI] [PubMed] [Google Scholar]
- 31.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 32.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 33.Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12(6):371–9. [DOI] [PubMed] [Google Scholar]
- 34.Ware J, Snoww K, Kosinski MA, Gandek BG. SF-36. Health survey: manual and interpretation guide. Health Assessment Lab; 1993.
- 35.Bruser C, Stadlthanner K, Brauers A, Leonhardt S. Applying machine learning to detect individual heart beats in ballistocardiograms. 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology. 2010:1926-9. [DOI] [PubMed]
- 36.Chinoy ED, Cuellar JA, Huwa KE, Jameson JT, Watson CH, Bessman SC et al. Performance of seven consumer sleep-tracking devices compared with polysomnography. Sleep. 2021;44(5). [DOI] [PMC free article] [PubMed]
- 37.Kainec KA, Caccavaro J, Barnes M, Hoff C, Berlin A, Spencer RMC. Evaluating Accuracy in Five Commercial Sleep-Tracking Devices Compared to Research-Grade Actigraphy and Polysomnography. Sens (Basel). 2024;24(2). [DOI] [PMC free article] [PubMed]
- 38.Rosenberg K. Weighted Blankets for Insomnia in Patients with Psychiatric Disorders. Am J Nurs. 2021;121(1):55. [DOI] [PubMed] [Google Scholar]
- 39.Dyon NA, Sue JL, Tchakerian N, Fisher K. The use of weighted blankets as a novel approach for treatment of persistent vocalizations in late stage dementia. Geriatr Nurs. 2021;42(6):1253–6. [DOI] [PubMed] [Google Scholar]
- 40.Harris ML, Titler MG. Feasibility and Acceptability of a Remotely Delivered Weighted Blanket Intervention for People Living With Dementia and Their Family Caregivers. J Appl Gerontol. 2022;41(11):2316–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura M, Yamauchi N. A case of effective usage of a weighted blanket for a person with severe dementia. Psychogeriatrics. 2021;21(2):239–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baumgartner JN, Quintana D, Leija L, Schuster NM, Bruno KA, Castellanos JP, et al. Widespread Pressure Delivered by a Weighted Blanket Reduces Chronic Pain: A Randomized Controlled Trial. J Pain. 2022;23(1):156–74. [DOI] [PubMed] [Google Scholar]
- 43.Kennert BA, Harshorne TS, Kanouse S, Johnson C. Parent survey of sleep problems among children with CHARGE syndrome. Res Dev Disabil. 2020;101:103614. [DOI] [PubMed] [Google Scholar]
- 44.Eull D, Zachrison B, Nickel A. Feasibility trial of weighted blankets as an intervention for emergence delirium in postoperative pediatric patients. J Pediatr Nurs. 2022;62:30–5. [DOI] [PubMed] [Google Scholar]
- 45.Chellappa SL, Aeschbach D. Sleep and anxiety: From mechanisms to interventions. Sleep Med Rev. 2022;61:101583. [DOI] [PubMed] [Google Scholar]
- 46.Martinez-Calderon J, Flores-Cortes M, Morales-Asencio JM, Luque-Suarez A. Pain-Related Fear, Pain Intensity and Function in Individuals With Chronic Musculoskeletal Pain: A Systematic Review and Meta-Analysis. J Pain. 2019;20(12):1394–415. [DOI] [PubMed] [Google Scholar]
- 47.Alexander P, Charvy N, Christian FB, Stuart C, Susanna B, Richard W, et al. Exacerbation of Pain by Anxiety Is Associated with Activity in a Hippocampal Network. J Neurosci. 2001;21(24):9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Case LK, Liljencrantz J, McCall MV, Bradson M, Necaise A, Tubbs J, et al. Pleasant Deep Pressure: Expanding the Social Touch Hypothesis. Neuroscience. 2021;464:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krauss KE. The effects of deep pressure touch on anxiety. Am J Occup Ther. 1987;41(6):366–73. [DOI] [PubMed] [Google Scholar]
- 50.Messere A, Ceravolo G, Franco W, Maffiodo D, Ferraresi C, Roatta S. Increased tissue oxygenation explains the attenuation of hyperemia upon repetitive pneumatic compression of the lower leg. J Appl Physiol (1985). 2017;123(6):1451–60. [DOI] [PubMed] [Google Scholar]
- 51.Monteiro Rodrigues L, Rocha C, Ferreira HT, Silva HN. Lower limb massage in humans increases local perfusion and impacts systemic hemodynamics. J Appl Physiol (1985). 2020;128(5):1217–26. [DOI] [PubMed] [Google Scholar]
- 52.Gringras P, Green D, Wright B, Rush C, Sparrowhawk M, Pratt K, et al. Weighted blankets and sleep in autistic children–a randomized controlled trial. Pediatrics. 2014;134(2):298–306. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds S, Lane SJ, Mullen B. Effects of deep pressure stimulation on physiological arousal. Am J Occup Ther. 2015;69(3):p69033500101–5. [DOI] [PubMed] [Google Scholar]
- 54.Lönn M, Aili K, Svedberg P, Nygren J, Jarbin H, Larsson I. Experiences of Using Weighted Blankets among Children with ADHD and Sleeping Difficulties. Occup Ther Int. 2023;2023:1945290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lönn M, Svedberg P, Nygren J, Jarbin H, Aili K, Larsson I. The efficacy of weighted blankets for sleep in children with attention-deficit/hyperactivity disorder-A randomized controlled crossover trial. J Sleep Res. 2023:e13990. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request. Researchers with a specified purpose should send request email to Professor Zuyun Liu: Zuyun.liu@outlook.com.