Abstract
Background
The exact cause of vitiligo is still unknown. Genetic factors, self-destruction of melanocytes, the autoimmune process, and oxidative stress all can contribute to the pathogenesis of vitiligo.
Objectives
The aim of this study was to figure out the frequency of coexisting autoimmune and autoinflammatory diseases (AIIDs) in Egyptian patients with vitiligo and identify the associated risk factors.
Materials and methods
Egyptian children and adults with vitiligo and their parents were asked to answer a web-based survey. The survey consisted of multiple questions centered around demographic, clinical, and therapeutic data. The vitiligo disease activity (VIDA) score was evaluated for all the patients. Patients were also asked about the presence of co-existing AIIDs.
Results
There was a total of 294 participants, mostly females (54.8%), with a median age of 35 years and a median disease duration of 9 years. Nearly 27% had at least one AIID. The most common associated AIIDs were autoimmune thyroid disease (47 patients, 16%), followed by alopecia areata (14 patients,4.8%), then psoriasis and rheumatoid arthritis (11 patients, 3.7%). Univariate regression analysis revealed that age (OR 1.02, P = 0.036), female gender (OR 2.2, P = 0.004), disease duration (OR 1.04, P < 0.001), affected body surface area (OR 1.7, P = 0.048), and family history of AIIDs (OR 2.7, P < 0.001) were predictors for the presence of AIIDs in patients with vitiligo.
Conclusion
AIIDs are prevalent among vitiligo patients. Age, female gender, and family history of AIIDs are the main predictors of the presence of AIIDs in vitiligo patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41927-024-00427-1.
Keywords: Vitiligo, Autoimmune diseases, Autoinflammatory diseases, Egypt
Introduction
Vitiligo is the primary depigmentation of the skin, which may be generalized or circumscribed. Vitiligo affects nearly 1% of the general population, regardless of gender, ethnic group, or geographic area [1]. Quality of life is negatively affected in patients with vitiligo, mainly due to negative social stigma comparable to other oppressive skin diseases such as eczema and psoriasis. Uncovered parts like the face and the hands are commonly affected, leading to psychological distress and fear of the spread of the disease [2]. Unfortunately, there are limited therapeutic options; however, phototherapy remains the first therapeutic option [3].
The exact cause of vitiligo is still unknown. Genetic factors, self-destruction of melanocytes, the autoimmune process, and oxidative stress all can contribute to the pathogenesis of vitiligo [4]. The autoimmune mechanism is the most accepted, but the exact mechanism is still debatable. Vitiligo has several features of autoimmune diseases, such as identification of autoantibodies and lymphocyte infiltration in the affected areas, association with certain major histocompatibility haplotypes, association with certain autoimmune diseases, and response to immune suppressive treatment [5].
In patients with vitiligo, adaptation of melanocytes to stress is impaired, leading to the release of damage-associated molecular patterns (DAMPs), which leads to activation of innate immune cells as dendritic cells and subsequently T cell activation [6]. Recent evidence suggests that T cells play a central role in melanocyte destruction and interferon gamma exaggerates the immune response [7]. CD4 and CD8 lymphocytes were found in skin histology in addition to different circulating cytokines such as interferon (IFN) gamma, interleukin (IL)-1, transforming growth factor (TGF) beta, chemokines, antibodies, and markers of oxidative stress [8]. Interleukin 17 (IL17) was found to be high in patients with vitiligo and has a positive correlation with disease activity. Ultraviolet B phototherapy, which is used for treatment of vitiligo, can affect IL-17. Also, IL-17 has been involved in the pathogenesis of chronic skin inflammatory conditions such as psoriasis, alopecia areata and atopic dermatitis, in addition to other autoimmune diseases [9].
The association between vitiligo and autoimmune diseases is well known; however, the frequency and type of associated autoimmune diseases are variable according to the studied population [10]. The increased frequency of autoimmune diseases was found in both sporadic and familial cases of vitiligo, suggesting that immune intolerance with vitiligo is not related to genetic factors [11]. There is a reported association between vitiligo and thyroid diseases, type 1 diabetes mellitus, Addison disease, pernicious anemia, alopecia areata, systemic lupus erythematosus, rheumatoid arthritis, and inflammatory bowel disease. In addition, vitiligo is included in autoimmune polyglandular syndrome type 1 and type 2 [12]. Thyroid diseases are quite common in patients with vitiligo, and vitiligo may precede the onset of thyroid diseases by several years [13].
There is limited research in the literature that assesses how common autoimmune disorders are among individuals with vitiligo. Furthermore, the clinical significance of these coexisting autoimmune conditions must be elucidated to ensure the appropriate care of vitiligo in relation to long-term prognosis. Our aim of this study was to figure out the frequency of coexisting autoimmune conditions in Egyptian patients with vitiligo and identify the associated risk factors.
Patients and methods
Study design and setting
This cross-sectional study was conducted on vitiligo patients from Egypt. It was a survey-based study, and either the participants or their parents were required to complete a self-administered online questionnaire produced with Google Forms. All Egyptian patients who have been diagnosed with vitiligo were eligible to participate in the study. Patients with malignancies or neurological diseases were excluded from the study at the outset. The questionnaire was randomly disseminated to all potential subjects through social media platforms, including Facebook and WhatsApp, from January to May 2020.
Subsequently, they were directed to a website outlining the study’s objectives and instructions on how to fill out the questionnaire. Participants were guaranteed anonymity and the confidentiality of their data. All individuals who consented to partake in the research were redirected to the Google Form. Informed consent was obtained from all participants.
Sample size calculation
The online sample size calculator RaoSoftR was used to calculate the appropriate sample size. Based on an estimated population of Egyptian vitiligo patients of 1.5 million, 50% predicted response, 5% margin of error, and 90% confidence level, the minimum sample size was 271 participants.
Ethical consideration
The present study was carried out in adherence to the guidelines outlined in the Helsinki Declaration [14]. Informed consent was obtained from all participants. Furthermore, the Institutional Research Board of the Faculty of Medicine at Mansoura University granted approval for the study protocol (approval registration number: R.24.02.2494.R2).
Questionnaire structure
Baseline characteristics and clinical data
Patients were asked about demographic data, including age and gender. Clinical data was collected, including vitiligo duration, age of onset, and course of vitiligo, whether stationary, progressive, or regressive. Patients were asked about the percentage of body surface area affected, the distribution of vitiligo, whether affecting the body, face, or both, and the laterality of affection, whether unilateral, midline, or bilateral. Family history of vitiligo and family history of autoimmune diseases were asked about. Patients were asked about the treatment they are taking, whether local, systemic, or both.
Vitiligo disease activity (VIDA) score
VIDA (vitiligo disease activity score) was evaluated for all the patients. This is a six-point scale for assessing vitiligo activity. Patients were asked about their own opinion of the present disease activity over time. Expansion of existing lesions or appearance of new lesions indicates active vitiligo. Grading was done as follows: VIDA Score + 4 means active vitiligo within 6 weeks or less duration, + 3 means active vitiligo within 6 weeks to 3 months, + 2 means active vitiligo within 3 to 6 months, + 1 means active vitiligo within 6 to 12 months, 0 means stable disease for 1 year or more, and − 1 means stable disease with spontaneous regimentation for 1 year or more. A lower VIDA score indicates less activity, and a higher score indicates higher activity [15].
Autoimmune and autoinflammatory diseases (AIIDs)
Patients were also asked about the presence of co-existing AIIDs, provided that diagnosis of AIIDs was confirmed by a specialized physician, as shown in Supplementary File 1.
Statistical analysis
The data analysis was conducted using the Statistical Package for Social Science (SPSS) version 22 program. Quantitative data was presented using mean and standard deviations (SD) for parametric variables and median (min-max) for nonparametric variables. Qualitative data were presented utilizing percentages and numbers. The Shapiro-Wilk test was performed to see whether the variable’s distribution was normal. The independent samples t test was used to determine whether there was a statistically significant difference between two groups when the data were normally distributed; however, the Mann-Whitney test was utilized when the variables in question were not parametric. To make comparisons between qualitative variables, we employed either the Chi-square test or the Fisher exact test, as applicable. Univariate logistic regression analysis was used to identify factors linked with AIIDs in patients with vitiligo using the enter approach to assess the predictors of AIIDs. The goodness of fit for the model was tested using chi-square goodness of fit tests. A p value of less than 0.05 was considered statistically significant.
Results
This study was conducted on 294 patients. Their median age was 35 years, ranging from 3 years to 86 years. The most common age group was from 18 to 39 years (153 patients), followed by the age group from 40 to 59 years (88 patients), and the least common age group was more than 80 years (1 patient). Nearly 55% of patients were females (161 patients). The median duration of vitiligo was 9 years, ranging from 1 month to 54 years. The median age of onset of vitiligo was 24 years, ranging from 1 year to 84 years. As regards the course of vitiligo, it was progressive in 206 patients (70.1%), stationary in 67 (22.8%), and regressive in 21(7.1%) patients. Most patients have affected body surface from 1 to 25% (134 patients) and from 26 to 50% (131 patients). Only 16 patients had affected body surface area from 51 to 75%, and 13 patients had from 76 to 100% affected body surface area. More than 91% of patients had vitiligo in either the face or the body (268 patients), and the rest of patients had both face and body affection (26 patients). Unilateral or midline affection was found in 241 patients (82%), and bilateral affection was found in 53 patients (18%). A family history of vitiligo and AIIDs was found in 62 (21.1%) and 74(25.4%) of patients, respectively. As regards the vitiligo activity score, it was + 4 in 32 patients (10.9%), + 3 in 57 patients (19.4%), + 2 in 77 patients (26.2%), + 1 in 35 patients (11.9%), 0 in 32 patients (10.9%), and − 1 in 61 patients (20.7%). 48% of patients were not treated (141 patients). Local treatment was received by 131 patients (44.6%). Systemic treatment was received by 12 patients (4.1%), and 3.4% of patients received both local and systemic treatment (Table 1).
Table 1.
Demographic and clinical characteristics of study patients with vitiligo (n = 294)
| Variable n (%), median (min-max) |
The study sample (n = 294) |
|---|---|
| Age, years | 35 (3–86) |
| < 18 | 35 (11.9) |
| 18–39 | 153 (52.0) |
| 40–59 | 88 (29.9) |
| 60–79 | 17 (5.8) |
| ≥ 80 | 1 (0.3) |
| Sex | |
| Male | 133 (45.2) |
| Female | 161 (54.8) |
| Disease duration | 9 (0.10–54) |
| Age at vitiligo onset | 24 (1–84) |
| Course of the disease | |
| Stationary | 67 (22.8) |
| Regressive | 21 (7.1) |
| Progressive | 206 (70.1) |
|
Affected BSA, % 1–25 |
134 (45.6) |
| 26–50 | 131 (44.6) |
| 51–75 | 16 (5.4) |
| 76–100 | 13 (4.4) |
| Distribution | |
| Face or body | 268 (91.2) |
| Face and body | 26 (8.8) |
| Laterality | |
| Unilateral/midline | 241 (82.0) |
| Bilateral | 53 (18.0) |
| Family history of vitiligo | 62 (21.1) |
| Family history of AIIDs | 74 (25.2) |
| VIDA | |
| + 4 | 32 (10.9) |
| + 3 | 57 (19.4) |
| + 2 | 77 (26.2) |
| + 1 | 35 (11.9) |
| 0 | 32 (10.9) |
| -1 | 61 (20.7 |
| Treatment | |
| No | 141 (48) |
| Local | 131 (44.6) |
| Systemic | 12 (4.1) |
| Both local and systemic | 10 (3.4) |
AIIDs: autoimmune and autoinflammatory diseases, BSA: body surface area, VIDA: Vitiligo Disease Activity Score
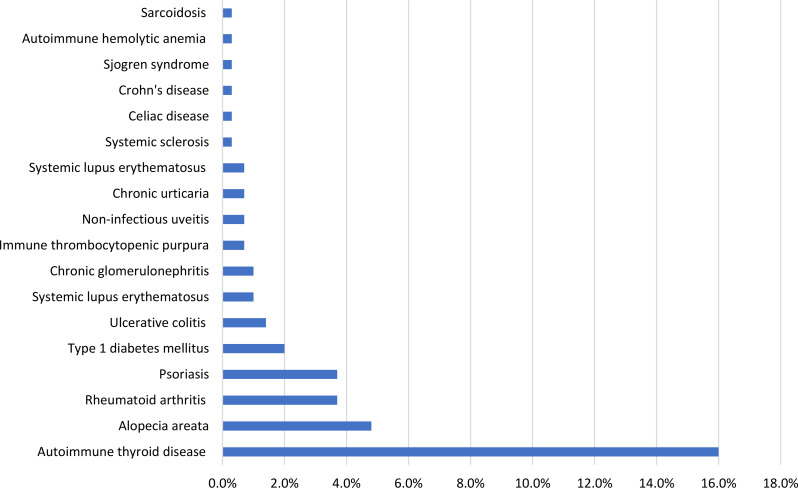
As shown in Fig. 1, associated AIIDs were found in 80 patients (27.2%). The most common associated AIID was autoimmune thyroid disease (47 patients, 16%), followed by alopecia areata (14 patients, 4.8%), then psoriasis and rheumatoid arthritis (11 patients, 3.7%). The least common associated AIIDs were Crohn’s disease, systemic sclerosis, celiac disease, Sjogren syndrome, autoimmune hemolytic anemia, and sarcoidosis (1 patient, 0.3%). Table 2 illustrates the number of associated AIIDs per individual in the study of vitiligo patients.
Fig. 1.
Distribution of associated autoimmune and autoinflammatory diseases among the study patients with vitiligo (n = 294)
Table 2.
Number of autoimmune and autoinflammatory diseases per individual among the studied vitiligo patients (n = 294)
| Associated autoimmune and autoinflammatory disease | The study sample (n = 294) n (%) |
|---|---|
|
No autoimmune disease At least 1 AIID At least 2 AIIDs At least 3 AIIDs More than 3 AIIDs |
214 (72.8) 58 (19.7) 16 (5.4) 4 (1.4) 2 (0.7) |
AIIDs: autoimmune and autoinflammatory diseases
Table 3 shows the difference between vitiligo patients with and without AIIDs. Vitiligo patients with AIIDs were significantly older than patients without AIIDs (36.5, 35 years, P < 0.001). The percentage of females in patients with AIIDs was significantly higher than in patients without AIIDs (68.8%, 49.5%, P = 0.003). Vitiligo disease duration was significantly higher in patients with AIIDs than in patients without AIIDs (11.5, 8 years, P = 0.001). There was no significant difference between vitiligo patients with and without AIIDs as regard age of onset and disease course. As regards vitiligo distribution, there was no significant difference between both groups as regard body surface area affected, face or body affection, and laterality. Family history of AIIDs was significantly higher in vitiligo patients with AIIDs than in patients without AIIDs (40%, 19.6%, P < 0.001). However, there was no significant difference between both groups as regard family history of vitiligo, VIDA score, and treatment.
Table 3.
Demographic and clinical data of the study vitiligo patients with and without autoimmune and autoinflammatory disease (n = 294)
| Variable n (%), median (min-max) |
Autoimmune and autoinflammatory diseases | P | |
|---|---|---|---|
| Without (n = 214) |
With (n = 80) |
||
| Age, years | 35 (3–86) | 36.50 (8–78) | < 0.001* |
|
< 18 18–39 40–59 60–79 ≥ 80 |
29 (13.6) 111 (51.9) 64 (29.9) 9 (4.2) 1 (0.5) |
6 (7.5) 42 (52.5) 24 (30.0) 8 (10.0) - |
0.235 |
| Sex | |||
|
Male Female |
108 (50.5) 106 (49.5) |
25 (31.3) 55 (68.8) |
0.003* |
| Disease duration | 8 (0.10–54) | 11.50 (0.30–50) | 0.001* |
| Age at vitiligo onset | 24 (1–84) | 20.50 (2–75) | 0.331 |
| Course of the disease | |||
|
Stationary Regressive Progressive |
50 (23.4) 14 (6.5) 150 (70.1) |
17 (21.3) 7 (8.8) 56 (70) |
0.774 |
| Affected BSA, % | |||
|
1–25 26–50 51–75 76–100 |
90 (42.1) 98 (45.8) 15 (7) 11 (5.1) |
44 (55) 33 (41.3) 1 (1.3) 2 (2.5) |
0.076 |
| Distribution | |||
|
Face or body Face and body |
192 (89.7) 22 (10.3) |
76 (95) 4 (5) |
0.175 |
| Laterality | |||
|
Unilateral/midline Bilateral |
170 (79.4) 44 (20.6) |
71 (88.8) 9 (11.3) |
0.065 |
| Family history of vitiligo | 44 (20.6) | 18 (22.5) | 0.717 |
| Family history of AIIDs | 42 (19.6) | 32 (40) | < 0.001* |
| VIDA | |||
|
+ 4 + 3 + 2 + 1 0 -1 |
39 (18.2) 23 (10.7) 27 (12.6) 57 (26.6) 45 (21.0) 23 (10.7) |
22 (27.5) 9 (11.3) 8 (10.0) 20 (25.0) 12 (15.0) 9 (11.3) |
0.560 |
| Treatment | |||
| No | 95 (44.4) | 46 (57.5) | 0.149 |
| Local | 100 (46.7) | 31 (38.8) | |
| Systemic | 11 (5.1) | 1 (1.3) | |
| Both local and systemic | 8 (3.7) | 2 (2.5) | |
*p < 0.05
AIIDs: autoimmune and autoinflammatory diseases
Univariate regression analysis revealed that age (OR 1.02, P = 0.036), female gender (OR 2.2, P = 0.004), disease duration (OR 1.04, P < 0.001), affected body surface area (OR 1.7, P = 0.048), and family history of AIIDs (OR 2.7, P < 0.001) were predictors for the presence of AIIDs in patients with vitiligo. With multivariate regression analysis, family history of AIIDs was found to be the main predictor for the presence of AIIDs in patients with vitiligo (OR 2.4, P = 0.005) as shown in Table 4.
Table 4.
Univariate and multivariate regression analyses of predictive factors of the presence of autoimmune and autoinflammatory diseases in patients with vitiligo (n = 294)
| Variable | Univariate regression | Multivariate regression | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Age, years | 1.019 | 1.001–1.037 | 0.036* | 0.850 | 0.236–3.056 | 0.803 |
| Female gender | 2.242 | 1.302–3.860 | 0.004* | 1.470 | 0.801–2.697 | 0.213 |
| Disease duration | 1.040 | 1.018–1.061 | < 0.001* | 1.240 | 0.345–4.456 | 0.742 |
| Age at vitiligo onset | 0.992 | 0.974–1.009 | 0.353 | 1.188 | 0.330–4.269 | 0.792 |
| Affected BSA (1–25%) | 1.684 | 1.004–2.825 | 0.048* | 0.947 | 0.508–1.763 | 0.863 |
| Family history of vitiligo | 1.122 | 0.603–2.087 | 0.717 | 0.788 | 0.376–1.652 | 0.529 |
| Family history of AIIDs | 2.730 | 1.559–4.781 | < 0.001* | 2.436 | 1.310–4.530 | 0.005* |
| VIDA | 1.120 | 0.959–1.308 | 0.152 | 1.161 | 0.978–1.377 | 0.088 |
| Constant | - | - | - | 0.085 | - | < 0.001 |
*p < 0.05
AIIDs: autoimmune and autoinflammatory diseases, VIDA: Vitiligo Disease Activity Score
Discussion
This study was conducted to evaluate AIIDs associated with vitiligo. Nearly 27% of patients had at least one AIID. The most common AIID associated with vitiligo was autoimmune thyroid disease, followed by alopecia areata. There was a significant difference between vitiligo patients with and without AIIDs as regard age, gender, vitiligo duration, and family history of AIIDs. The family history of AIIDs was the main predictor for the development of AIIDs.
The prevalence of AIIDs in this study was 27.2%. A lower percentage was reported in another retrospective study conducted on 209 Indian patients (2.9%) [16]. About 20% of vitiligo patients were reported to have associated AIIDs in other retrospective and cross-sectional studies [12, 17, 18]. However, in another study conducted on 175 patients, more than 40% were found to have associated AIIDs. This difference may be caused by the use of thyroid profile and autoantibodies beside clinical evaluation for detection of associated AIIDs in the previous study [19].When it comes to general population, estimates of the prevalence of AIIDs, indicate that all AIIDs collectively impact approximately one in ten individuals [20].In general, the estimates of the annual increases in the overall global incidence and prevalence of AIIDs in general population are 19.1% and 12.5%, respectively [21].
In this study, the most commonly associated AIID was autoimmune thyroid diseases. Other previous retrospective and cross-sectional studies in which data was collected either from patient charts, patient clinical evaluation, or clinical and laboratory evaluation demonstrated that autoimmune thyroid diseases were also the most common associated AIIDs [12, 17–19, 22]. About 16% of patients in this study had autoimmune thyroid diseases. A higher percentage was found in other studies that used the laboratory beside clinical evaluation (20.4%, 37%) [19, 22]. However, a lower percentage (around 12%) was reported in other studies [12, 17, 18, 23]. This difference in the results may be related to differences in the method of evaluation and differences in ethnic groups. In another cross-sectional study conducted on more than 73 thousand patients, vitiligo patients were at higher risk for Grave’s disease and Hashimoto thyroiditis than the general population [24]. Similarly, thyroid autoantibodies and thyroid disorders were significantly higher in patients with vitiligo than in healthy persons in other different case-control studies [25–30].
As regard the association between vitiligo and other autoimmune skin disease diseases, alopecia areata was the second most common associated AIID and the most common associated autoimmune skin disease, followed by psoriasis and then chronic urticaria in this study. Similarly, alopecia areata was the second most common associated AIID in another cross-sectional study [18]. Alopecia areata was found in nearly 5% of vitiligo patients, and this percentage was close to the percentage in a previous study conducted on 133 Japanese patients [17]. In another retrospective study, autoimmune skin diseases such as morphea, alopecia areata, discoid lupus, and pemphigus were the main AIIDs associated with vitiligo [16]. Vitiligo patients were at higher risk for alopecia areata, psoriasis, and discoid lupus compared to healthy persons, as reported by several studies [27, 31–33]. Similarly, patients with alopecia areata were at higher risk of developing vitiligo than the healthy persons [34].
In this study, rheumatoid arthritis emerged as the most common associated connective tissue disease, followed by SLE, systemic sclerosis, and Sjogren syndrome. Multiple previous studies reported that vitiligo patients were at higher risk of having RA and SLE compared to healthy persons [18, 27, 31]. Sjogren syndrome and sarcoidosis were found in 1 patient (0.3%). In a previous cross-sectional study and retrospective cohort, there was a significant association between vitiligo and Sjogren syndrome [18, 35]. In another study conducted to estimate the incidence and prevalence of vitiligo and associated conditions, vitiligo patients were at higher risk of developing Sjogren syndrome than healthy persons [18, 31]. The association between vitiligo and sarcoidosis is not common and was reported in some case reports [36–40].
In the present study, type 1 DM was reported in 2% of vitiligo. DM was more common in vitiligo patients compared to the healthy persons in other studies [27, 30, 31]. Similarly, a significant association between vitiligo and type 1, type 2 DM, hypertension, and obesity was confirmed in previous 2 meta-analyses and a case control study [41–43].
In this study, ulcerative colitis was diagnosed in 1.4% of vitiligo patients; however, Crohn’s disease was diagnosed in 0.3% of patients. Vitiligo patients were at higher risk of developing ulcerative colitis and Crohn’s disease than healthy persons in 2 previous cross-sectional studies conducted to evaluate the relation between vitiligo and associated AIIDs [18, 31]. However, there was no increased risk of inflammatory bowel disease in patients with vitiligo in another large cohort study, which was done to compare the incidence of IBD in patients with and without chronic inflammatory skin diseases [44]. Also, the risk of vitiligo was reported to increase during treatment with biological agents in patients with inflammatory bowel diseases and ankylosing spondylitis in other studies [45–47].
As regards blood diseases, immune thrombocytopenia was found in 2 patients, and autoimmune hemolytic anemia was found in 1 patient. The association of vitiligo with immune thrombocytopenia and with autoimmune hemolytic anemia was reported in some case reports [48, 49]. An association between vitiligo and pernicious anemia was reported in a previous cross-sectional study [50]. Additionally, pernicious anemia was found in 1.3% of vitiligo patients in a previous retrospective study [12]. The association between noninfectious uveitis and vitiligo was found in 2 patients (0.7%). The association between vitiligo and uveitis is known in cases of Vogt Koyanagi Harada syndrome [51].
This study found that nearly 20% of vitiligo patients had at least one associated AIID. More than one AIID was found in 7% of patients in this study. A lower percentage of patients with more than one AIID (2.8%) was reported in another cross-sectional study conducted on more than one thousand vitiligo patients [18]. This difference may be related to different ethnic groups.
Vitiligo patients associated with AIIDs were significantly older than patients without AIIDs in this study. The percentage of females in vitiligo patients having AIIDs was higher than patients without AIIDs. These results were similar to the results in two previous cross-sectional studies conducted to evaluate the relation between vitiligo and multiple AIIDs [18, 50]. However, in another study conducted to evaluate the relation between vitiligo and associated autoimmune thyroid diseases, the association was stronger for males and younger patients [24].
In this study, there was no difference in the affected BSA between vitiligo patients with and without AIIDs. This was different from the result of a previous study that reported that vitiligo patients associated with AIIDs had higher BSA compared to vitiligo patients without associated AIIDs. This difference may be related to the higher number of patients in the other study [18].
Positive family history of AIIDs was significantly higher in vitiligo patients with AIIDs than those without. Family history of AIIDs was the main predictor for the co-existence of AIIRDs with vitiligo in our cohort. In another study conducted on Japanese vitiligo patients, family history of generalized vitiligo was found in 15% of patients; however, family history of other AIIDs was found in 20% of patients. This result can suggest that there is a genetic susceptibility not only to vitiligo but also to the associated AIIDs [17]. In another study, there was a significant association between gender, family history of AIIDs, and family history of autoimmune thyroid disease and the development of autoimmune thyroid disease in vitiligo patients [52].
This study provides up-to-date information on the frequency of AIIDs in Egyptian individuals with vitiligo. The study’s significant strength was its comparatively large sample size. Our findings enhance the understanding of epidemiology, thereby aiding in the comprehension and management of the disease.
However, it is important to acknowledge some limitations of this study. One major limitation was the cross-sectional design of the study, preventing the establishment of causal relationships. It is crucial to incorporate AIIDs in longitudinal studies to comprehend causality. Secondly, we failed to account for potential confounders like polypharmacy, disease awareness, and social support. Due to the omission of certain factors, we cannot confirm their correlations with multimorbidity and their potential influence on the study results. We did not analyze the specific categories of AIIDs that were grouped together, which could have had varying impacts on vitiligo. Thirdly, it would be better if vitiligo patients receiving treatment either local or systemic were excluded from the start as the treatment would certainly affect the body surface area and VIDA score. Finally, the estimation of the BSA by patients may not be an accurate enough method to evaluate the extension of the disease.
Conclusion
In conclusion, AIIDs are common among vitiligo patients. Age, female gender, and family history of AIIDs are the main predictors of the presence of AIIDs in vitiligo patients. So, early identification and treatment are highly recommended.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Emad Elshebiny (Rheumatology &Immunology Division, Faculty of Medicine, Menoufia) for his assistance.
Author contributions
Conceptualization: ST, FH, Investigation: all authors, Data curation, formal analysis: SH, MKN, writing–original draft: ST, FH, writing–review & editing: All authors.
Funding
Not applicable.
Data availability
Data is provided within the manuscript and supplementary information file.
Declarations
Ethics approval and consent to participate
The present study was carried out in adherence to the guidelines outlined in the Helsinki Declaration [14].Furthermore, the Institutional Research Board of the Faculty of Medicine at Mansoura University granted approval for the study protocol. (approval registration number: R.24.02.2494.R2). All individuals who consented to partake in the research were redirected to the Google Form. Informed consent was obtained from all participants.
Clinical trial number
Not applicable.
Consent for publication
Not Applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang Y, Cai Y, Shi M, Jiang S, Cui S, Wu Y, et al. The prevalence of vitiligo: a meta-analysis. PLoS ONE. 2016;11(9):e0163806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elbuluk N, Ezzedine K. Quality of life, burden of disease, co-morbidities, and systemic effects in vitiligo patients. Dermatol Clin. 2017;35(2):117–28. [DOI] [PubMed] [Google Scholar]
- 3.Zubair R, Hamzavi IH. Phototherapy for vitiligo. Dermatol Clin. 2020;38(1):55–62. [DOI] [PubMed] [Google Scholar]
- 4.Feng Y, Lu Y. Advances in vitiligo: update on therapeutic targets. Front Immunol. 2022;13:986918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kemp EH, Waterman EA, Weetman AP. Autoimmune aspects of vitiligo. Autoimmunity. 2001;34(1):65–77. [DOI] [PubMed] [Google Scholar]
- 6.Cui T, Zhang W, Li S, Chen X, Chang Y, Yi X, et al. Oxidative stress–induced HMGB1 release from melanocytes: a paracrine mechanism underlying the cutaneous inflammation in vitiligo. J Invest Dermatology. 2019;139(10):2174–84. e4. [DOI] [PubMed] [Google Scholar]
- 7.Frisoli ML, Essien K, Harris JE. Vitiligo: mechanisms of pathogenesis and treatment. Annu Rev Immunol. 2020;38:621–48. [DOI] [PubMed] [Google Scholar]
- 8.Speeckaert R, Speeckaert M, De Schepper S, van Geel N. Biomarkers of disease activity in vitiligo: a systematic review. Autoimmun rev. 2017;16(9):937–45. [DOI] [PubMed] [Google Scholar]
- 9.Bernardini N, Skroza N, Tolino E, Mambrin A, Anzalone A, Balduzzi V, et al. IL-17 and its role in inflammatory, autoimmune, and oncological skin diseases: state of art. Int J Dermatol. 2020;59(4):406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanioka M, Yamamoto Y, Katoh M, Takahashi K, Miyachi Y. Vitiligo Vulgaris and autoimmune diseases in Japan: a report from vitiligo clinic in Kyoto University Hospital. Dermato-Endocrinology. 2009;1(1):43–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Li S, Li C. Clinical features, immunopathogenesis, and therapeutic strategies in vitiligo. Clin Rev Allergy Immunol. 2021;61(3):299–323. [DOI] [PubMed] [Google Scholar]
- 12.Sawicki J, Siddha S, Rosen C. Vitiligo and associated autoimmune disease: retrospective review of 300 patients. J Cutan Med Surg. 2012;16(4):261–6. [DOI] [PubMed] [Google Scholar]
- 13.Dahir AM, Thomsen SF. Comorbidities in vitiligo: comprehensive review. Int J Dermatol. 2018;57(10):1157–64. [DOI] [PubMed] [Google Scholar]
- 14.Association GAotWM. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. 2014;81(3):14–8. [PubMed] [Google Scholar]
- 15.Njoo M, Das P, Bos J, Westerhof W. Association of the Köbner phenomenon with disease activity and therapeutic responsiveness in vitiligo vulgaris. Arch Dermatol. 1999;135(4):407–13. [DOI] [PubMed] [Google Scholar]
- 16.Poojary S. Vitiligo and associated autoimmune disorders: a retrospective hospital-based study in Mumbai, India. Allergol Immunopathol. 2011;39(6):356–61. [DOI] [PubMed] [Google Scholar]
- 17.Narita T, Oiso N, Fukai K, Kabashima K, Kawada A, Suzuki T. Generalized vitiligo and associated autoimmune diseases in Japanese patients and their families. Allergology Int. 2011;60(4):505–8. [DOI] [PubMed] [Google Scholar]
- 18.Gill L, Zarbo A, Isedeh P, Jacobsen G, Lim HW, Hamzavi I. Comorbid autoimmune diseases in patients with vitiligo: a cross-sectional study. J Am Acad Dermatol. 2016;74(2):295–302. [DOI] [PubMed] [Google Scholar]
- 19.Ingordo V, Cazzaniga S, Raone B, Digiuseppe MD, Musumeci ML, Fai D, et al. Circulating autoantibodies and autoimmune comorbidities in vitiligo patients: a multicenter Italian study. Dermatology. 2014;228(3):240–9. [DOI] [PubMed] [Google Scholar]
- 20.Conrad N, Misra S, Verbakel JY, Verbeke G, Molenberghs G, Taylor PN, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. 2023;401(10391):1878–90. [DOI] [PubMed] [Google Scholar]
- 21.Miller FW. The increasing prevalence of autoimmunity and autoimmune diseases: an urgent call to action for improved understanding, diagnosis, treatment, and prevention. Curr Opin Immunol. 2023;80:102266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunes DH, Esser LMH. Vitiligo epidemiological profile and the association with thyroid disease. An Bras Dermatol. 2011;86:241–8. [DOI] [PubMed] [Google Scholar]
- 23.Vachiramon V, Harnchoowong S, Onprasert W, Chanprapaph K. Prevalence of thyroid abnormalities in thai patients with vitiligo. BioMed research international. 2017;2017. [DOI] [PMC free article] [PubMed]
- 24.Bae JM, Lee JH, Yun JS, Han B, Han TY. Vitiligo and overt thyroid diseases: a nationwide population-based study in Korea. J Am Acad Dermatol. 2017;76(5):871–8. [DOI] [PubMed] [Google Scholar]
- 25.Kılıc S, Şehitoğlu H, Gül C. Assessment of ADAM17 and ADAM10 proteins with CXCL10 and thyroid autoimmunity in vitiligo pathogenesis. Adv Dermatology Allergology/Postępy Dermatologii i Alergologii. 2022;39(2):397–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasumagic-Halilovic E, Prohic A, Begovic B, Ovcina-Kurtovic N. Association between vitiligo and thyroid autoimmunity. Journal of thyroid research. 2011;2011. [DOI] [PMC free article] [PubMed]
- 27.Lee JH, Ju HJ, Seo JM, Almurayshid A, Kim GM, Ezzedine K, et al. Comorbidities in patients with vitiligo: a systematic review and meta-analysis. J Invest Dermatology. 2023;143(5):777–89. e6. [DOI] [PubMed] [Google Scholar]
- 28.Khiangte L, Lalrindik C. Study of thyroid disorders in vitiligo. J Family Med Prim Care. 2023;12(4):619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prashant P, Garg R, Bansal P, Praveen S. PRASHANT P. Thyroid autoimmunity in Vitiligo: a case-control study. Cureus. 2023;15(1). [DOI] [PMC free article] [PubMed]
- 30.Yang Y, Huang G, Yan X, Qing Z. Clinical analysis of thyroglobulin antibody and thyroid peroxidase antibody and their association with vitiligo. Indian J Dermatology. 2014;59(4):357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang H, Lee S. Prevalence and incidence of vitiligo and associated comorbidities: a nationwide population-based study in Korea. Clin Exp Dermatol. 2023;48(5):484–9. [DOI] [PubMed] [Google Scholar]
- 32.Yen H, Chi C-C. Association between psoriasis and vitiligo: a systematic review and meta-analysis. Am J Clin Dermatol. 2019;20:31–40. [DOI] [PubMed] [Google Scholar]
- 33.Kridin K, Lyakhovitsky K, Onn E, Lyakhovitsky A, Ludwig R, Weinstein O, et al. Investigating the epidemiological relationship between vitiligo and psoriasis: a population-based study. Arch Dermatol Res. 2023;315(3):395–400. [DOI] [PubMed] [Google Scholar]
- 34.Holmes S, Harries M, Macbeth AE, Chiu WS, de Lusignan S, Messenger AG, et al. Alopecia Areata and risk of atopic and autoimmune conditions: population-based cohort study. Clin Exp Dermatol. 2023;48(4):325–31. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Chen Y, Hwang C, Lin M, Chen T, Chen C, et al. Comorbidity profiles in association with vitiligo: a nationwide population-based study in Taiwan. J Eur Acad Dermatol Venereol. 2015;29(7):1362–9. [DOI] [PubMed] [Google Scholar]
- 36.Barnadas A. Subcutaneous sarcoidosis associated with vitiligo, pernicious anaemia and autoimmune thyroiditis. Clin Exp Dermatol. 2000;25(1):55–6. [DOI] [PubMed] [Google Scholar]
- 37.Chhabra N, Pandhi D, Verma P, Singal A. Pleomorphic cutaneous sarcoidosis confined to lesions of vitiligo vulgaris in a patient with type 1 diabetes mellitus. Indian J Dermatol Venereol Leprol. 2012;78:754. [DOI] [PubMed] [Google Scholar]
- 38.Demirkoek SS, Arzuhal N, Devranoğlu G, Demirkesen C, Tuezuen Y. Recurrent sarcoidosis on a scar associated with vitiligo. J Dermatol. 2007;34(12):829–33. [DOI] [PubMed] [Google Scholar]
- 39.Terunuma A, Watabe A, Kato T, Tagami H. Coexistence of vitiligo and sarcoidosis in a patient with circulating autoantibodies. Int J Dermatol. 2000;39(7):551–3. [DOI] [PubMed] [Google Scholar]
- 40.Thrash BT, Pantlin PG, Mize BC, Rutner CC, Shaw CA, McCarron RE. Acute Vitiligo Repigmentation in the setting of suspected pulmonary sarcoidosis. Ochsner J. 2022;22(3):249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang H-C, Lin M-H, Huang Y-C, Hou T-Y. The association between vitiligo and diabetes mellitus: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;81(6):1442–5. [DOI] [PubMed] [Google Scholar]
- 42.Kang P, Zhang WG, Ji ZH, Shao ZJ, Li CY. Association between Vitiligo and relevant components of metabolic syndrome: a systematic review and meta-analysis. JDDG: J Der Deutschen Dermatologischen Gesellschaft. 2022;20(5):629–41. [DOI] [PubMed] [Google Scholar]
- 43.Ibrahim S, El-Tahlawi S, Mogawer RM, El Ansary M, Esmat S, El‐Hawary M. Different vitiligo characteristics as predictors of increased risk of metabolic syndrome and insulin resistance: a case–control study. J Cosmet Dermatol. 2022;21(12):7170–7. [DOI] [PubMed] [Google Scholar]
- 44.Schneeweiss MC, Kirchgesner J, Wyss R, Jin Y, York C, Merola JF, et al. Occurrence of inflammatory bowel disease in patients with chronic inflammatory skin diseases: a cohort study. Br J Dermatol. 2022;187(5):692–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Méry-Bossard L, Bagny K, Chaby G, Khemis A, Maccari F, Marotte H, et al. New‐onset vitiligo and progression of pre‐existing vitiligo during treatment with biological agents in chronic inflammatory diseases. J Eur Acad Dermatol Venereol. 2017;31(1):181–6. [DOI] [PubMed] [Google Scholar]
- 46.Lambert JL, De Schepper S, Speeckaert R. Cutaneous manifestations in biological-treated inflammatory bowel disease patients: a narrative review. J Clin Med. 2021;10(5):1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bae JM, Kim M, Lee HH, Kim K-J, Shin H, Ju HJ, et al. Increased risk of vitiligo following anti-tumor necrosis factor therapy: a 10-year population-based cohort study. J Invest Dermatology. 2018;138(4):768–74. [DOI] [PubMed] [Google Scholar]
- 48.Alhebshi A, Abbas H, Alotaibi HM, Attaf M, Al-Yamani A, Al-Hebshi A. A Saudi child with chronic Immune Thrombocytopenia and Vitiligo. Cureus. 2020;12(7). [DOI] [PMC free article] [PubMed]
- 49.Nguyen KT, Gwinn CC, Vary JC Jr. Rituximab treatment for dermatitis herpetiformis in the setting of type 1 diabetes mellitus, celiac disease, vitiligo, autoimmune hemolytic anemia, and autoimmune thrombocytopenia. JAAD Case Rep. 2020;6(2):122–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rios-Duarte JA, Sanchez-Zapata MJ, Silverberg JI. Association of vitiligo with multiple cutaneous and extra-cutaneous autoimmune diseases: a nationwide cross-sectional study. Arch Dermatol Res. 2023;315(9):2597–603. [DOI] [PubMed] [Google Scholar]
- 51.Abu El-Asrar AM, Van Damme J, Struyf S, Opdenakker G. New perspectives on the immunopathogenesis and treatment of uveitis associated with Vogt-Koyanagi-Harada disease. Front Med. 2021;8:705796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ma Z, Cao P, Cai M, Lin Q, Long X, Ge M et al. Characteristics of vitiligo patients with versus without associated autoimmune thyroid disease. Int J Dermatol. 2023. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript and supplementary information file.