Abstract
Background
Chemo-endocrine therapy can lead to various side effects associated with ovarian dysfunction. Predicting menstrual recovery is necessary to discuss the treatment-related issues regarding fertility and premature menopause with patients.
Methods
In the ASTRRA trial, patients who resumed ovarian function within 2 years after chemotherapy were randomized to receive tamoxifen for 5 years or OFS with tamoxifen for 2 years. With these 1298 patients, we developed a model that predicts when menstrual recovery will occur within a 3-year period after chemotherapy using variables including age, body mass index, chemotherapy regimen and duration, and serum estradiol and follicle-stimulating hormone levels.
Results
The data of 957 patients were used to develop the prediction model, and those of 341 patients were used for validation. In the development group, menstruation resumed in 450 patients (47.0%) within 5 years. In multivariable analysis, younger age (< 35 vs. 45, HR 7.85, 95% CI 4.63–13.30, p < 0.0001), anthracycline-based chemotherapy without taxane (vs. with taxane, HR 1.81, 95% CI 1.37–2.38, p < 0.0001), and chemotherapy duration (≤ 90 days vs. > 90 days, HR 1.32, 95% CI 1.01–1.72, p = 0.045) correlated with menstrual recovery. Using combined age, regimen, and duration of chemotherapy, we developed a simplified scoring system to estimate recovery chances and used a concordance index of 0.679 overall and 0.744 at 3 years for validation.
Conclusion
This model predicted timing and probability of menstrual recovery, based on their individual age, type and duration of chemotherapy in premenopausal women diagnosed with breast cancer who received tamoxifen after chemotherapy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13058-024-01903-9.
Keywords: Breast neoplasms, Chemotherapy, Menstruation, Premenopause, Tamoxifen
Introduction
Management of premenopausal women with breast cancer, especially in hormone-receptor positive, human epidermal growth factor negative and axillary lymph-node positive can be complex and challenging. Addition of ovarian function suppression (OFS) to conventional endocrine therapy improves the survival of premenopausal women with moderate-to-high-risk hormone receptor-positive breast cancer, especially in those who receive chemotherapy [1–3]. Consequently there is a high likelihood of recommending both chemotherapy and escalated endocrine therapy for these women. Ovarian dysfunction is a common side-effect of systemic treatment for premenopausal women with breast cancer. Women often experience vasomotor symptoms, sexual dysfunction, and impaired fertility when they undergo an abrupt menopause due to anticancer therapy [4]. In high-risk hormone-receptor positive tumors, endocrine therapy is often escalated up to 10 years or adding OFS to adjuvant tamoxifen or aromatase inhibitor which may potentially lead to distressing short and long-term side effects. This can result in both reduced quality of life for patients and decrease in treatment adherence, alongside the chemotherapy [5]. In particular, in younger patients under 35 years of age, the discontinuation rate of endocrine therapy reaches 20–25% [6]. In young breast cancer patients, fertility preservation is a high concern. Over the last few decades, various methods have been suggested to reduce the side effects of treatment and preserve fertility, including assisted reproductive techniques, ovarian tissue cryopreservation, and the concurrent use of GnRH agonists during chemotherapy. These options are now widely used in clinical practice and are strongly recommended to be offered to young breast cancer patients before starting treatment [7].
The evaluation of ovarian function recovery involves various methods, and there is no single absolute method. Along with biomarkers such as follicle stimulating hormone (FSH), estradiol (E2), and anti mullerian hormone (AMH), the restoration of menstruation has been traditionally used as a surrogate indicator for assessing ovarian function recovery [8]. Factors associated with ovarian function recovery after breast cancer treatment have been well elucidated in previous studies, notably including age and the use of alkylating agents such as cyclophosphamide, with anthracycline-based chemotherapy also increasing the likelihood of experiencing amenorrhea [9]. In addition to the gonadotoxicity associated with anthracycline-based chemotherapy, additional gonadotoxicity has been reported as an adverse effect of adding taxane to anthracycline-based chemotherapy [10]. In high-risk young patients with hormone-receptor positive breast cancer, in addition to gonadotoxic chemotherapy, tamoxifen is administered for an extended duration, which can affect menstrual recovery [11, 12]. Individualized prediction of the possibility and timing of menstrual recoveryto gain a better perspective for an individual adjuvant treatment plan based on the patient’s risk and age and also desire for fertility. This study aimed to analyze the factors affecting menstrual recovery in premenopausal patients who received chemotherapy and endocrine therapy using the data of the patient group in the Addition of Ovarian Suppression to Tamoxifen in Young Women with Hormone-Sensitive Breast Cancer Who Remain Premenopausal or Regain Vaginal Bleeding After Chemotherapy (ASTRRA; ClinicalTrials.gov identifier: NCT00912548) trial. Furthermore, we developed a model to predict the timing of menstrual cycle recovery during treatment.
Methods
Brief introduction to the ASTRRA study
The ASTRRA trial aimed to determine the survival benefits of concurrent OFS using goserelin and tamoxifen in premenopausal women after completing chemotherapy. Briefly, premenopausal women aged 45 years or younger with estrogen receptor-positive operable breast cancer (stage I to III) were enrolled after completing neoadjuvant or adjuvant chemotherapy. Patients who recovered ovarian function were randomized to receive 2 years of goserelin plus 5 years of tamoxifen treatment or 5 years of tamoxifen alone. Recovery of ovarian function was defined as a serum FSH level < 30 mU/mL or evidence of vaginal bleeding. Ovarian function was assessed at the time of enrollment and every 6 months thereafter for 2 years based on FSH levels and menstruation history.
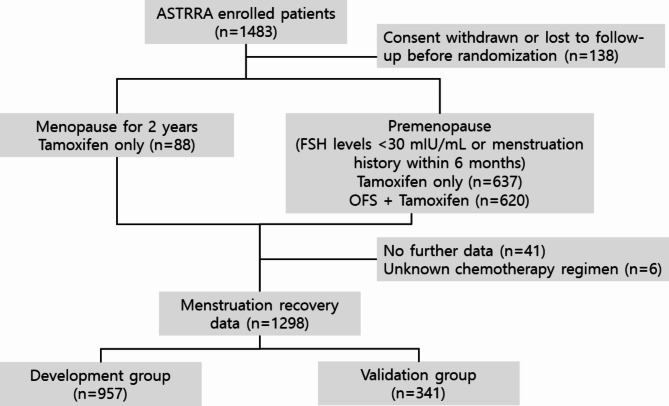
This study aimed to predict the timing of menstrual recovery in premenopausal women with hormone receptor-positive breast cancer who completed planned chemotherapy. From the population of the original study (ASTRRA), we identified 1395 women who had information on their menstrual recovery status. From these patients, we excluded six who received other than anthracycline- and/or taxane-based chemotherapy. Chemotherapy regimens were stratified into anthracycline-based and taxane-based protocols, with the taxane-based group including regimens that also incorporated anthracyclines. (Supplementary Table 1) We further identified and excluded 41 patients for whom follow-up data were unavailable. All patients started taking tamoxifen immediately after chemotherapy (baseline). Those who experienced menstruation recovery or had FSH levels below 30 mIU/mL at baseline were randomly assigned in a 1:1 ratio to receive additional OFS. If menstruation resumed or FSH levels were confirmed to be below 30 mIU/mL within two years during the follow-up period, the participants were randomized to receive either additional OFS or continue tamoxifen alone. In this study, we included and analyzed women who did not recover menstruation within two years. For patients who received OFS, menstruation recovery events were censored at the time of OFS administration. Most patients received the chemotherapy regimen mentioned above, and only a small number of patients received additional 5-fluorouracil (5-FU) and/or trastuzumab. Menstrual recovery was defined as normal vaginal bleeding after interruption due to prior chemotherapy, regardless of serum E2 or FSH levels. Because the primary study enrolled patients from 35 cancer centers nationwide in South Korea, we included patients enrolled from 2009 to 2011 in the development dataset and those enrolled in 2012 and 2013 in the validation dataset. (Fig. 1)
Fig. 1.
CONSORT flow diagram showing enrollment, and follow-up process with the respective patient numbers and reasons for exclusion
Patient age at diagnosis, body mass index (BMI), serum FSH and E2 levels at baseline (3 months within completion of adjuvant chemotherapy), chemotherapy regimen, and duration of chemotherapy were included in the primary univariate analysis to identify factors that influence menstrual recovery. Unfortunately, we were unable to obtain separate data on tamoxifen adherence and lacked information on whether ovarian or hysterectomy procedures were performed during the follow-up period, which could not be reflected in our study results. Descriptive statistics were calculated for these variables. Using a Cox proportional hazards model, we identified the most relevant factors determining the timing of menstrual recovery.
After risk modeling with Cox proportional hazards, we used a simple scoring system to estimate the yearly chance of menstrual recovery by assigning hazard ratio-related points compared with the reference over a 3-year period. The estimated risk was then validated using a concordance index (C-index) for each development, internal, and validation set to demonstrate the discrimination potential of the model. Internal validation was performed using the optimism-corrected C-index and validation was performed using the validation dataset. Calibration curves for each year’s probability of resuming menstruation in both the development and validation sets are shown to visualize the accuracy of the model.
All statistical analyses were performed using SAS software.
This study was approved by the institutional review boards of all participating institutions. The requirement for informed consent was waived and the study was approved by the institutional review board of each institution owing to the retrospective nature of the study.
Results
A total of 1298 patients enrolled between March 2009 and March 2014 were included in the primary analysis to estimate the timing of menstrual recovery. The median age at enrollment was 40 years (range, 24 to 45). Overall, 517 patients (39.8%) were aged < 40 years. Data on BMI at baseline and FSH and E2 levels at baseline and 6 months after enrollment were collected. More than half (59.6%) of patients received anthracycline with taxane-based chemotherapy. The use of 5-FU and trastuzumab, and the duration of chemotherapy were also included in the univariate analysis. The baseline patient characteristics are shown in Table 1.
Table 1.
Baseline characteristics of all patients
| Development (N = 957) | Validation (N = 341) | Total (N = 1298) | |||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Age at enrollment, years (median) | 39.8 | 40.1 | 39.9 | ||||
| Age groups | < 35 | 113 | 11.8 | 39 | 11.4 | 152 | 11.7 |
| 35–39 | 277 | 28.9 | 88 | 25.8 | 365 | 28.1 | |
| 40–44 | 490 | 51.2 | 180 | 52.8 | 670 | 51.6 | |
| 45 | 77 | 8.1 | 34 | 10.0 | 111 | 8.6 | |
| BMI (mean) | 23.25 | 23.31 | 23.27 | ||||
| BMI GROUP | < 18.5 | 40 | 4.3 | 16 | 4.8 | 56 | 4.5 |
| 18.5–25 | 647 | 69.9 | 215 | 64.6 | 862 | 68.5 | |
| ≥ 25 | 239 | 25.8 | 102 | 30.6 | 341 | 27.1 | |
| FSH baseline | < 30 | 76 | 8.0 | 23 | 6.8 | 99 | 7.7 |
| (mIU/mL) | ≥ 30 | 876 | 92.0 | 316 | 93.2 | 1192 | 92.3 |
| E2 baseline | < 40 | 647 | 93.4 | 291 | 93.6 | 938 | 93.4 |
| (pg/mL) | ≥ 40 | 46 | 6.6 | 20 | 6.4 | 66 | 6.6 |
| FSH at 6mo | < 30 | 576 | 63.4 | 233 | 70.6 | 809 | 65.3 |
| (mIU/mL) | ≥ 30 | 333 | 36.6 | 97 | 29.4 | 430 | 34.7 |
| E2 at 6mo | < 40 | 461 | 68.9 | 212 | 70.2 | 673 | 69.3 |
| (pg/mL) | ≥ 40 | 208 | 31.1 | 90 | 29.8 | 298 | 30.7 |
| Chemotherapy regimen | A based | 363 | 41.0 | 123 | 38.9 | 486 | 40.4 |
| T based | 523 | 59.0 | 193 | 61.1 | 716 | 59.6 | |
| Additional 5-FU | 98 | 11.1 | 36 | 11.4 | 134 | 11.2 | |
| HER2 targeted agents | 14 | 1.6 | 15 | 4.8 | 29 | 2.4 | |
| Chemotherapy duration | ≤ 90d | 297 | 31.2 | 93 | 27.8 | 390 | 30.3 |
| > 90d | 654 | 68.8 | 242 | 72.2 | 896 | 69.7 | |
Abbreviations: BMI, body mass index; FSH, follicle-stimulating hormone; E2, estradiol; A, anthyracyclines; T, taxanes; 5-FU, 5-fluorouracil; HER2, human epidermal growth factor receptor; d, days
Predictive model for menstrual recovery
Data from patients enrolled between 2009 and 2011 were used to estimate the time of menstrual recovery. Of the 957 patients, 450 (47.0%) experienced menstrual recovery during the 5-year follow-up period. Univariate analysis revealed that younger age, lower FSH and higher E2 levels at baseline, an anthracycline-only regimen, and shorter chemotherapy duration were predictive of early menstrual recovery. BMI and the additional use of 5-FU and/or trastuzumab were not associated with menstrual recovery in this study cohort. In multivariable analysis, younger age (< 35, hazard ratio (HR) 7.848, 95% CI 4.633–13.295, p < 0.0001), anthracycline-based regimen without taxane (HR 1.809, 95% CI 1.374–2.381, p < 0.001), and shorter (≤ 90 days) chemotherapy duration were predictive factors for menstrual recovery (Table 2).
Table 2.
Prediction model for menstruation recovery in all patients
| Univariate | Multivariable | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | HR | 95% CI | P value | Estimate | HR | 95% CI | P value | Points | |||
| Age at enrollment | -0.123 | 0.885 | 0.867 | 0.903 | 0.000 | ||||||
| < 35 | 1.972 | 7.185 | 4.233 | 12.194 | 0.000 | 2.060 | 7.848 | 4.633 | 13.295 | < 0.0001 | 5 |
| 35–39 | 1.521 | 4.577 | 2.769 | 7.566 | 0.000 | 1.600 | 4.951 | 3.005 | 8.157 | < 0.0001 | 4 |
| 40–44 | 0.879 | 2.408 | 1.467 | 3.952 | 0.001 | 0.867 | 2.380 | 1.456 | 3.891 | 0.001 | 2 |
| 45 | 1 | < 0.001 | 1 | < 0.001 | 0 | ||||||
| BMI | 0.000 | 1.000 | 0.973 | 1.029 | 0.975 | ||||||
| < 18.5 | 0.352 | 1.422 | 0.945 | 2.138 | 0.091 | ||||||
| 18.5–25 | 1 | 0.239 | |||||||||
| ≥ 25 | 0.036 | 1.037 | 0.836 | 1.287 | 0.741 | ||||||
| FSH < 30 | 0.711 | 2.036 | 1.500 | 2.763 | 0.000 | ||||||
| E2 ≥ 40 | 0.980 | 2.665 | 1.801 | 3.946 | 0.000 | ||||||
| Chemo-regimen | |||||||||||
| Anthracycline based | 0.666 | 1.946 | 1.611 | 2.351 | 0.000 | 0.593 | 1.809 | 1.374 | 2.381 | < 0.0001 | 1 |
| Taxane based | 1 | 1 | 0 | ||||||||
| 5-FU | 0.238 | 1.269 | 0.954 | 1.688 | 0.101 | ||||||
| Herceptin | 0.105 | 1.111 | 0.496 | 2.488 | 0.798 | ||||||
| Chemo-duration | |||||||||||
| ≤ 90 days | 0.579 | 1.784 | 1.473 | 2.162 | 0.000 | 0.275 | 1.317 | 1.006 | 1.723 | 0.045 | 1 |
| > 90 days | 1 | 1 | 0 | ||||||||
Abbreviations: BMI, body mass index; FSH, follicle-stimulating hormone; E2, estradiol; 5-FU, 5-fluorouracil;
Scoring system
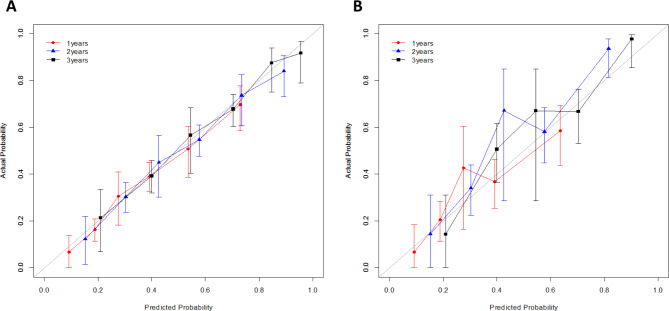
The simplified scoring system estimates menstrual recovery for each risk factor. The scoring for the menstrual recovery model displayed the overall menstrual recovery estimated at the time of completion of chemotherapy using three factors (age, chemotherapy regimen, and chemotherapy duration). For example, a patient aged 34 years at the time of chemotherapy (points = 5) received four cycles of anthracycline plus cyclophosphamide, followed by four cycles of docetaxel (points = 0 for chemotherapy regimen, points = 0 for chemotherapy duration). The risk estimation for each point total (= 5) shows that the patient has a 53.5%, 73.4%, and 84.6% chance of menstrual recovery within 1, 2, and 3 years, respectively, after the end of chemotherapy (Tables 2 and 3, Supplementary Table 2). The C-index of the model was 0.683 (95% CI 0.659–0.707) for the development set and 0.680 (95% CI 0.656–0.704) for the internal validation. Validation with 341 patients showed a C-index of 0.67 (95% CI 0.369–0.719) overall and 0.744 (95% CI 0.678–0.810) at 3 years. The discrimination of the predictions using the C-index and the calibration curve for each set is shown in Supplementary Tables 3 and Fig. 2(a) and 2(b).
Table 3.
Menstruation recovery estimation using the simplified scoring system
| Development group | Validation group | Estimate of menstruation recovery | |||
|---|---|---|---|---|---|
| Points total | N | N | 1 year | 2 years | 3 years |
| 0 | 47 | 17 | 0.0839 | 0.1406 | 0.1931 |
| 1 | 10 | 6 | 0.1264 | 0.2085 | 0.2818 |
| 2 | 285 | 113 | 0.1882 | 0.3028 | 0.3999 |
| 3 | 77 | 27 | 0.2750 | 0.4268 | 0.5451 |
| 4 | 317 | 107 | 0.3912 | 0.5763 | 0.7034 |
| 5 | 107 | 39 | 0.5350 | 0.7341 | 0.8467 |
| 6 | 86 | 30 | 0.6931 | 0.8705 | 0.9446 |
| 7 | 28 | 2 | 0.8384 | 0.9573 | 0.9885 |
Fig. 2.
Calibration curve for the prediction of menstrual recovery in all patients. (a) Internal validation with development set of patients enrolled in 2009–2011 (n = 957). (b) Validation with patients enrolled in 2012 and 2013 (n = 341)
Discussion
This predictive model estimated the timing of menstrual recovery in premenopausal women with hormone receptor-positive breast cancer treated with chemotherapy and adjuvant tamoxifen. In this study, the data of approximately 1,300 patients on menstruation resumption in a randomized controlled trial were analyzed, and the timing of menstrual resumption was accurately recorded. The main objective of this study was to determine the probability of menstrual recovery over time from the end of chemotherapy. The annual likelihood of menstrual recovery in the three-year follow-up after chemotherapy was mostly related to the patient’s age and the type and duration of chemotherapy. Younger age at the end of chemotherapy, anthracycline-based chemotherapy without taxanes, and shorter duration of chemotherapy were predictors of early menstrual recovery. Using these predictors, the probability of menstrual recovery per year after completing chemotherapy could be calculated using a simplified scoring system.
The factors used in this menstrual recovery prediction model were patient age and the type and duration of chemotherapy. Several factors were known to be related to menstrual recovery after chemotherapy induced amenorrhea (CIA), which have been reported in various studies. Age was the strongest predictor of menstrual recovery in this study, consistent with the findings of previous studies [13–19]. Given that the possibility of menstrual recovery after CIA was extremely low in those aged 45 years or older, this study is one of the largest-scale population studies that analyzed more than 1,000 women with a median age of 40 years and represents a subset of patients who require menstrual recovery prediction [14, 18]. Factors other than age showed conflicting results in different studies owing to the diversity of the design and population characteristics of each study. This study subjects received an anthracycline with or without taxane regimen, with 95% of them concurrently receiving cyclophosphamide [20]. Anthracycline/cyclophosphamide regimen is associated with a low to intermediate risk of amenorrhea, and this risk is known to be increased in women aged 40 or older [21]. Although some studies have shown conflicting results [22, 23], a large-scale meta-analysis has shown that risk of CIA increased when taxane is added to anthracycline [24]. In our study, we have also confirmed that the addition of taxane predicts a reduced likelihood of menstrual recovery. In addition to chemotherapy regimen, chemotherapy duration was identified as an independent factor predicting menstrual recovery in this study [25]. The duration of chemotherapy is also known as a risk factor for CIA. However, our study could not conclusively determine whether this result is due to the addition of taxanes or the absence of cyclophosphamide. We assume that the impact of the duration of chemotherapy, when combined with age and the regimen, on predicting menstrual recovery is influenced by complex interactions with other factors. Currently, there is a shifting trend in preferred chemotherapy regimens for hormone receptor-positive breast cancer patients from anthracycline to taxane/cyclophosphamide. This shift may lead to potential challenges in accurately reflecting this model to the treatment of future patients. However, it’s essential to note that this trend can vary by region and institution. In countries or institutions that continue to use anthracycline-based regimens, predicting menstrual recovery and discussing related factors remain significant considerations [26].
In terms of biological markers, despite those 6–8% of patients in this study met the criteria for premenopause based on FSH and E2 levels within three months after chemotherapy, we could not identify baseline FSH and E2 as independent predictors of menstrual recovery. This may be due to the limited number of cases, making it challenging to establish statistical significance. Additionally, since age is a strong factor influencing E2 and FSH changes, its effect may have offset the differences. Also, according to the study protocol, baseline serum analysis including FSH and E2 were taken within 3 months after chemotherapy. Therefore, the lack of reflection of the results of biomarkers before starting chemotherapy is considered a significant limitation that influenced the findings of this study. Obesity has been shown to be associated earlier resumption of menstruation; however, we could not identify a positive effect of higher BMI on menstrual recovery in this study [27]. The rate of obesity defined as BMI > 30 kg/m2 is nearly 5–15-fold lower among Asian populations and the rate of overweight (BMI 25–30 kg/m2) is also lower (up to 10% compared with that in the United States) [28]. We suggest that the relatively low prevalence of overweight or obese patients with breast cancer in this study population is responsible for the lack of statistical relevance. Therefore, there are limitations to applying and interpreting this model in patients from regions with different BMI distributions. Also, in regions with similar BMI distributions, caution should be exercised when applying this model to predict menstrual recovery in patients with higher BMI.
A significant proportion of premenopausal patients are reproductive-aged women (< 40 years). Some patients want to achieve ovarian function suppression from chemotherapy and anti-hormone therapy to improve the oncologic outcome of breast cancer, whereas others want to obtain a chance to resume menstruation and attempt pregnancy and childbirth. Therefore, sufficient consultation between healthcare providers and patients is required, and a tool to predict the changes and timing of menstrual recovery is crucial. While some patients may experience symptoms related to ovarian dysfunction despite menstrual recovery, evaluating more objective factors such as Anti-Mullerian Hormone (AMH) or antral follicle count may be helpful [29]. However, the advantage of this model is that the resume of menstruation is a more intuitive surrogate indicator of ovarian function for patients. It allows the prediction of probabilities based on easily accessible factors like age, regimen of chemotherapy agents used, and the duration of chemotherapy. This model may help predict the timing for patients who desire to have children and wish to attempt natural conception. Current guidelines and consensus consistently emphasize the importance of providing fertility counseling to women with breast cancer not only at childbearing age but also in all premenopausal women who desire conception. Clinical evidence for discontinuing antihormone therapy during pregnancy is still lacking; however, according to the recent result, temporary interruption of endocrine therapy is not inferior in terms of short-term disease-free survival, thus encouraging active onco-fertility counseling [30]. The probability of CIA occurrence associated with tamoxifen intake is conflicting. In the IBCSG trial 13–93, CIA was significantly associated with older age, regardless of whether they received tamoxifen [17]. However, in the NSABP B-30 trial, premenopausal women receiving tamoxifen experienced prolonged age-dependent amenorrhea in the doxorubicin, cyclophosphamide, and docetaxel arms [31]. Patients undergoing tamoxifen therapy may experience amenorrhea or irregular menses, which should not be interpreted as an indicator of infertility. While the restoration of menses is a necessary condition for natural conception, it does not guarantee successful pregnancy outcomes. Given that tamoxifen was initially developed as a fertility agent and possesses potential teratogenic effects, there remains a risk of unintended pregnancy even in the absence of menstruation. Consequently, it is imperative to advise the use of non-hormonal contraception during tamoxifen treatment, irrespective of menstrual status. Our study population reflects these patients, especially those who received tamoxifen in one of the largest cohort prospective analyses of ovarian function restoration, with the longest follow-up period to date. Predicting whether and when menstrual recovery will occur in these patients will greatly assist with CIA issues. Although most patients received adjuvant tamoxifen, information on the discontinuation of endocrine therapy, which may have influenced menstruation, was unavailable. This study does not include patients treated with aromatase inhibitors after CIA. Aromatase inhibitors can lead to the recovery of ovarian function and are more commonly associated with relatively young age [32]. Therefore, there are limitations to applying this model to premenopausal women taking aromatase inhibitors. As the study population comprised Korean women, direct application to other populations should be carefully considered. Yet, clinical decision-making for individual patients often requires nuanced consideration of their unique circumstances, medical history, preferences, and response to treatment. Therefore, while this scoring systems can offer valuable insights into overall menstruation recovery possibilities, direct application to guiding specific clinical interventions for individual patients may be limited. Although the model showed reasonable predictive accuracy (C-index 0.683 for development and 0.680 for internal validation), its performance was less reliable in certain probability ranges, with modest discrimination overall (C-index 0.67 for external validation). While useful, the model should be applied cautiously, particularly for patients with moderate menstrual recovery probabilities. Clinicians must integrate this scoring system outputs with their clinical expertise and patient-specific factors to make informed decisions tailored to each patient’s needs.
In conclusion, our prediction model demonstrated that young age, anthracycline without taxanes, and short chemotherapy duration were positively correlated with earlier menstrual recovery. This prediction model will help guide clinicians in counseling premenopausal women regarding fertility preservation and CIA issues. Further refinement of the model and additional external validation studies may be necessary to improve predictive accuracy across all probability ranges. These steps would help enhance the reliability of the model and its utility in diverse clinical settings.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank all patients who participated in the ASTRRA trial, the participating investigators, and the Korean Breast Cancer Study Group.
Author contributions
YJL and HJK; Data curation: All authors; Roles/Writing - original draft: YJL, SK, and HJK; Writing - review and editing: All authors.
Funding
This research was supported by a grant of Patient-Centered Clinical Research Coordinating Center (PACEN) funded by the Ministry of Health & Welfare, Republic of Korea (grant number : RS-2024-00396822).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the institutional review boards of all participating institutions. The requirement for informed consent was waived by the institutional review board of each institution owing to the retrospective nature of the study.
Consent for publication
Not applicable, as no individually identifiable information is included.
Competing interests
The authors declare no competing interests.
Declarations of generative AI in scientific writing
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Francis PA, Regan MM, Fleming GF, Lang I, Ciruelos E, Bellet M, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015;372:436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim H-A, Lee JW, Nam SJ, Park B-W, Im S-A, Lee ES, et al. Adding ovarian suppression to tamoxifen for premenopausal breast cancer: a randomized phase III trial. J Clin Oncol. 2020;38:434–43. [DOI] [PubMed] [Google Scholar]
- 3.Pagani O, Regan MM, Walley BA, Fleming GF, Colleoni M, Lang I, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014;371:107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta P, Sturdee DW, Palin SL, Majumder K, Fear R, Marshall T, Paterson I. Menopausal symptoms in women treated for breast cancer: the prevalence and severity of symptoms and their perceived effects on quality of life. Climacteric. 2006;9(1):49–58. [DOI] [PubMed] [Google Scholar]
- 5.Franzoi MA, Agostinetto E, Perachino M, Del Mastro L, de Azambuja E, Vaz-Luis I, et al. Evidence-based approaches for the management of side-effects of adjuvant endocrine therapy in patients with breast cancer. Lancet Oncol. 2021;22:e303–13. [DOI] [PubMed] [Google Scholar]
- 6.Saha P, Regan MM, Pagani O, Francis PA, Walley BA, Ribi K, et al. Treatment efficacy, adherence, and quality of life among women younger than 35 years in the international breast cancer study group TEXT and SOFT adjuvant endocrine therapy trials. J Clin Oncol. 2017;35:3113–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Massarotti C, Scaruffi P, Lambertini M, Sozzi F, Remorgida V, Anserini P. Beyond fertility preservation: role of the oncofertility unit in the reproductive and gynecological follow-up of young cancer patients. Hum Reprod. 2019;34:1462–9. [DOI] [PubMed] [Google Scholar]
- 8.Silva C, Caramelo O, Almeida-Santos T, Ribeiro Rama AC. Factors associated with ovarian function recovery after chemotherapy for breast cancer: a systematic review and meta-analysis. Hum Reprod, 31(12), 2737–49. [DOI] [PubMed]
- 9.Walshe JM, Denduluri N, Swain SM. Amenorrhea in premenopausal women after adjuvant chemotherapy for breast cancer. J Clin Oncol. 2006;24:5769–79. [DOI] [PubMed] [Google Scholar]
- 10.Kramer R, Tham YL, Sexton K, Friedman L, Weiss H. Chemotherapy-induced amenorrhea is increased in patients treated with adjuvant doxorubicin and cyclophosphamide (AC) followed by a taxane (T). J Clin Oncol. 2005;23:s41–s. [DOI] [PubMed] [Google Scholar]
- 11.Swain SM, Land SR, Sundry R, Ritter M, Costantino J, Wolmark N, et al. Amenorrhea in premenopausal women on the doxorubicin (A) and cyclophosphamide (C)-> docetaxel (T) arm of NSABP B-30: preliminary results. J Clin Oncol. 2005;23:s13–s. [Google Scholar]
- 12.International Breast Cancer, Study G, Colleoni M, Gelber S, Goldhirsch A, Aebi S, Castiglione-Gertsch M, et al. Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: international breast Cancer Study Group Trial 13–93. J Clin Oncol. 2006;24:1332–41. [DOI] [PubMed] [Google Scholar]
- 13.Rosendahl M, Ahlgren J, Andersen J, Bergh J, Blomquist C, Lidbrink E, et al. The risk of amenorrhoea after adjuvant chemotherapy for early stage breast cancer is related to inter-individual variations in chemotherapy-induced leukocyte nadir in young patients: data from the randomised SBG 2000-1 study. Eur J Cancer. 2009;45:3198–204. [DOI] [PubMed] [Google Scholar]
- 14.Pérez-Fidalgo JA, Roselló S, García-Garré E, Jordá E, Martín-Martorell P, Bermejo B, et al. Incidence of chemotherapy-induced amenorrhea in hormone-sensitive breast cancer patients: the impact of addition of taxanes to anthracycline-based regimens. Breast Cancer Res Treat. 2010;120:245–51. [DOI] [PubMed] [Google Scholar]
- 15.Swain SM, Jeong J-H, Geyer CE Jr, Costantino JP, Pajon ER, Fehrenbacher L, et al. Longer therapy, iatrogenic amenorrhea, and survival in early breast cancer. N Engl J Med. 2010;362:2053–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pagani O, O’Neill A, Castiglione M, Gelber R, Goldhirsch A, Rudenstam C-M, et al. Prognostic impact of amenorrhoea after adjuvant chemotherapy in premenopausal breast cancer patients with axillary node involvement: results of the international breast Cancer Study Group (IBCSG) Trial VI. Eur J Cancer. 1998;34:632–40. [DOI] [PubMed] [Google Scholar]
- 17.Colleoni M, Gelber S, Goldhirsch A, Aebi S, Castiglione-Gertsch M, Price KN, et al. Tamoxifen after adjuvant chemotherapy for premenopausal women with lymph node-positive breast cancer: international breast Cancer Study Group Trial 13–93. J Clin Oncol. 2006;24:1332–41. [DOI] [PubMed] [Google Scholar]
- 18.Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: understanding women’s health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–14. [DOI] [PubMed] [Google Scholar]
- 19.Canney P, Coleman R, Morden J, Barrett-Lee P, Banerji J, Wardley A et al. 200 TACT2 trial in early breast cancer (EBC): differential rates of amenorrhoea in premenopausal women following adjuvant epirubicin (E) or accelerated epirubicin (aE) followed by capecitabine (X) or CMF (CRUK/05/019). Eur J Cancer. 2012:S102.
- 20.Kim HA, Ahn SH, Nam SJ, Park S, Ro J, Im SA, Jung YS, Yoon JH, Hur MH, Choi YJ, Lee SJ. The role of the addition of ovarian suppression to tamoxifen in young women with hormone-sensitive breast cancer who remain premenopausal or regain menstruation after chemotherapy (ASTRRA): study protocol for a randomized controlled trial and progress. BMC Cancer. 2016;16(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology Recommendations on Fertility Preservation in Cancer patients. J Clin Oncol. 2006;24(18):2917–31. [DOI] [PubMed] [Google Scholar]
- 22.Najafi S, Djavid GE, Mehrdad N, Rajaii E, Alavi N, Olfatbakhsh A, et al. Taxane-based regimens as a risk factor for chemotherapy-induced amenorrhea. Menopause. 2011;18:208–12. [DOI] [PubMed] [Google Scholar]
- 23.Martin M, Pienkowski T, Mackey J, Pawlicki M, Guastalla J-P, Weaver C, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–13. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Li Y, Liang J, Zhang N, Yang Q. Chemotherapy-induced amenorrhea and its prognostic significance in premenopausal women with breast cancer: an updated meta-analysis. Front Oncol. 2022;12. [DOI] [PMC free article] [PubMed]
- 25.Sukumvanich P, Case LD, Van Zee K, Singletary SE, Paskett ED, Petrek JA, Naftalis E, Naughton MJ. Incidence and time course of bleeding after long-term amenorrhea after breast cancer treatment: a prospective study. Cancer. 2010;116(13):3102–11. [DOI] [PubMed] [Google Scholar]
- 26.Harbeck N, Burstein HJ, Hurvitz SA, Johnston S, Vidal GA. A look at current and potential treatment approaches for hormone receptor-positive, HER2‐negative early breast cancer. Cancer. 2022;128:2209–23. [DOI] [PubMed] [Google Scholar]
- 27.Pistilli B, Mazouni C, Zingarello A, Faron M, Saghatchian M, Grynberg M, et al. Individualized prediction of menses recovery after chemotherapy for early-stage breast cancer: a nomogram developed from UNICANCER PACS04 and PACS05 trials. Clin Breast Cancer. 2019;19:63–70. [DOI] [PubMed] [Google Scholar]
- 28.Ramachandran A, Chamukuttan S, Shetty SA, Arun N, Susairaj P. Obesity in Asia–is it different from rest of the world. Diabetes Metab Res Rev. 2012;28(Suppl 2):47–51. [DOI] [PubMed] [Google Scholar]
- 29.Kim HA, Choi J, Park CS, Seong MK, Hong SE, Kim JS, Noh WC, et al. Post-chemotherapy serum anti-Müllerian hormone level predicts ovarian function recovery. Endocr Connections. 208;78:949–56. [DOI] [PMC free article] [PubMed]
- 30.Partridge AH, Niman SM, Ruggeri M, Peccatori FA, Azim HA Jr, Colleoni M, Saura C, Shimizu C, Sætersdal AB, Kroep JR, Mailliez A. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med. 2023;388(18):1645–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swain SM, Land SR, Ritter MW, Costantino JP, Cecchini RS, Mamounas EP, et al. Amenorrhea in premenopausal women on the doxorubicin-and-cyclophosphamide-followed-by-docetaxel arm of NSABP B-30 trial. Breast Cancer Res Treat. 2009;113:315–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henry NL, Xia R, Banerjee M, Gersch C, McConnell D, Giacherio D, Schott AF, Pearlman M, Stearns V, Partridge AH, Hayes DF. Predictors of recovery of ovarian function during aromatase inhibitor therapy. Ann Oncol. 2013;24(8):2011–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.