Abstract
Neurodegenerative diseases, including Alzheimer’s Disease (AD) and Parkinson’s Disease (PD) are common complications of diabetes, arising from insulin resistance, inflammation, and other pathological processes in the central nervous system. The potential of numerous antidiabetic agents to modify neurodegenerative disease progression, both preclinically and clinically, has been assessed. These agents may provide additional therapeutic benefits beyond glycemic control. Introduced in the twenty-first century, glucagon-like peptide-1 receptor agonists (GLP-1RAs) are a class of antidiabetic drugs noted not only for their potent glucose-lowering effects but also for their cardiovascular and renal protective benefits. Various GLP-1RAs have been demonstrated to have significant benefits in in vitro and in vivo models of neurodegenerative diseases through modulating a variety of pathogenic mechanisms, including neuroinflammation, autophagy, mitochondrial dysfunction, and the abnormal phosphorylation of pathognomonic proteins. These agents also have substantial protective effects on cognitive and behavioral functions, such as motor function. However, clinical trials investigating GLP-1RAs in diseases such as AD, PD, mild cognitive impairment, psychiatric disorders, and diabetes have yielded mixed results for cognitive and motor function. This review examines the link between diabetes and neurodegenerative diseases, explores the effects of antidiabetic agents on neurodegeneration, provides a concise overview of the GLP-1 pathway, and discusses both preclinical and clinical trial outcomes of GLP-1RAs for neurodegenerative diseases, including their effects on cognition in AD and PD. This review also proposed new strategies for the design of future clinical trials on GLP-1 RAs for both AD and PD.
Keywords: Neurodegenerative diseases, Alzheimer’s disease, Cognition, Parkinson’s disease, Diabetes, Glucagon-like peptide-1 receptor agonists, Neuroprotection
Introduction
Alzheimer’s disease (AD)
The global increase in the prevalence of neurodegenerative diseases, particularly AD and PD, as a result of an aging population poses a significant challenge to health-care systems worldwide [1, 2]. AD is the most common cause of dementia which is characterized by progressive cognitive decline and neurodegeneration. The hallmark pathologies in AD are the accumulation of amyloid-beta (Aβ) plaques and tau tangles in the brain, leading to neuronal loss and brain atrophy [3, 4]. The risk of AD significantly increases with age, particularly after 65 years old [5]. Given the global trend of population aging, the number of individuals living with AD is expected to increase remarkedly, necessitating a shift in the care provided to these individuals and the resources re-allocated [6].
Parkinson’s disease (PD)
PD is another prevalent neurodegenerative disorder; it primarily affects motor function due to the loss of dopamine-producing neurons in the substantia nigra, a region of the midbrain. Its symptoms include tremors, rigidity, bradykinesia (slowness of movement), and postural instability. Similar to AD, the prevalence of PD increases with age and is expected to increase with population aging [7]. Cellular senescence, oxidative stress, and mitochondrial dysfunction are common aging-related processes that exacerbate the neurodegenerative pathways in both diseases [8, 9]. The increased prevalence indicates the need for novel therapeutic strategies and supportive care to manage the symptoms of these neurodegenerative diseases and to improve the quality of life for the affected individuals.
Urgent need of treatment for AD and PD
Despite considerable advances, existing AD and PD treatments primarily address the symptoms rather than the cause. Disease-modifying therapies that can halt or slow the progression of these neurodegenerative disorders are urgently required. Researchers are currently focusing on the molecular and cellular mechanisms underlying AD and PD, which are key to targeted therapies. Therapies for AD should reduce Aβ accumulation, prevent tau pathology, and protect neuronal function. For PD, the aim is to protect dopaminergic neurons, improve mitochondrial function, reduce α-synuclein accumulation and formulate gene therapies. However, these diseases are difficult to treat given their complexity and the challenges of drug delivery to the brain [10, 11].
Diabetes and neurodegenerative diseases
A growing body of evidence supports the link between diabetes and an increased risk of neurodegenerative diseases such as AD and PD. This link is believed to be primarily mediated by the metabolic, vascular, and inflammatory pathways associated with diabetes, which contribute to the pathogenesis of neurodegeneration. The pathways include inflammation, vascular damage, mitochondrial dysfunction, the effects of insulin resistance (IR) on metabolism in the brain, the formation of advanced glycation end products (AGEs), and the aggregation of pathological proteins such as Aβ and α-synuclein [12].
Diabetes and AD
Some researchers have termed AD “type 3 diabetes,” reflecting the observed link between IR (a hallmark of type 2 diabetes) and AD [13]. IR in the brain impairs glucose metabolism, which is critical for the provision of neuronal energy and the maintenance of synaptic function. This impairment can lead to neuronal damage and increased Aβ accumulation, a key feature of AD pathology [14, 15]. In diabetes, high glucose levels lead to the formation of AGEs, which can accumulate in the brain and contribute to Aβ aggregation and tau phosphorylation, both of which play pivotal roles in AD pathology. AGEs promote oxidative stress and inflammation, leading to the further damage of neurons [16]. Diabetes causes vascular damage through atherosclerosis, leading to impaired blood flow and reduced oxygen supply to the brain. This vascular dysfunction may cause the development of cerebral amyloid angiopathy and may contribute to the neurodegenerative processes observed in AD [17].
Diabetes and PD
Diabetes induces mitochondrial dysfunction, which is a key feature of PD pathology [18, 19]. Impaired mitochondrial function in dopaminergic neurons in the substantia nigra can lead to increased oxidative stress, neuronal damage, and neuronal death, contributing to PD progression [20]. Chronic systemic inflammation, a consequence of diabetes, can exacerbate neuroinflammation, which is implicated in the pathogenesis of PD. Inflammatory mediators can cross the blood–brain barrier, activating the microglia and leading to the release of neurotoxic factors with adverse effects on dopaminergic neurons [21]. Evidence suggests that diabetes exacerbates the aggregation of α-synuclein, a protein that accumulates in the brain in patients with PD, forming Lewy bodies. Hyperglycemia and IR have been linked to increased α-synuclein aggregation, further implicating the role of diabetes in the pathogenesis of PD [22].
Diabetes and pathogeneses of AD and PD
The evidence linking diabetes to the increased risks of AD and PD underscores the complex mechanisms through which metabolic diseases influence brain health and function. Understanding these links is crucial for developing preventive strategies and treatments that address the metabolic basis of neurodegeneration. Moreover, the aforementioned findings highlight the importance of managing diabetes not only to prevent its physical health complications but also to mitigate the risk of neurodegenerative diseases.
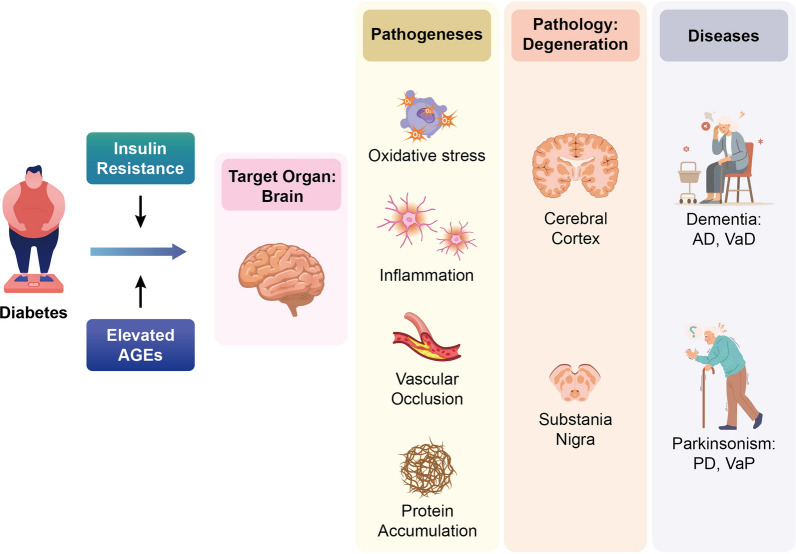
In summary, the potential mechanism by which diabetes contributes to neurodegeneration in AD and PD may involve the accumulation of AGE and cellular IR. This leads to neuroinflammation, vascular occlusion, mitochondrial dysfunction, and the aggregation of pathognomonic proteins, which, in turn, trigger neurodegeneration in the cerebral cortex for AD and the substantia nigra for PD (illustrated in Fig. 1).
Fig. 1.
The postulated mechanism by which diabetes contributes to neurodegeneration in Alzheimer’s disease (AD) and Parkinson’s disease (PD) may involve the accumulation of advanced glycation end products (AGE) and cellular insulin resistance (IR). For the target organ, brain, these two phenomenon lead to inflammation, vascular occlusion, oxidative stress, and the aggregation of pathognomonic proteins, which, in turn, trigger neurodegeneration in the cerebral cortex for dementia, such as AD and vascular dementia (VaD), and the substantia nigra for parkinsonism, such as PD and vascular parkinsonism (VaP)
Neuroprotective effect of antidiabetic agents
Metformin and neuroprotection
Considering the link between diabetes and the increased risks of AD and PD, researchers have investigated the possible neuroprotective effect of antidiabetic agents. Epidemiological evidence has increasingly suggested that metformin, a commonly prescribed medication for type 2 diabetes, protects against the development of neurodegenerative diseases [23]. One candidate mechanism underlying this neuroprotective effect lies in the ability of metformin to improve insulin sensitivity, reduce chronic inflammation, and exert direct effects on brain cells [24]. Furthermore, research has indicated that metformin may improve cognitive performance in individuals with type 2 diabetes [25]. This cognitive benefit may stem from metformin’s ability to improve glucose metabolism in the brain and reduce IR, which are the risk factors for AD. Epidemiological studies have also demonstrated that the use of metformin in individuals with type 2 diabetes is associated with a lower risk of PD [26]. This association suggests that the metabolic benefits of metformin extend to the protection of dopaminergic neurons. Compelling epidemiological evidence links metformin prescription to the reduced risks of AD and PD, indicating the potential repurposing of this widely used diabetes drug for neuroprotection. A clinical trial investigated the effect of 8-week metformin treatment on aging individuals with mild cognitive impairment (MCI) or early AD, and the results revealed that metformin is associated with improved executive function, companied by improvement in learning/memory and attention [27]. However, more large-scale randomized controlled trials (RCTs) are required to verify the neuroprotective effects of metformin.
Thiazolidinediones (TZDs) and neuroprotection
TZDs, including pioglitazone and rosiglitazone (which are often referred to by the broader term “thioglitazones” [TZDs]), are a class of medications primarily used to treat type 2 diabetes. They function as insulin sensitizers by activating the peroxisome proliferator-activated receptor gamma (PPAR-γ), a type of nuclear receptor that plays a crucial role in the regulation of glucose and lipid metabolism. Epidemiological research has been conducted to examine the potential link between the prescription of TZDs and the reduced risks of AD [28, 29] and PD [30, 31], and studies have explored their anti-inflammatory and neuroprotective effects [32]. A few RCTs have investigated the effects of pioglitazone on cognitive decline in patients with AD [33–36]. A pilot study demonstrated that pioglitazone led to cognitive and metabolic improvements in patients with diabetes with AD or MCI [34]. Nevertheless, a phase III study found no significant differences in cognition between rosiglitazone treatment and placebo in APOE-ε4-non-carriers or overall [37]. Early trials have provided implication of the neuroprotective benefits of pioglitazone, which are potentially mediated by its anti-inflammatory and insulin-sensitizing effects. Varied results have been obtained, and more robust and larger-scale RCTs are necessary to draw definitive conclusions. Some clinical trials have been conducted on the repurposing of pioglitazone for PD treatment. A notable RCT is the pioglitazone in early PD trial, which failed to demonstrate the possibility of pioglitazone delaying disease progression in early-stage PD patients [38]. The repurposing of metformin or TZDs for neuroprotection in AD and PD remains a promising, but not yet conclusively proven, strategy. Although both drug classes could theoretically offer neuroprotective benefits based on their mechanisms of action, RCTs have provided mixed or inconclusive results. Thus, more targeted, well-designed clinical trials are required for providing clearer evidence on the efficacy, optimal dose, and safety profiles of these drugs in AD and PD. Table 1 summarized the clinical trials of metformin and TZDs on cognition, MCI, AD, and PD.
Table 1.
Summary of published clinical trials of metformin and thiazolidinedione on cognition in mild cognitive impairment (MCI), Alzheimer’s disease (AD), and Parkinson’s disease (PD)
| Anti-diabetic agents [Ref.] | Population | Treatment duration | Phase | Outcome measurements (primary/secondary) | Final results |
|---|---|---|---|---|---|
| Metformin [27] | Age 55–80, MCI or early AD (CDR ≤ 1.0), MMSE > 19, at least one positive AD biomarker | 8 weeks | 2 | CSF Aβ, tau, p-tau/ADAS-Cog, CANTAB, TMT-B, DS, BNT, ASL-MRI | No change of CSF biomarkers, improvement executive function |
| Pioglitazone [34] | Mild to moderate AD (MMSE 16–26) and amnestic MCI with DM | 6 months | NA | MMSE, ADAS-JCog, WMS-R, FAB, CF, rCBF | Improvement of ADAS-Jcog scores |
| Rosiglitazone [37] | Age 50–90, probable AD (MMSE 10–23) | 24 weeks | 3 | ADAS-Cog, CIBIC+/NPI, DAD, MMSE | No evidence of efficacy |
| Pioglitazone [36] | Probable AD, MMSE 12–26, CDR 1 or 2, with cholinesterase inhibitors | 6 months | NA | CDR-SB, ADAS-Cog, NPI | No significant treatment effect |
| Pioglitazone [33] | Age 65–83, CDR 0, MMSE ≥ 25, CVLT-LDFR or BVMT-DR > − 1.5 SDs | 5 years | 3 | Time to diagnosis of MCI/ADCS-ADL | No delay the onset of MCI |
| Pioglitazone [38] | Age > 30, PD < 5 years, H&Y ≤ 2 | 44 weeks | 2 | UPDRS/PDQ-39, SEADL, UPSIT | No significant treatment effect |
ADAS-Cog Alzheimer’s disease assessment scale-cognitive subscale, ADAS-JCog Alzheimer disease assessment scale (Japanese version) cognitive subscale, ADCS Alzheimer’s disease cooperative study, ADL activities of daily living, ASL arterial spin labeling, BNT Boston naming test, BVMT-DR brief visuospatial memory test-revised, CANTAB Cambridge neuropsychological test automated battery, CDR clinical dementia rating, CDR-SB clinical dementia rating-sum of boxes, CF category fluency, CIBIC+ clinician’s interview-based impression of change with caregiver’s input, CSF cerebrospinal fluid, CVLT-LDFR California verbal learning test-long delay free recall, DAD disability assessment for dementia, DS digit span, DM diabetes mellitus, FAB frontal assessment battery, H&Y Hoehn and Yahr stages, MCI mild cognitive impairment, MRI magnetic resonance imaging, NPI neuropsychiatric inventory, PDQ-39 Parkinson’s disease questionnaire-39, rCBF regional cerebral blood flow, SEADL self-efficacy for activities of daily living, TMT trail making test, UPDRS unified Parkinson’s disease rating scale, UPSIT University of Pennsylvania smell identification test, WMS-R Wechsler memory scale-revised
Overview of glucagon-like peptide-1 and its role in glucose metabolism
New-generation antidiabetic agents, particularly those targeting the glucagon-like peptide-1 (GLP-1) pathway, represent a significant advancement in the treatment of type 2 diabetes. GLP-1 is an incretin hormone that is secreted in response to food intake, and it exerts multiple actions conducive to glycemic control, including enhancing glucose-dependent insulin secretion, suppressing glucagon release, and delaying gastric emptying [39]. GLP-1 receptor agonists (GLP-1RAs) are designed to mimic the action of endogenous GLP-1 but have a longer duration of action, making them effective therapeutic agents for type 2 diabetes [40]. Studies have demonstrated the efficacy of GLP-1RAs for glycemic control and their potential cardiovascular [41] and renal protective effects [42]. These effects are particularly relevant given the increased risks of cardiovascular and kidney diseases in patients with diabetes. Several landmark cardiovascular outcome trials have demonstrated that GLP-1RAs can significantly reduce the risk of major adverse cardiovascular events (MACE), which include cardiovascular death, nonfatal myocardial infarction (heart attack), and nonfatal stroke [43]. The cardiovascular benefits of GLP-1RAs extend beyond their effects on blood glucose levels. Potential mechanisms include improvements in blood pressure, lipid profiles, body weight, inflammation, and endothelial function as well as direct cardioprotective effects on the cardiac muscle and vasculature [41]. GLP-1RAs can reduce the incidence of MACEs and delay the progression of kidney disease; thus, they are an attractive therapeutic option for patients with type 2 diabetes, especially those with existing cardiovascular disease or at high risk for those complications.
Molecular mechanisms of GLP-1 agonists for neuroprotection
GLP-1 receptors are functionally expressed in insulin-releasing neuroglia form cells in the rat cerebral cortex, and hyperglycemia significantly increases GLP-1 receptor expression. Additionally, GLP-1 receptor activation by GLP-1RAs can mimic effects on these cells similar to those on pancreatic beta cells, suggesting that this pathway also modulates neural insulin production [44].
Insulin and GLP-1 signaling pathway
The molecular mechanism of neuroprotection of several GLP-1RAs has been explored using in vivo and in vitro models. Existing findings indicate that the protective mechanism is mainly attributed to the GLP-1 signaling pathway, which involves cyclic adenosine monophosphate (cAMP), protein kinase A (PKA) and cAMP response element-binding protein (CREB). A study demonstrated the neuroprotective effects of liraglutide, a commercially available GLP-1RA, on neuronal cells from H2O2-induced oxidative stress and glutamate-induced excitotoxicity, enhancing cell viability. The neuroprotective effects are linked to the activation of the cAMP/PKA/pCREB pathway [45]. The neuroprotective benefits of GLP-1RAs may also result from the insulin signaling pathway. Exendin-4 (Ex-4), another GLP-1RA that is homologous to commercially available exenatide, ameliorated IR in primary neurons through insulin receptor substrate-1 (IRS-1), AKT, and glycogen synthase kinase-3β (GSK-3β) pathways; GSK-3β significantly contributes to neuroprotection given its role in tau hyperphosphorylation in AD pathology [46].
Mitochondria and anti-oxidants
The neuroprotection of GLP-1RAs is attributed to the restoration of mitochondrial function, reduction of oxidative stress, and prevention of subsequent apoptosis. Ex-4 enhances the activities of key antioxidant enzymes in hippocampal mitochondria, including catalase, manganese superoxide dismutase, and glutathione peroxidase, reversing AGE-dependent decline. Ex-4 treatment upregulates the expression of the proteins critical for mitochondrial biogenesis, including peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), nuclear respiratory factor-1 (NRF-1), and transcription factor a, mitochondrial (Tfam). It also reduces GSK-3β activity [47]. In addition, Ex-4 provides neuroprotection against ischemia-induced damage in the hippocampus by downregulating hypoxia-inducible factor-1α and modulating apoptosis through the increased B-cell lymphoma 2 (Bcl-2)/Bcl-2-associated X-protein (Bax) ratio, which is mediated by the GLP-1 receptor [48]. The protective effects of liraglutide against Aβ-induced neurotoxicity in neuroblastoma cells are attributed to the activation of the phosphoinositide 3-kinase (PI3K)/Akt signaling pathway, as evidenced by the increased phosphorylation of Akt and the altered expression of the apoptosis-related proteins Bcl-2/Bax, and cytochrome c [49].
Neuroinflammation
Neuroinflammation is a critical pathogenesis of neurodegeneration, and diabetes significantly and systemically enhances inflammation, including that in the brain. EX-4 affects the microglia (immune cells in the brain) through an autocrine mechanism, in which interleukin-10 (IL-10) mediates β-endorphin expression. EX-4 stimulates the expression of anti-inflammatory M2 microglial markers (including IL-10, IL-4, arginase 1, and CD206), but not that of proinflammatory M1 markers, which are linked to neuroprotection and antinociception in neuropathic rats [50]. Ex-4 significantly reduces neuroinflammation by converting proinflammatory M1 microglia into anti-inflammatory M2 microglia both in vitro and in vivo through the rescue of the expression of Arf and Rho GAP adapter protein 3 (ARAP3); this finding suggests that the PI3K/ARAP3/(Ras homolog family member A (RhoA) signaling pathway plays a crucial role in Ex-4-mediated anti-inflammation [51]. In an AD mouse model, liraglutide reduces the activation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome in the microglia, leading to the decreased production of proinflammatory cytokines and Aβ plaques [52]. Semaglutide, a GLP-1RA, mitigates seizure severity and cognitive impairment through the inhibition of the lipopolysaccharide (LPS)- and nigericin-induced NLRP3 inflammasome pathway, reducing lactate dehydrogenase (LDH) release by microglial cells [53].
Autophagy
The impairment of autophagy and the clearance of accumulated, unwanted proteins play crucial roles in the development of neurodegeneration. Liraglutide treatment results in the increased phosphorylation of AMP-activated protein kinase (AMPK) and the reduced phosphorylation of mammalian target of rapamycin (mTOR); this result suggests that autophagy pathways are activated in high-glucose-treated hippocampal neurons. Liraglutide-induced increases in the microtubule-associated protein 1A/1B-light chain 3 (LC3)-II expression and p62 degradation were prevented by the compound C-mediated inhibition of AMPK; thus, liraglutide-induced autophagy in hippocampal neurons is induced by the AMPK/mTOR pathway [54]. By enhancing the autophagy flux and reducing oxidative stress and mitochondrial dysfunction, semaglutide and liraglutide protect neuroblastoma against 6-hydroxydopamine (6-OHDA)-induced toxicity, a common toxin model of PD. Semaglutide significantly increases the levels of the autophagy-related proteins LC3-II/LC3-I, Beclin1, and autophagy-related 7 (Atg7) and decreases p62 levels, indicating enhanced autophagy [55]. Some studies have also demonstrated the neuroprotective effect of GLP-1RAs through broader mechanisms.
Growth and apoptosis
Ex-4 significantly improves the survival of rat cortical neurons under oxygen/glucose deprivation (OGD) conditions through reduced apoptosis rates, increased expression of glucose-regulated protein 78, and decreased levels of C/EBP-homologous protein, indicating the role of Ex-4 in modulating unfolded protein response for protecting neurons from ischemic damage [56]. Ex-4 also promotes neurite outgrowth and survival in neurons in the dorsal root ganglion of adult rats through the activation of the PI3K signaling pathway, which in turn suppresses RhoA activity, a negative regulator of nerve regeneration [57]. Liraglutide protects neuroblastoma cells against neurotoxicity induced by the organophosphate pesticide mipafox by enhancing neuritogenesis, increasing glucose uptake, and elevating the levels of cytoskeleton proteins and synaptic-plasticity modulators. It also reduces the levels of the proinflammatory cytokine IL-1β and decreases caspase-3 activity [58].
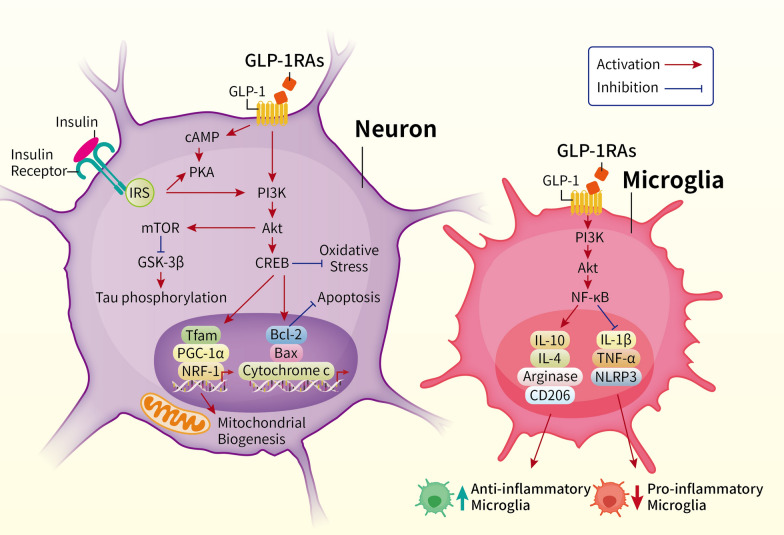
In summary, in in vitro models, the protective mechanisms of GLP-1RAs are complex. In neurons, it involves the activation of PI3K/Akt signaling pathway, inhibition of GSK-3β, reducing tau phosphorylation, promotion of mitochondrial biogenesis, activation of the Bcl-2 family proteins, and inhibiting apoptosis. In microglia, GLP-1R activation via PI3K/Akt signaling also reduces neuroinflammation by modulating NF-κB activity, decreases the production of pro-inflammatory cytokines, increases anti-inflammatory markers such as IL-10, IL-4, Arginase, and CD206, and the transformation of microglia into an anti-inflammatory state. Thus, the dual role of GLP-1RAs in reducing neurodegeneration by improving neuronal survival and modulating microglial activity, providing a potential therapeutic approach for neurodegenerative diseases (summarized in Fig. 2).
Fig. 2.
This figure illustrates the neuroprotective mechanisms of GLP-1 receptor agonists (GLP-1RAs) in neurons and microglia, highlighting the key molecular pathways involved in neurodegeneration and inflammation. In neurons, the binding of GLP-1 to its receptor (GLP-1R) activates the cyclic AMP (cAMP) and protein kinase A (PKA) signaling pathway, which in turn activates PI3K/Akt signaling. This activation leads to the downstream phosphorylation and inhibition of GSK-3β, reducing tau phosphorylation—a critical event in neurodegenerative diseases such as Alzheimer’s disease. Additionally, Akt activation promotes mitochondrial biogenesis by upregulating transcription factors such as Tfam, PGC-1α, and NRF-1, leading to enhanced energy production and reduced oxidative stress. The Akt/CREB pathway also activates Bcl-2 family proteins, inhibiting apoptosis by preventing cytochrome c release. Overall, these signaling events protect neurons from oxidative stress and apoptosis, which are central to neurodegeneration. In microglia, GLP-1R activation via PI3K/Akt signaling also reduces neuroinflammation by modulating NF-κB activity. This pathway decreases the production of pro-inflammatory cytokines, including IL-1β, TNF-α, and NLRP3, and increases anti-inflammatory markers such as IL-10, IL-4, arginase, and CD206. This shift in cytokine balance promotes the transformation of microglia into an anti-inflammatory state, which is neuroprotective, reducing overall inflammation and damage in the brain. The figure highlights the dual role of GLP-1RAs in reducing neurodegeneration by improving neuronal survival and modulating microglial activity, providing a potential therapeutic approach for neurodegenerative diseases such as AD and PD. AGE advanced glycation end products, Akt protein kinase B, Bax Bcl-2 associated X protein, Bcl-2 B-cell lymphoma 2, cAMP cyclic adenosine monophosphate, CREB cAMP response element-binding protein, GSK-3β glycogen synthase kinase-3 beta, IL-1β interleukin 1 beta, IL-4 interleukin 4, IL-10 interleukin 10, IRS insulin receptor substrate, IR insulin resistance, mTOR mammalian target of rapamycin, NF-κB nuclear factor kappa-light-chain-enhancer of activated B cells, NLRP3 NOD-, LRR-, and pyrin domain-containing protein 3, NRF-1 nuclear respiratory factor 1, PGC-1α peroxisome proliferator-activated receptor gamma coactivator 1-alpha, PI3K phosphatidylinositol 3-kinase, PKA protein kinase A, Tfam mitochondrial transcription factor A, TNF-α tumor necrosis factor alpha
Neuroprotective effects of GLP-1RAs in AD, PD, and other in vivo disease models
Effects on wild type and diabetes in vivo models
The neuroprotection of GLP-1RAs in in vivo models is underpinned by the ability of GLP-1RAs to cross the blood–brain barrier (BBB) and by the presence of GLP-1 receptors in the brain. The BBB-crossing ability of liraglutide and lixisenatide has been successfully demonstrated, and they subsequently activate GLP-1 receptors in the brain. This activation leads to neuroprotection and the promotion of neuronal progenitor proliferation [59]. Initially, the neuroprotection of GLP-1RAs was tested in in vivo models of diabetes. The subcutaneous administration of liraglutide not only improves hyperglycemia and peripheral IR but also reverses insulin signaling impairment in the brain, leading to decreased AD-associated tau hyperphosphorylation [60]. In addition, liraglutide improves learning and memory in diabetic mice [61]. Interestingly, the benefit of liraglutide for tau phosphorylation in diabetic mice cannot be replicated by insulin treatment, suggesting that the unique neuroprotective effects of GLP-1RAs are medicated by pathways other than insulin signaling pathways [62].
The chronic subcutaneous administration of Ex-4 attenuates the peripheral symptoms of type 2 diabetes in rats, including hyperglycemia and IR, and enhances the levels of brain cortical GLP-1 and insulin-like growth factor (IGF)-1 and their downstream signaling pathways. It also upregulates brain cortical autophagy and ameliorates apoptosis [63]. Ex-4 also promotes brain-derived insulin production and enhances insulin signaling through the Wnt/β-catenin/NeuroD1 signaling pathway, which subsequently reduces tau hyperphosphorylation and cognitive dysfunction in diabetic mice [64]. EX-4 treatment reduces tau hyperphosphorylation levels in the hippocampus, a key area for memory and AD development [65]. Lastly, Ex-4 also protects the AGE-induced activation of receptor AGE signaling pathway, which is responsible for the inflammation and oxidative stress induced by AGEs [66].
Aging models
Given the close association between diabetes, aging, and neurodegeneration, GLP-1RAs have been tested in in vivo aging models. Liraglutide, but not metformin, prevents insulin-induced neuronal senescence, including abnormal electrophysiological activity, and impairs glycolysis, favoring the production of p25; p25 induces the hyperactivation of cyclin-dependent kinase 5 and disrupts GSK3β-mediated processes [67]. Ex-4 also reverses endothelial cell aging in the mouse brain, which is characterized by zonation-dependent transcriptomic changes; thus, Ex-4 treatment improves BBB integrity and reduces the microglial priming associated with aging [68]. Systemic exenatide treatment can reverse aging-associated transcriptomic changes in various brain cell types in mice, including glial and neurovascular cells. This genome-wide reversal influences the common and cell type-specific pathways involved in aging and neurodegeneration. Additionally, aging leads to a reduction in AD-associated transcriptomic signatures in the microglia [69].
Neurodegeneration and AD models: exenatide
Considering the successful neuroprotection of GLP-1RAs in in vivo diabetic models, researchers have directly tested GLP-1RAs in in vivo models of neurodegenerative diseases, including toxin-induced and transgenic models. Ex-4 regulates intracellular calcium homeostasis and prevents neuronal loss by preventing the Aβ1-42-induced calcium overload. Ex-4 can significantly rescue memory deficits and neuropathological changes in APP/PS1 AD mice, and it can downregulate the activities of N-acetylglucosaminyltransferase III and bisecting N-acetyl-d-glucosamine through the Akt/GSK-3β/β-catenin signaling pathway [70]. Ex-4 modulates apoptotic pathways in the hippocampus through the regulation of the expression levels of Bcl2, Bax, and caspase-3 [71], and it improves mitochondrial function and integrity in AD mice through the PI3K/Akt signaling pathway [72].
Ex-4 also modulates the influx of calcium through L-type voltage-dependent calcium channels (L-VDCCs) and N-methyl-d-aspartate receptors, and it maintains the expression of phosphorylated calcium/calmodulin-dependent protein kinase type IIα [73]. In a mouse model of AD, combining insulin with Ex-4 leads to the significant downregulation of the expression of insulin receptor signaling pathway genes in AD mice, which is associated with improved spatial learning. However, the reduction of Aβ levels is nonsignificant [74]. In 5xFAD transgenic mice, Ex-4 treatment significantly improves cognitive impairment, reduces Aβ1-42 deposition in the hippocampus, and alleviates synaptic degradation. Additionally, it restores mitochondrial morphology, mitochondrial energy production, and mitochondrial dynamics and inhibits oxidative stress [75]. Ex-4 also reverses the high-fat-diet-induced impairment of brain-derived neurotrophic factor (BDNF) signaling and the inflammatory response in the 3xTg-AD mouse model [76]. Lastly, EX-4 enhances cognitive function in APPxPS1-Ki AD mice by improving short- and long-term memory and increasing brain lactate levels, indicating a shift toward anaerobic glycolysis. However, exenatide has no effect on 3xTg-AD mice, suggesting that its benefits are model-specific [77].
Neurodegeneration and AD models: other GLP-1 RAs
Liraglutide effectively modulates tau hyperphosphorylation through the GSK-3β pathway; therefore, liraglutide treatment attenuates the learning and memory impairments induced by the exogenous application of Aβ1-42 in mice [78]. Long-term treatment with liraglutide significantly reduces tau pathology and improves motor function in a transgenic mouse model of tauopathy. Specifically, liraglutide treatment leads to a significant reduction in the phosphorylation of tau proteins, improving survival rates and reducing motor impairment in these mice [79]. More than neuron, liraglutide alleviates mitochondrial dysfunction in astrocytes through the cAMP/PKA signaling pathway, and it enhances the neuronal supportive capacity of astrocytes treated with Aβ [80]. However, similar to Ex-4, the responsiveness of liraglutide differs between AD models; further research is warranted to understand the mechanisms underlying these differences [81]. Lastly, in a nonhuman primate model of AD, liraglutide provides partial protection, reducing AD-related insulin receptor, synaptic, and tau pathology in specific brain regions [82].
Other novel GLP-1RAs have also been tested, whether used singly or in combination with other drugs. Treatment with liraglutide and a lipidized analog of prolactin-releasing peptide (Palm11-PrRP31) exerts neuroprotective effects in APP/PS1 mice. This combination treatment significantly reduces the Aβ plaque load, neuroinflammation, and tau phosphorylation in the brain [83]. In APP/PS1 transgenic mice, (D-Ser2)Oxm, a dual GLP-1 and Gcg receptor agonist, notably improves spatial memory and synaptic plasticity, reduces Aβ plaque deposition in the hippocampus, and modulates the PI3K/AKT1/GSK3β signaling pathway [84]. NLY01, an engineered Ex-4, effectively blocks the Aβ-induced activation of the microglia and prevents the conversion of the microglia into reactive astrocytes in transgenic AD mouse models (5xFAD and 3xTg-AD) [85]. A novel GLP-1/GIP/Gcg triagonist significantly improves cognitive deficits and reduces AD pathology in 3xTg-AD mice. This treatment also enhances long-term spatial memory, working memory, and hippocampal synaptic plasticity. It also decreases Aβ and phosphorylated tau aggregates in the hippocampus [86].
Neurodegeneration and PD models
The neuroprotection of GLP-1RAs has also been tested in in vivo models of PD, the second most common neurodegenerative disease. Mice with PD induced by 1,2,3,6 tetrahydro-1-methyl-4-phenylpyridine (MPTP) were treated with liraglutide and lixisenatide, which are GLP-1RA mimetics. Both drugs prevented MPTP-induced motor impairment, protected dopaminergic neurons in the substantia nigra, reduced the expression of the proapoptotic signaling molecule Bax, and increased the levels of the antiapoptotic molecule Bcl-2 [87]. In MPTP-induced PD models, liraglutide treatment reduced the levels of neuroinflammatory markers in the substantia nigra through AMPK activation and NF-κB reduction [88]. In toxin-induced PD mouse models, DA-JC1, a novel dual GLP-1/GIP receptor agonist, improved motor impairment, increased the number of dopaminergic neurons in the substantia nigra, enhanced BDNF levels, and modulated apoptosis signaling pathways by altering the expression of Bax and Bcl-2 [89, 90].
In summary, the neuroprotective effects of GLP-1RAs have been demonstrated in various in vivo models of diabetes, aging, AD, and PD. GLP-1RAs can cross the blood–brain barrier and activate brain GLP-1 receptors, leading to neuroprotection by improving insulin signaling, reducing tau hyperphosphorylation, and enhancing cognitive function in diabetic and aging models. Exenatide (Ex-4) and liraglutide are effective in modulating key pathways involved in neurodegeneration, such as apoptosis, mitochondrial function, and autophagy, in models of AD and PD. While the results in AD models are mixed, with benefits varying between specific models, GLP-1RAs show consistent neuroprotective effects in PD models, protecting dopaminergic neurons and reducing inflammation.
Clinical studies on neuroprotection of GLP-1RAs
After numerous preclinical studies have examined the neuroprotective effects of GLP-1RAs, new clinical trials have been initiated. These trials have targeted various groups, including individuals with diabetes, focusing on cognitive effects, and those with or at risk of AD or PD (summarized in Table 2 for AD and Table 3 for PD).
Table 2.
Summary of registered clinical trials of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) on cognition, mild cognitive impairment (MCI) and Alzheimer’s disease (AD)
| GLP-1 RAs | Population | Treatment duration | Phase | Outcome measurements (primary/secondary) | Status | ClinicalTrials.gov identifier | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Clinical | Imaging | Biomarkers | |||||||
| Exenatide | MCI or early AD | 18 months | II | Tolerability/MMSE, ADAS-Cog, CDR | Nil | CSF (tau, p181 tau, Aβ42) | Terminated | NCT01255163 | [95] |
| Exenatide | MCI and dysglycemia/prediabetes | 32 weeks | III | ADAS-Cog/MMSE, VF, GDS, CDR, NPI, ADL, IADL | Nil | Nil | Unknown | NCT02847403 | Nil |
| Liraglutide | MMSE > 27 | 12 weeks | N/A | BVRT, CVLT-II, D-KEFS, RCFT, PP, WAIS-III, WASI | Resting state fMRI | Nil | Completed | N/A | [94] |
| Liraglutide | Aging adults with prediabetes | 90 days | I | ACT, BVRT, BNT, BFSRT, D-KEFS, color-word subtest, PP, ROCFT, TCFT, WASI, WAIS | Nil | Nil | Completed | NCT02140983 | Nil |
| Liraglutide | Bipolar disorders or depressive disorders with below-average performance in the TMT-B | 4 weeks | III | TMT-B | Resting state fMRI | Nil | Completed | NCT02423824 | Nil |
| Liraglutide | HIV and TIIDM | 6 months | IV | Global cognitive scores | Nil | Nil | Terminated | NCT02743598 | Nil |
| Liraglutide | TIIDM | 12 weeks | III | DST, RAVL, LDFR, TMT, ANT, CDT, MMSE, MES | Functional NIRS | Nil | Completed | NCT03707171 | [92] |
| Liraglutide | TIIDM, MMSE > 24 | 16 weeks | N/A | MoCA | Change of olfactory brain activation by fMRI | Nil | Completed | NCT03961659 | [91] |
| Liraglutide | TIIDM with MCI | 48 weeks | N/A | MoCA/RBANS | fMRI | Nil | Recruiting | NCT05313529 | Nil |
| Liraglutide | TIIDM | 12 weeks | III | MMSE | Nil | Blood (tau, p181 tau, Aβ42, TDP-43, IL-6, IL-8, TNF-α) | Completed | NCT05360147 | Nil |
| Dulaglutide | TIIDM, HbA1c ≤ 9.5%, age > 50 years old | 8 years | III | MoCA, DSST | Nil | Nil | Completed | NCT01394952 | [93] |
| Dulaglutide | Bipolar disorders | 24 weeks | N/A | CBCT | Nil | Nil | Recruiting | NCT06331286 | Nil |
| Semaglutide | Major depression disorder | 16 weeks | II | DSST, stroop test, spatial n-back/RAVLT, TMT-A, PDQ | Nil | Nil | Recruiting | NCT04466345 | Nil |
| Semaglutide | MCI and metabolic syndrome | 12 months | II | Trails B, DST, CF, ADAS-Cog, LMS I-WMT/CDR, ADL, IADL | CBF, FDG-PET/WHH and GM in MRI | Plasma Aβ42/Aβ40 ratio, p-tau181, p-tau 231, NfL, GFAP | Recruiting | NCT06072963 | Nil |
| Semaglutide | Healthy volunteers | Single dose | N/A | Reward/emotional processing, emotional impulsivity, memory | Nil | Nil | Recruitment pending | NCT06363487 | Nil |
ADAS-Cog Alzheimer’s disease assessment scale-cognitive subscale, ADL activities of daily living, ANT attention network test, BDNF brain-derived neurotrophic factor, BFSRT Buschke–Fuld selective reminding test, BNT Boston naming test, BVRT Benton visual retention test, CBCT Chinese brief cognitive test, CBF cerebral blood flow, CDR clinical dementia rating, CDT clock-drawing test, CF category fluency, CSF cerebrospinal fluid, CT computed tomography, CVLT-II California verbal learning test second edition, D-KEFS Delis–Kaplan executive function system, DSST digit symbol substitution test, DST digital symbol test, FDG-PET fluorodeoxyglucose-positron emission tomography, fMRI functional magnetic resonance imaging, GDS geriatric depression scale, GFAP Glial fibrillary acidic protein, GM grey matter, IADL instrumental activities of daily living, IL-6 interleukin 6, IL-8 interleukin 8, LDFR long delay free recall, LMS I-WMT logical memory story I from the Wechsler memory test, M1 primary motor cortex, MES memory and executive screening, MMSE mini-mental status examination, MoCA Montreal cognitive assessment, NfL neurofilament light chain, NIRS near-infrared spectroscopy, NPI neuropsychiatric inventory, PHQ-9 patient health questionnaire, PP Purdue pegboard, RAVL Rey auditory verbal learning, RBANS repeatable battery for the assessment of neuropsychological status, RCFT Rey complex figure test and recognition trial, ROCFT Rey–Osterrieth complex figure test, SMA supplementary motor area, TCFT Taylor complex figure task, TDP-43 TAR DNA-binding protein 43,TMT trail making test, TNF-α tumor necrosis factor alpha, VF verbal fluency, WAIS-III Wechsler adult intelligence scale-third edition, WASI Wechsler abbreviated scale of intelligence, WLM Wechsler logical memory, WMH White matter hyperintensity
Table 3.
Summary of registered clinical trials of glucagon-like peptide-1 receptor agonists (GLP-1 RAs) on Parkinson’s disease (PD)
| GLP-1 RAs | Population | Treatment duration | Phase | Outcome measurements (primary/secondary) | Status | ClinicalTrials.gov identifier | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Clinical | Imaging | Biomarkers | |||||||
| Exenatide | PD H&Y 2 to 2.5 | 48 weeks | II | MDS-UPDRS III/UDRS, MADRS, PDQ39, NMSS, LED | DaTSCAN | CSF-BDNF | Completed | NCT01971242 | [97] |
| Exenatide | Not applicable | 1 year | I | Nil | Free-water accumulation in the substantia nigra, BOLD signal in the posterior putamen, M1, and SMA | Nil | Completed | NCT03456687 | Nil |
| NLY01, a pegylated form of exenatide | PD | 36 weeks | II | MDS UPDRS II and III | Nil | Nil | Active, not recruiting | NCT04154072 | [98] |
| Exenatide | PD H&Y ≤ 2.5 | 2 years | III | MDS UPDRS III/MDS UPDRS I, II, VI, PET, MoCA-K, K-PDQ39, K-NMSS, LED, H&Y | Nil | Nil | Active, not recruiting | NCT04232969 | Nil |
| Exenatide, sustained release | PD H&Y ≤ 2.5 | 48 weeks | II | MDS UPDRS III/MDS UPDRS I, II, VI, timed walk assessment, MoCA, UDRS, PHQ-9, PDQ39, NMSS, LED | Nil | Nil | Unknown | NCT04269642 | Nil |
| Exenatide | PD H&Y ≤ 2 | 18 months | II | MDS UPDRS I–VI, H&Y, MoCA, PDQ-39, NMSQuest, LED, ESS, MADRS, B-SIT | FDG-PET network analysis | Nil | Active, not recruiting | NCT04305002 | Nil |
| Liraglutide | PD within 2 years of diagnosis | 54 weeks | II | UPDRS III, NMSS, MDRS-2/PDQ39, UPDRS I, II, VI | Nil | Nil | Completed | NCT02953665 | Nil |
| Semaglutide | PD | 48 months | II | MDS-UPDRS III in OFF | Nil | Nil | Recruitment pending | NCT03659682 | Nil |
| Lixisenatide | PD H&Y < 3, within 3 years of diagnosis | 12 months | II | MDS-UPDRS III | Nil | Nil | Completed | NCT03439943 | [99] |
B-SIT brief smell identification test, DaTscan dopamine transporter scan, LED levodopa equivalent dose, M1 primary motor cortex, MADRS montgomery-asberg depression rating scale, MDRS-2 mattis dementia rating scale 2, MDS movement disorder society, MES memory and executive screening, MMSE mini-mental status examination, MoCA montreal cognitive assessment, NMSQuest non-motor symptoms questionnaire, NMSS non-motor symptoms scale for Parkinson’s disease, PDQ39 Parkinson’s disease questionnaire-39, PET positron emission tomography, SMA supplementary motor area, UDRS unified dykinesia rating scale, UPDRS unified Parkinson’s disease rating scale
Cognition-orientated clinical trials: people with diabetes
In patients with type 2 diabetes without dementia [defined by a Mini Mental Status Examination (MMSE) score of > 24], treatment with liraglutide, but not dapagliflozin or acarbose, over 16 weeks significantly enhanced impaired odor-induced hippocampal activation, as assessed by functional magnetic resonance imaging (fMRI). Although no significant improvement was found in overall cognitive performance, as evaluated by the MMSE and the Montreal Cognitive Assessment (MoCA), notable enhancements were observed in the delayed memory, attention, and executive function subdomains of the MoCA. These enhancements were speculated to be a direct effect of liraglutide, rather than an effect of decreased blood glucose, which was achieved by dapagliflozin and acarbose [91]. Similarly, a prospective, parallel-assignment, open-label, phase III study examined changes in MMSE scores following 12-week liraglutide treatment in individuals with diabetes (glycated hemoglobin [HbA1c] > 7.0% and on oral antidiabetic drugs or insulin for at least 3 months). Multiple cognitive assessments were conducted, involving memory, executive function, attention, and verbal function evaluation. Compared with the control group, the liraglutide group derived greater cognitive benefit on the improvements in MMSE scores and category naming scores. Additionally, liraglutide significantly enhanced brain activation in the dorsolateral prefrontal cortex and orbitofrontal cortex during a verbal fluency task. The increases in blood flow and brain activation in the dorsolateral prefrontal cortex and orbitofrontal cortex were strongly associated with improvements in cognitive performance [92].
The Researching Cardiovascular Events with a Weekly Incretin in Diabetes (REWIND) trial focused on adults aged ≥ 50 years with either established or newly diagnosed type 2 diabetes and additional cardiovascular risk factors, and this trial investigated subjects with HbA1c ≤ 9.5%, receiving up to two oral glucose-lowering drugs with or without basal insulin, and a body mass index of at least 23 kg/m2. The REWIND trial mainly assessed the cardiovascular outcomes of dulaglutide over up to 8 years. Interestingly, the trial also revealed a positive correlation between dulaglutide treatment and a reduced risk of cognitive impairment. With the adjustment for baseline cognition, participants who had undergone dulaglutide treatment for approximately 5 years exhibited a 14% lower hazard ratio of substantive cognitive impairment (SCI) adjusted for baseline scores during follow-up [93].
cognition-orientated clinical trials: people with risk or diagnosed AD
In a clinical trial in individuals at risk of AD, participants aged 45 to 70 years who did not have diabetes or a history of neurological disorders were recruited. All participants exhibited SCI but maintained a MMSE score of ≥ 27. The results revealed that 12-week treatment with liraglutide led to significant improvements in intrinsic connectivity within the default mode network, as assessed by resting-state fMRI. Despite these changes on neuroimages, no observable cognitive differences were noted between the treatment and placebo groups over the study period. These findings highlight the potential of liraglutide to enhance brain connectivity in populations at risk of AD, suggesting its neuroprotective effect [94]. Regarding the participants with MCI or AD, defined based on a clinical dementia rating (CDR) global score of 0.5 or 1, although the 18-month treatment with exenatide was safe and well-tolerated, it did not significantly affect clinical and cognitive measures, nor did it affect MRI cortical thickness and volume or most biomarkers in the cerebrospinal fluid (CSF), plasma, and plasma neuronal extracellular vesicles (EVs). The only notable positive outcome was the reduction of Aβ42 in plasma neuronal EVs, which may reflect the neuronal condition in the systemic circulation. Unfortunately, the study was prematurely terminated and underpowered, which precludes a conclusive determination of the disease-modifying effects of exenatide for MCI or early AD in clinical settings [95].
PD clinical trials
The neuroprotective effects of GLP-1RAs, particularly exenatide, seem to be more promising in PD. Exenatide treatment for 12 months was generally well-tolerated in individuals with mild PD, which was defined as Hoen and Yahr (H&Y) stages 2 to 2.5. PD symptoms significantly improved, including motor function assessed by the Movement Disorder Society-Unified Parkinson’s Disease Rating Score (MDS-UPDRS) Part III motor scores and cognitive function assessed by the Mattis Dementia Rating Scale-2 (Mattis DRS-2). These improvements were noted in the exenatide treatment group compared with the control group of PD patients. Crucially, these improvements persisted even after a 2-month washout period, suggesting the potential long-term benefits [96]. A subsequent larger-scale phase II study further investigated the effect of exenatide on PD. Participants were treated with exenatide for 48 weeks. The study found a statistically significant improvement in off-medication MDS-UPDRS Part III motor scores; at the end of the treatment, a 2.3 point improvement was noted in the exenatide group compared with a 1.7 point deterioration in the placebo group. This improved score persisted after a 12-week washout period as well. However, no significant differences were detected in other outcomes, including nonmotor symptoms, cognition, finger tapping, walking speed, and levodopa equivalent dose [97]. By analyzing serum neuron-derived EVs, the study further explored how exenatide may modify brain insulin signaling pathways. Exenatide treatment led to the augmented activation of proteins involved in insulin signaling in the brain and of effectors, such as Akt and mTOR. This increased activation was correlated with clinical improvements in PD symptoms. These findings suggest the potential neuroprotective effects of exenatide and indicate its utility as a biomarker of the treatment response in PD [98]. However, NLY01, a brain-penetrant, pegylated, longer-lasting version of exenatide, provided no significant benefits in PD patients with a H&Y stage of ≤ 2.5 and an average disease duration of 1 year, as assessed by the MDS-UPDRS Parts II and III scores. A subgroup analysis revealed significant improvement in PD patients aged < 60 years, suggesting the age-dependent efficacy of NLY01 in PD [99].
A phase II RCT of lixisenatide in early PD, which was defined as patients recruited within 2 years of diagnosis, also demonstrated the promising disease-modifying effect of lixisenatide, particularly regarding motor disability. After treatment for 12 months, MDS-UPDRS Part III scores improved slightly by 0.04 points in the lixisenatide group and decreased by 3.04 points in the placebo group. Following a 2-month washout period, the mean MDS-UPDRS motor scores in the off-medication state were 2.9 points higher in the placebo group, underscoring the potential benefits of lixisenatide in early PD [100]. These results indicate a class effect of GLP-1RAs on neuroprotection in PD. At present, a large-scale phase III RCT of 2-year exenatide treatment in patients with PD, defined as a Hoen and Yahr (H&Y) stage of ≤ 2.5, is ongoing; the results are expected to be reported in 2025.
Summary of completed and ongoing clinical trials of GLP-1RAs for neurodegeneration
In summary, clinical trials investigating GLP-1RAs in neurodegenerative diseases have yielded contrasting outcomes. In individuals with AD, GLP-1RAs did not exert disease-modifying effects on cognition. However, in patients with diabetes, treatment with GLP-1RAs offered cognitive benefits beyond their blood glucose-lowering effect. In PD, several GLP-1RAs have demonstrated promising disease-modifying effects in phase II RCTs, and the results of phase III trials are anticipated. These discrepancies might be attributed to the timing of GLP-1RA intervention in the disease course. AD is characterized by a decade-long prodromal stage, and dementia diagnosis typically occurs in the late biological stage of AD, by which time substantial amounts of Aβ and tau proteins have accumulated in the cortex [101]. Individuals at risk of AD or with diabetes may be in the early or middle biological stage of AD, offering a greater opportunity for GLP-1RAs to provide neuroprotection. By contrast, PD, which also has a prolonged prodromal stage similar to AD before the onset of motor symptoms, has a different progression of Lewy body pathology, which may take another decade to pervade the entire brain and cause significant, widespread neurodegeneration [102]. Moreover, the complex pathogenesis of PD makes it a suitable target for GLP-1RAs, which modulate numerous intracellular signaling pathways, as demonstrated by in vitro and in vivo studies.
Challenges and future directions
Currently, GLP-1RAs have shown potential for mitigating neurodegeneration. Regarding the underlying mechanism, IR in the brain significantly contributes to neurodegenerative processes, and GLP-1RAs modulate downstream signaling pathways, including mitochondrial biogenesis, autophagy, inflammation, and cell survival. Additionally, the safety and tolerability profiles of GLP-1RAs have been well-established; thus, GLP-1RAs are favorable candidates for repurposing for the treatment of neurodegenerative diseases. However, several challenges remain. Unfavorable outcomes from AD clinical trials underscore the importance of the timing of treatment; GLP-1RAs may not effectively reverse disease progression in mid-to-late stages. Moreover, the persistence of GLP-1RAs’ beneficial effects remains uncertain. In PD clinical trials, the beneficial effects have been observed for only 1 year—a substantially short period considering the lifelong, progressive nature of neurodegeneration. Thus, future clinical trials must assess the long-term effect of GLP-1RA treatment. Lastly, the systemic effects of GLP-1RAs, such as body weight loss [103] and potential reduction in muscle mass [104], pose significant challenges for elderly individuals with neurodegenerative diseases. Lastly, the limited evidence on the effects of GLP-1 RAs on key pathognomonic proteins, Aβ and α-synuclein, weakens the scientific basis for considering GLP-1 RAs as major game-changers for neurodegenerative diseases. These factors necessitate the careful consideration of the use of GLP-1RAs in the broader context of managing and treating these neurodegenerative diseases.
The design of future clinical trials for GLP-1 receptor agonists (GLP-1RAs) targeting AD and PD may necessitate distinct approaches. In the context of AD, the focus should be on individuals at risk, such as those with MCI, subjective cognitive decline, elders with positive Aβ or tau biomarkers, APOE-ε4-carriers or healthy elderly individuals with high-risk scores [105]. Given the pronounced effect of tau elimination observed in in vitro and in vivo studies, selecting patients who test positive for tau biomarkers (CSF, blood, or positron emission tomography) could enhance the likelihood of achieving significant disease modification. Currently, AD patients with positive tau pathology exhibit poor responsiveness to anti-Aβ antibody treatments, which are ineffective in reducing tau levels [106]. In addition, it is recommended that the duration of future clinical trials of GLP-1RAs for AD extend to 2 to 3 years to evaluate long-term outcomes, including changes in cognition, activities of daily living, instrumental activities of daily living, and biomarkers for tau and Aβ.
In contrast, current promising clinical trials for PD primarily focus on early-stage patients with short disease duration and H&Y stage of ≤ 2.5. It may also be beneficial to consider individuals at high risk for prodromal PD. However, unlike the relatively predictable conversion from MCI to AD, the progression from hyposmia or rapid eye movement sleep behavior disorder to PD is variable [107], complicating the assessment of GLP-1RAs’ efficacy in preventing phenotype conversion. The development of novel techniques for ultra-sensitive α-synuclein detection, particularly real-time quaking-induced conversion [108], could improve the accuracy of identifying prodromal PD and serve as reliable biomarkers for treatment response. In addition, LRRK2 G2019S carriers without clinical diagnosed PD may also be ideal as prodromal PD candidates for clinical trials.
In summary, future clinical trials should prioritize enrolling individuals at risk, followed by those in the early stages of the disease, as this offers a higher likelihood of demonstrating neuroprotective and disease-modifying effects. GLP-1 RAs may need to be combined with other agents targeting the clearance of Aβ or α-synuclein to achieve a more effective disease-modifying outcome. For AD specifically, given the well-established effects of GLP-1 RAs on tau hyperphosphorylation, we recommend that future GLP-1 RA clinical trials focus on the tau-positive subgroup of patients.
Conclusion
Beyond their established role as potent antihyperglycemic agents, GLP-1RAs show potential for treating neurodegenerative diseases with disease-modifying effects. Both in vivo and in vitro studies provide substantial evidence of the neuroprotective benefits of GLP-1RAs through the modulation of inflammation, mitochondrial function, ROS, tau hyperphosphorylation, and anti-apoptotic pathways. However, while clinical trial results have been promising for PD, they have been less favorable in AD. In future RCTs, key considerations such as the optimal timing of intervention, treatment duration, specific patient subgroups based on molecular diagnosis, and potential systemic adverse events require further investigation.
Acknowledgements
The authors acknowledge the academic and science graphic illustration service provided by TMU Office of Research and Development.
Author contributions
Conceptualization, CT Hong and CJ Hu; resources, CJ Hu; data curation, CT Hong and JH Chen; writing—original draft preparation, CT Hong and JH Chen—review and editing, CJ Hu; funding acquisition, CJ Hu.
Funding
Ministry of Education (MOE) in Taiwan, Higher Education Sprout Project.
Availability of data and materials
Not applicable due to the nature of review. No new data was generated.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang Y, Li Y, Pan H, Han L. Global, regional, and national burden of neurological disorders in 204 countries and territories worldwide. J Glob Health. 2023;13:04160. 10.7189/jogh.13.04160. (Epub 20231129). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deuschl G, Beghi E, Fazekas F, Varga T, Christoforidi KA, Sipido E, et al. The burden of neurological diseases in Europe: an analysis for the Global Burden of Disease Study 2017. Lancet Public Health. 2020;5(10):e551–67. 10.1016/s2468-2667(20)30190-0. [DOI] [PubMed] [Google Scholar]
- 3.Malpetti M, Joie R, Rabinovici GD. Tau beats amyloid in predicting brain atrophy in Alzheimer disease: implications for prognosis and clinical trials. J Nucl Med. 2022;63(6):830–2. 10.2967/jnumed.121.263694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hampel H, Hardy J, Blennow K, Chen C, Perry G, Kim SH, et al. The amyloid-β pathway in Alzheimer’s disease. Mol Psychiatry. 2021;26(10):5481–503. 10.1038/s41380-021-01249-0. (Epub 20210830). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anstey KJ, Ee N, Eramudugolla R, Jagger C, Peters R. A systematic review of meta-analyses that evaluate risk factors for dementia to evaluate the quantity, quality, and global representativeness of evidence. J Alzheimers Dis. 2019;70(s1):S165–86. 10.3233/jad-190181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole CS, Dafoe A, Tietbohl CK, Jordan SR, Huebschmann AG, Lum HD, et al. Care challenges of home health patients living with dementia: a pathway forward with palliative care. BMC Palliat Care. 2023;22(1):122. 10.1186/s12904-023-01247-9. (Epub 20230829). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willis AW, Roberts E, Beck JC, Fiske B, Ross W, Savica R, et al. Incidence of Parkinson disease in North America. NPJ Parkinsons Dis. 2022;8(1):170. 10.1038/s41531-022-00410-y. (Epub 20221215). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15(10):565–81. 10.1038/s41582-019-0244-7. (Epub 20190909). [DOI] [PubMed] [Google Scholar]
- 9.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7(4):278–94. 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yiannopoulou KG, Papageorgiou SG. Current and future treatments in alzheimer disease: an update. J Cent Nerv Syst Dis. 2020;12:1179573520907397. 10.1177/1179573520907397. (Epub 20200229). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kieburtz K, Katz R, McGarry A, Olanow CW. A new approach to the development of disease-modifying therapies for PD; fighting another pandemic. Mov Disord. 2021;36(1):59–63. 10.1002/mds.28310. (Epub 20201007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santiago JA, Karthikeyan M, Lackey M, Villavicencio D, Potashkin JA. Diabetes: a tipping point in neurodegenerative diseases. Trends Mol Med. 2023;29(12):1029–44. 10.1016/j.molmed.2023.09.005. (Epub 20231010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de la Monte SM, Wands JR. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2(6):1101–13. 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Monte SM. Insulin resistance and neurodegeneration: progress towards the development of new therapeutics for Alzheimer’s disease. Drugs. 2017;77(1):47–65. 10.1007/s40265-016-0674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Femminella GD, Livingston NR, Raza S, van der Doef T, Frangou E, Love S, et al. Does insulin resistance influence neurodegeneration in non-diabetic Alzheimer’s subjects? Alzheimers Res Ther. 2021;13(1):47. 10.1186/s13195-021-00784-w. (Epub 20210217). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel VN, Chorawala MR, Shah MB, Shah KC, Dave BP, Shah MP, et al. Emerging pathophysiological mechanisms linking diabetes mellitus and Alzheimer’s disease: an old wine in a new bottle. J Alzheimers Dis Rep. 2022;6(1):349–57. 10.3233/adr-220021. (Epub 20220622). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vargas-Soria M, Ramos-Rodriguez JJ, Del Marco A, Hierro-Bujalance C, Carranza-Naval MJ, Calvo-Rodriguez M, et al. Accelerated amyloid angiopathy and related vascular alterations in a mixed murine model of Alzheimer’s disease and type two diabetes. Fluids Barriers CNS. 2022;19(1):88. 10.1186/s12987-022-00380-6. (Epub 20221107). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong CT, Chen KY, Wang W, Chiu JY, Wu D, Chao TY, et al. Insulin resistance promotes Parkinson’s disease through aberrant expression of α-synuclein, mitochondrial dysfunction, and deregulation of the polo-like kinase 2 signaling. Cells. 2020;9(3):740. 10.3390/cells9030740. (Epub 20200317). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H, Sun T, He X, Wang Z, Zhao K, An J, et al. Association between Parkinson’s disease and diabetes mellitus: from epidemiology, pathophysiology and prevention to treatment. Aging Dis. 2022;13(6):1591–605. 10.14336/ad.2022.0325. (Epub 20221201). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henrich MT, Oertel WH, Surmeier DJ, Geibl FF. Mitochondrial dysfunction in Parkinson’s disease—a key disease hallmark with therapeutic potential. Mol Neurodegener. 2023;18(1):83. 10.1186/s13024-023-00676-7. (Epub 20231111). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tansey MG, Wallings RL, Houser MC, Herrick MK, Keating CE, Joers V. Inflammation and immune dysfunction in Parkinson disease. Nat Rev Immunol. 2022;22(11):657–73. 10.1038/s41577-022-00684-6. (Epub 20220304). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lv YQ, Yuan L, Sun Y, Dou HW, Su JH, Hou ZP, et al. Long-term hyperglycemia aggravates α-synuclein aggregation and dopaminergic neuronal loss in a Parkinson’s disease mouse model. Transl Neurodegener. 2022;11(1):14. 10.1186/s40035-022-00288-z. (Epub 20220307). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Zhang Y, Shi X, Han J, Lin B, Peng W, et al. Metformin and the risk of neurodegenerative diseases in patients with diabetes: a meta-analysis of population-based cohort studies. Diabet Med. 2022;39(6): e14821. 10.1111/dme.14821. (Epub 20220308). [DOI] [PubMed] [Google Scholar]
- 24.Cao G, Gong T, Du Y, Wang Y, Ge T, Liu J. Mechanism of metformin regulation in central nervous system: progression and future perspectives. Biomed Pharmacother. 2022;156: 113686. 10.1016/j.biopha.2022.113686. (Epub 20221013). [DOI] [PubMed] [Google Scholar]
- 25.Zhang QQ, Li WS, Liu Z, Zhang HL, Ba YG, Zhang RX. Metformin therapy and cognitive dysfunction in patients with type 2 diabetes: a meta-analysis and systematic review. Medicine. 2020;99(10): e19378. 10.1097/md.0000000000019378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahlqvist ML, Lee M-S, Hsu C-C, Chuang S-Y, Lee J-T, Tsai H-N. Metformin-inclusive sulfonylurea therapy reduces the risk of Parkinson’s disease occurring with type 2 diabetes in a Taiwanese population cohort. Parkinsonism Relat Disord. 2012;18(6):753–8. 10.1016/j.parkreldis.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Koenig AM, Mechanic-Hamilton D, Xie SX, Combs MF, Cappola AR, Xie L, et al. Effects of the insulin sensitizer metformin in Alzheimer disease: pilot data from a randomized placebo-controlled crossover study. Alzheimer Dis Assoc Disord. 2017;31(2):107–13. 10.1097/wad.0000000000000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim WJ, Noh JH, Han K, Park CY. The association between second-line oral antihyperglycemic medication on types of dementia in type 2 diabetes: a nationwide real-world longitudinal study. J Alzheimers Dis. 2021;81(3):1263–72. 10.3233/jad-201535. [DOI] [PubMed] [Google Scholar]
- 29.Tang X, Brinton RD, Chen Z, Farland LV, Klimentidis Y, Migrino R, et al. Use of oral diabetes medications and the risk of incident dementia in US veterans aged ≥60 years with type 2 diabetes. BMJ Open Diabetes Res Care. 2022;10(5): e002894. 10.1136/bmjdrc-2022-002894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussain S, Singh A, Baxi H, Taylor B, Burgess J, Antony B. Thiazolidinedione use is associated with reduced risk of Parkinson’s disease in patients with diabetes: a meta-analysis of real-world evidence. Neurol Sci. 2020;41(12):3697–703. 10.1007/s10072-020-04494-3. (Epub 20200609). [DOI] [PubMed] [Google Scholar]
- 31.Zhao H, Zhuo L, Sun Y, Shen P, Lin H, Zhan S. Thiazolidinedione use and risk of Parkinson’s disease in patients with type 2 diabetes mellitus. NPJ Parkinsons Dis. 2022;8(1):138. 10.1038/s41531-022-00406-8. (Epub 20221021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapadia R, Yi JH, Vemuganti R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front Biosci. 2008;13:1813–26. 10.2741/2802. (Epub 20080101). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burns DK, Alexander RC, Welsh-Bohmer KA, Culp M, Chiang C, O’Neil J, et al. Safety and efficacy of pioglitazone for the delay of cognitive impairment in people at risk of Alzheimer’s disease (TOMMORROW): a prognostic biomarker study and a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2021;20(7):537–47. 10.1016/s1474-4422(21)00043-0. [DOI] [PubMed] [Google Scholar]
- 34.Hanyu H, Sato T, Kiuchi A, Sakurai H, Iwamoto T. Pioglitazone improved cognition in a pilot study on patients with Alzheimer’s disease and mild cognitive impairment with diabetes mellitus. J Am Geriatr Soc. 2009;57(1):177–9. 10.1111/j.1532-5415.2009.02067.x. [DOI] [PubMed] [Google Scholar]
- 35.Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T. Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging. 2011;32(9):1626–33. 10.1016/j.neurobiolaging.2009.10.009. (Epub 20091117). [DOI] [PubMed] [Google Scholar]
- 36.Geldmacher DS, Fritsch T, McClendon MJ, Landreth G. A randomized pilot clinical trial of the safety of pioglitazone in treatment of patients with Alzheimer disease. Arch Neurol. 2011;68(1):45–50. 10.1001/archneurol.2010.229. (Epub 20100913). [DOI] [PubMed] [Google Scholar]
- 37.Gold M, Alderton C, Zvartau-Hind M, Egginton S, Saunders AM, Irizarry M, et al. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: results from a randomized, double-blind, placebo-controlled phase III study. Dement Geriatr Cogn Disord. 2010;30(2):131–46. 10.1159/000318845. (Epub 20100821). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pioglitazone in early Parkinson’s disease: a phase 2, multicentre, double-blind, randomised trial. Lancet Neurol. 2015;14(8):795–803. 10.1016/s1474-4422(15)00144-1. (Epub 20150623). [DOI] [PMC free article] [PubMed]
- 39.MacDonald PE, El-kholy W, Riedel MJ, Salapatek AMF, Light PE, Wheeler MB. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51(suppl_3):S434–42. 10.2337/diabetes.51.2007.S434. [DOI] [PubMed] [Google Scholar]
- 40.Nachawi N, Rao PP, Makin V. The role of GLP-1 receptor agonists in managing type 2 diabetes. Clevel Clin J Med. 2022;89(8):457–64. 10.3949/ccjm.89a.21110. (Epub 20220801). [DOI] [PubMed] [Google Scholar]
- 41.Del Olmo-Garcia MI, Merino-Torres JF. GLP-1 receptor agonists and cardiovascular disease in patients with type 2 diabetes. J Diabetes Res. 2018;2018:4020492. 10.1155/2018/4020492. (Epub 20180402). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Granata A, Maccarrone R, Anzaldi M, Leonardi G, Pesce F, Amico F, et al. GLP-1 receptor agonists and renal outcomes in patients with diabetes mellitus type 2 and diabetic kidney disease: state of the art. Clin Kidney J. 2022;15(9):1657–65. 10.1093/ckj/sfac069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giugliano D, Scappaticcio L, Longo M, Caruso P, Maiorino MI, Bellastella G, et al. GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 2021;20(1):189. 10.1186/s12933-021-01366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Csajbók ÉA, Kocsis ÁK, Faragó N, Furdan S, Kovács B, Lovas S, et al. Expression of GLP-1 receptors in insulin-containing interneurons of rat cerebral cortex. Diabetologia. 2019;62(4):717–25. 10.1007/s00125-018-4803-z. (Epub 2019/01/15). [DOI] [PubMed] [Google Scholar]
- 45.Li Y, Bader M, Tamargo I, Rubovitch V, Tweedie D, Pick CG, et al. Liraglutide is neurotrophic and neuroprotective in neuronal cultures and mitigates mild traumatic brain injury in mice. J Neurochem. 2015;135(6):1203–17. 10.1111/jnc.13169. (Epub 20150618). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang M, Yoon G, Song J, Jo J. Exendin-4 improves long-term potentiation and neuronal dendritic growth in vivo and in vitro obesity condition. Sci Rep. 2021;11(1):8326. 10.1038/s41598-021-87809-4. (Epub 20210415). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.An FM, Chen S, Xu Z, Yin L, Wang Y, Liu AR, et al. Glucagon-like peptide-1 regulates mitochondrial biogenesis and tau phosphorylation against advanced glycation end product-induced neuronal insult: studies in vivo and in vitro. Neuroscience. 2015;300:75–84. 10.1016/j.neuroscience.2015.05.023. (Epub 2015/05/20). [DOI] [PubMed] [Google Scholar]
- 48.Jin J, Kang HM, Jung J, Jeong JW, Park C. Related expressional change of HIF-1α to the neuroprotective activity of exendin-4 in transient global ischemia. NeuroReport. 2014;25(1):65–70. 10.1097/wnr.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 49.Liu XY, Wang LX, Chen Z, Liu LB. Liraglutide prevents beta-amyloid-induced neurotoxicity in SH-SY5Y cells via a PI3K-dependent signaling pathway. Neurol Res. 2016;38(4):313–9. 10.1080/01616412.2016.1145914. (Epub 20160423). [DOI] [PubMed] [Google Scholar]
- 50.Wu HY, Tang XQ, Mao XF, Wang YX. Autocrine interleukin-10 mediates glucagon-like peptide-1 receptor-induced spinal microglial β-endorphin expression. J Neurosci. 2017;37(48):11701–14. 10.1523/jneurosci.1799-17.2017. (Epub 20171030). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qian Z, Chen H, Xia M, Chang J, Li X, Ye S, et al. Activation of glucagon-like peptide-1 receptor in microglia attenuates neuroinflammation-induced glial scarring via rescuing Arf and Rho GAP adapter protein 3 expressions after nerve injury. Int J Biol Sci. 2022;18(4):1328–46. 10.7150/ijbs.68974. (Epub 20220116). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Song S, Guo R, Mehmood A, Zhang L, Yin B, Yuan C, et al. Liraglutide attenuate central nervous inflammation and demyelination through AMPK and pyroptosis-related NLRP3 pathway. CNS Neurosci Ther. 2022;28(3):422–34. 10.1111/cns.13791. (Epub 20220105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y, Glotfelty EJ, Karlsson T, Fortuno LV, Harvey BK, Greig NH. The metabolite GLP-1 (9-36) is neuroprotective and anti-inflammatory in cellular models of neurodegeneration. J Neurochem. 2021;159(5):867–86. 10.1111/jnc.15521. (Epub 20211021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kong FJ, Wu JH, Sun SY, Ma LL, Zhou JQ. Liraglutide ameliorates cognitive decline by promoting autophagy via the AMP-activated protein kinase/mammalian target of rapamycin pathway in a streptozotocin-induced mouse model of diabetes. Neuropharmacology. 2018;131:316–25. 10.1016/j.neuropharm.2018.01.001. (Epub 20180103). [DOI] [PubMed] [Google Scholar]
- 55.Liu DX, Zhao CS, Wei XN, Ma YP, Wu JK. Semaglutide protects against 6-OHDA toxicity by enhancing autophagy and inhibiting oxidative stress. Parkinsons Dis. 2022;2022:6813017. 10.1155/2022/6813017. (Epub 20220713). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang MD, Huang Y, Zhang GP, Mao L, Xia YP, Mei YW, et al. Exendin-4 improved rat cortical neuron survival under oxygen/glucose deprivation through PKA pathway. Neuroscience. 2012;226:388–96. 10.1016/j.neuroscience.2012.09.025. (Epub 20120919). [DOI] [PubMed] [Google Scholar]
- 57.Tsukamoto M, Niimi N, Sango K, Takaku S, Kanazawa Y, Utsunomiya K. Neurotrophic and neuroprotective properties of exendin-4 in adult rat dorsal root ganglion neurons: involvement of insulin and RhoA. Histochem Cell Biol. 2015;144(3):249–59. 10.1007/s00418-015-1333-3. (Epub 20150531). [DOI] [PubMed] [Google Scholar]
- 58.Fernandes LS, Dos Santos NAG, Emerick GL, Dos Santos AC. The antidiabetic drug liraglutide minimizes the non-cholinergic neurotoxicity of the pesticide mipafox in SH-SY5Y cells. Neurotox Res. 2019;35(1):150–9. 10.1007/s12640-018-9941-z. (Epub 20180807). [DOI] [PubMed] [Google Scholar]
- 59.Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:33. 10.1186/1471-2202-13-33. (Epub 20120323). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang Y, Zhang J, Ma D, Zhang M, Hu S, Shao S, et al. Subcutaneous administration of liraglutide ameliorates Alzheimer-associated tau hyperphosphorylation in rats with type 2 diabetes. J Alzheimers Dis. 2013;37(3):637–48. 10.3233/jad-130491. (Epub 2013/08/21). [DOI] [PubMed] [Google Scholar]
- 61.Xiong H, Zheng C, Wang J, Song J, Zhao G, Shen H, et al. The neuroprotection of liraglutide on Alzheimer-like learning and memory impairment by modulating the hyperphosphorylation of tau and neurofilament proteins and insulin signaling pathways in mice. J Alzheimers Dis. 2013;37(3):623–35. 10.3233/jad-130584. (Epub 2013/09/07). [DOI] [PubMed] [Google Scholar]
- 62.Ma DL, Chen FQ, Xu WJ, Yue WZ, Yuan G, Yang Y. Early intervention with glucagon-like peptide 1 analog liraglutide prevents tau hyperphosphorylation in diabetic db/db mice. J Neurochem. 2015;135(2):301–8. 10.1111/jnc.13248. (Epub 2015/07/18). [DOI] [PubMed] [Google Scholar]
- 63.Candeias E, Sebastião I, Cardoso S, Carvalho C, Santos MS, Oliveira CR, et al. Brain GLP-1/IGF-1 signaling and autophagy mediate exendin-4 protection against apoptosis in type 2 diabetic rats. Mol Neurobiol. 2018;55(5):4030–50. 10.1007/s12035-017-0622-3. (Epub 2017/06/03). [DOI] [PubMed] [Google Scholar]
- 64.Kang X, Wang D, Zhang L, Huang T, Liu S, Feng X, et al. Exendin-4 ameliorates tau hyperphosphorylation and cognitive impairment in type 2 diabetes through acting on Wnt/β-catenin/NeuroD1 pathway. Mol Med. 2023;29(1):118. 10.1186/s10020-023-00718-2. (Epub 2023/09/05). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu W, Yang Y, Yuan G, Zhu W, Ma D, Hu S. Exendin-4, a glucagon-like peptide-1 receptor agonist, reduces Alzheimer disease-associated tau hyperphosphorylation in the hippocampus of rats with type 2 diabetes. J Investig Med. 2015;63(2):267–72. 10.1097/jim.0000000000000129. (Epub 2014/12/06). [DOI] [PubMed] [Google Scholar]
- 66.Chen S, Yin L, Xu Z, An FM, Liu AR, Wang Y, et al. Inhibiting receptor for advanced glycation end product (AGE) and oxidative stress involved in the protective effect mediated by glucagon-like peptide-1 receptor on AGE induced neuronal apoptosis. Neurosci Lett. 2016;612:193–8. 10.1016/j.neulet.2015.12.007. (Epub 2015/12/19). [DOI] [PubMed] [Google Scholar]
- 67.Chow HM, Shi M, Cheng A, Gao Y, Chen G, Song X, et al. Age-related hyperinsulinemia leads to insulin resistance in neurons and cell-cycle-induced senescence. Nat Neurosci. 2019;22(11):1806–19. 10.1038/s41593-019-0505-1. (Epub 2019/10/23). [DOI] [PubMed] [Google Scholar]
- 68.Zhao L, Li Z, Vong JSL, Chen X, Lai HM, Yan LYC, et al. Pharmacologically reversible zonation-dependent endothelial cell transcriptomic changes with neurodegenerative disease associations in the aged brain. Nat Commun. 2020;11(1):4413. 10.1038/s41467-020-18249-3. (Epub 2020/09/06). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li Z, Chen X, Vong JSL, Zhao L, Huang J, Yan LYC, et al. Systemic GLP-1R agonist treatment reverses mouse glial and neurovascular cell transcriptomic aging signatures in a genome-wide manner. Commun Biol. 2021;4(1):656. 10.1038/s42003-021-02208-9. (Epub 2021/06/04). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Chen S, Xu Z, Chen S, Yao W, Gao X. GLP-1 receptor agonists downregulate aberrant GnT-III expression in Alzheimer’s disease models through the Akt/GSK-3β/β-catenin signaling. Neuropharmacology. 2018;131:190–9. 10.1016/j.neuropharm.2017.11.048. (Epub 2017/12/11). [DOI] [PubMed] [Google Scholar]
- 71.Jia XT, Ye T, Yuan L, Zhang GJ, Liu ZQ, Di ZL, et al. Exendin-4, a glucagon-like peptide 1 receptor agonist, protects against amyloid-β peptide-induced impairment of spatial learning and memory in rats. Physiol Behav. 2016;159:72–9. 10.1016/j.physbeh.2016.03.016. (Epub 2016/03/20). [DOI] [PubMed] [Google Scholar]
- 72.Garabadu D, Verma J. Exendin-4 attenuates brain mitochondrial toxicity through PI3K/Akt-dependent pathway in amyloid beta (1-42)-induced cognitive deficit rats. Neurochem Int. 2019;128:39–49. 10.1016/j.neuint.2019.04.006. (Epub 2019/04/21). [DOI] [PubMed] [Google Scholar]
- 73.Wang X, Wang L, Jiang R, Yuan Y, Yu Q, Li Y. Exendin-4 antagonizes Aβ1-42-induced suppression of long-term potentiation by regulating intracellular calcium homeostasis in rat hippocampal neurons. Brain Res. 2015;1627:101–8. 10.1016/j.brainres.2015.09.015. (Epub 2015/09/24). [DOI] [PubMed] [Google Scholar]
- 74.Robinson A, Lubitz I, Atrakchi-Baranes D, Licht-Murava A, Katsel P, Leroith D, et al. Combination of insulin with a GLP1 agonist is associated with better memory and normal expression of insulin receptor pathway genes in a mouse model of Alzheimer’s disease. J Mol Neurosci. 2019;67(4):504–10. 10.1007/s12031-019-1257-9. (Epub 2019/01/13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.An J, Zhou Y, Zhang M, Xie Y, Ke S, Liu L, et al. Exenatide alleviates mitochondrial dysfunction and cognitive impairment in the 5×FAD mouse model of Alzheimer’s disease. Behav Brain Res. 2019;370: 111932. 10.1016/j.bbr.2019.111932. (Epub 2019/05/15). [DOI] [PubMed] [Google Scholar]
- 76.Bomba M, Granzotto A, Castelli V, Onofrj M, Lattanzio R, Cimini A, et al. Exenatide reverts the high-fat-diet-induced impairment of BDNF signaling and inflammatory response in an animal model of Alzheimer’s disease. J Alzheimers Dis. 2019;70(3):793–810. 10.3233/jad-190237. (Epub 2019/07/01). [DOI] [PubMed] [Google Scholar]
- 77.Bomba M, Ciavardelli D, Silvestri E, Canzoniero LM, Lattanzio R, Chiappini P, et al. Exenatide promotes cognitive enhancement and positive brain metabolic changes in PS1-KI mice but has no effects in 3xTg-AD animals. Cell Death Dis. 2013;4(5): e612. 10.1038/cddis.2013.139. (Epub 2013/05/04). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Qi L, Ke L, Liu X, Liao L, Ke S, Liu X, et al. Subcutaneous administration of liraglutide ameliorates learning and memory impairment by modulating tau hyperphosphorylation via the glycogen synthase kinase-3β pathway in an amyloid β protein induced Alzheimer disease mouse model. Eur J Pharmacol. 2016;783:23–32. 10.1016/j.ejphar.2016.04.052. (Epub 2016/05/02). [DOI] [PubMed] [Google Scholar]
- 79.Hansen HH, Barkholt P, Fabricius K, Jelsing J, Terwel D, Pyke C, et al. The GLP-1 receptor agonist liraglutide reduces pathology-specific tau phosphorylation and improves motor function in a transgenic hTauP301L mouse model of tauopathy. Brain Res. 2016;1634:158–70. 10.1016/j.brainres.2015.12.052. (Epub 2016/01/10). [DOI] [PubMed] [Google Scholar]
- 80.Xie Y, Zheng J, Li S, Li H, Zhou Y, Zheng W, et al. GLP-1 improves the neuronal supportive ability of astrocytes in Alzheimer’s disease by regulating mitochondrial dysfunction via the cAMP/PKA pathway. Biochem Pharmacol. 2021;188: 114578. 10.1016/j.bcp.2021.114578. (Epub 2021/04/26). [DOI] [PubMed] [Google Scholar]
- 81.Hansen HH, Fabricius K, Barkholt P, Kongsbak-Wismann P, Schlumberger C, Jelsing J, et al. Long-term treatment with liraglutide, a glucagon-like peptide-1 (GLP-1) receptor agonist, has no effect on β-amyloid plaque load in two transgenic APP/PS1 mouse models of Alzheimer’s disease. PLoS ONE. 2016;11(7): e0158205. 10.1371/journal.pone.0158205. (Epub 2016/07/16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Batista AF, Forny-Germano L, Clarke JR, Lyra ESNM, Brito-Moreira J, Boehnke SE, et al. The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease. J Pathol. 2018;245(1):85–100. 10.1002/path.5056. (Epub 2018/02/13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holubová M, Hrubá L, Popelová A, Bencze M, Pražienková V, Gengler S, et al. Liraglutide and a lipidized analog of prolactin-releasing peptide show neuroprotective effects in a mouse model of β-amyloid pathology. Neuropharmacology. 2019;144:377–87. 10.1016/j.neuropharm.2018.11.002. (Epub 2018/11/15). [DOI] [PubMed] [Google Scholar]
- 84.Wang ZJ, Han YF, Zhao F, Yang GZ, Yuan L, Cai HY, et al. A dual GLP-1 and Gcg receptor agonist rescues spatial memory and synaptic plasticity in APP/PS1 transgenic mice. Horm Behav. 2020;118: 104640. 10.1016/j.yhbeh.2019.104640. (Epub 2019/11/26). [DOI] [PubMed] [Google Scholar]
- 85.Park JS, Kam TI, Lee S, Park H, Oh Y, Kwon SH, et al. Blocking microglial activation of reactive astrocytes is neuroprotective in models of Alzheimer’s disease. Acta Neuropathol Commun. 2021;9(1):78. 10.1186/s40478-021-01180-z. (Epub 2021/04/28). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li T, Jiao JJ, Hölscher C, Wu MN, Zhang J, Tong JQ, et al. A novel GLP-1/GIP/Gcg triagonist reduces cognitive deficits and pathology in the 3xTg mouse model of Alzheimer’s disease. Hippocampus. 2018;28(5):358–72. 10.1002/hipo.22837. (Epub 2018/02/24). [DOI] [PubMed] [Google Scholar]
- 87.Liu W, Jalewa J, Sharma M, Li G, Li L, Hölscher C. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience. 2015;303:42–50. 10.1016/j.neuroscience.2015.06.054. (Epub 2015/07/05). [DOI] [PubMed] [Google Scholar]
- 88.Cao B, Zhang Y, Chen J, Wu P, Dong Y, Wang Y. Neuroprotective effects of liraglutide against inflammation through the AMPK/NF-κB pathway in a mouse model of Parkinson’s disease. Metab Brain Dis. 2022;37(2):451–62. 10.1007/s11011-021-00879-1. (Epub 2021/11/25). [DOI] [PubMed] [Google Scholar]
- 89.Jalewa J, Sharma MK, Gengler S, Hölscher C. A novel GLP-1/GIP dual receptor agonist protects from 6-OHDA lesion in a rat model of Parkinson’s disease. Neuropharmacology. 2017;117:238–48. 10.1016/j.neuropharm.2017.02.013. (Epub 2017/02/23). [DOI] [PubMed] [Google Scholar]
- 90.Ji C, Xue GF, Lijun C, Feng P, Li D, Li L, et al. A novel dual GLP-1 and GIP receptor agonist is neuroprotective in the MPTP mouse model of Parkinson’s disease by increasing expression of BNDF. Brain Res. 2016;1634:1–11. 10.1016/j.brainres.2015.09.035. (Epub 2015/10/11). [DOI] [PubMed] [Google Scholar]
- 91.Cheng H, Zhang Z, Zhang B, Zhang W, Wang J, Ni W, et al. Enhancement of impaired olfactory neural activation and cognitive capacity by liraglutide, but not dapagliflozin or acarbose, in patients with type 2 diabetes: a 16-week randomized parallel comparative study. Diabetes Care. 2022;45(5):1201–10. 10.2337/dc21-2064. (Epub 2022/03/10). [DOI] [PubMed] [Google Scholar]
- 92.Li Q, Jia M, Yan Z, Li Q, Sun F, He C, et al. Activation of glucagon-like peptide-1 receptor ameliorates cognitive decline in type 2 diabetes mellitus through a metabolism-independent pathway. J Am Heart Assoc. 2021;10(14): e020734. 10.1161/jaha.120.020734. (Epub 20210710). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cukierman-Yaffe T, Gerstein HC, Colhoun HM, Diaz R, García-Pérez LE, Lakshmanan M, et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol. 2020;19(7):582–90. 10.1016/s1474-4422(20)30173-3. (Epub 2020/06/21). [DOI] [PubMed] [Google Scholar]
- 94.Watson KT, Wroolie TE, Tong G, Foland-Ross LC, Frangou S, Singh M, et al. Neural correlates of liraglutide effects in persons at risk for Alzheimer’s disease. Behav Brain Res. 2019;356:271–8. 10.1016/j.bbr.2018.08.006. (Epub 2018/08/14). [DOI] [PubMed] [Google Scholar]
- 95.Mullins RJ, Mustapic M, Chia CW, Carlson O, Gulyani S, Tran J, et al. A pilot study of exenatide actions in Alzheimer’s disease. Curr Alzheimer Res. 2019;16(8):741–52. 10.2174/1567205016666190913155950. (Epub 2019/09/14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, et al. Exenatide and the treatment of patients with Parkinson’s disease. J Clin Invest. 2013;123(6):2730–6. 10.1172/jci68295. (Epub 2013/06/04). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390(10103):1664–75. 10.1016/s0140-6736(17)31585-4. (Epub 2017/08/07). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Athauda D, Gulyani S, Karnati HK, Li Y, Tweedie D, Mustapic M, et al. Utility of neuronal-derived exosomes to examine molecular mechanisms that affect motor function in patients with parkinson disease: a secondary analysis of the exenatide-PD trial. JAMA Neurol. 2019;76(4):420–9. 10.1001/jamaneurol.2018.4304. (Epub 2019/01/15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McGarry A, Rosanbalm S, Leinonen M, Olanow CW, To D, Bell A, et al. Safety, tolerability, and efficacy of NLY01 in early untreated Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2024;23(1):37–45. 10.1016/s1474-4422(23)00378-2. [DOI] [PubMed] [Google Scholar]
- 100.Meissner WG, Remy P, Giordana C, Maltête D, Derkinderen P, Houéto JL, et al. Trial of lixisenatide in early Parkinson’s disease. N Engl J Med. 2024;390(13):1176–85. 10.1056/NEJMoa2312323. [DOI] [PubMed] [Google Scholar]
- 101.Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9(1):119–28. 10.1016/s1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318(1):121–34. 10.1007/s00441-004-0956-9. (Epub 20040824). [DOI] [PubMed] [Google Scholar]
- 103.Wilbon SS, Kolonin MG. GLP1 receptor agonists-effects beyond obesity and diabetes. Cells. 2023;13(1):65. 10.3390/cells13010065. (Epub 20231228). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yajima T, Yajima K, Takahashi H, Yasuda K. The effect of dulaglutide on body composition in type 2 diabetes mellitus patients on hemodialysis. J Diabetes Complicat. 2018;32(8):759–63. 10.1016/j.jdiacomp.2018.05.018. (Epub 20180601). [DOI] [PubMed] [Google Scholar]
- 105.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5(9):735–41. 10.1016/s1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 106.Guiloff AE, Rudge P. Amyloid antibody therapy for early-stage Alzheimer’s disease: a critical review of three recent trials. J Neurol. 2024;271(5):2914–6. 10.1007/s00415-024-12361-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ahadiat S-A, Hosseinian Z. A look back at the prodromal findings in Parkinson’s disease. Bull Natl Res Centre. 2023;47(1):167. 10.1186/s42269-023-01143-5. [Google Scholar]
- 108.van Rumund A, Green AJE, Fairfoul G, Esselink RAJ, Bloem BR, Verbeek MM. α-Synuclein real-time quaking-induced conversion in the cerebrospinal fluid of uncertain cases of parkinsonism. Ann Neurol. 2019;85(5):777–81. 10.1002/ana.25447. (Epub 20190325). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable due to the nature of review. No new data was generated.