Abstract
Background
Drug-coated balloons (DCB) have promising results in the management of in-stent restenosis (ISR), still their role remains a major challenge, and not well established in contemporary clinical practice.
Aims
To provide a comprehensive appraisal of the efficacy and safety of DCBs in patients with in-stent restenosis (ISR).
Methods
We searched PubMed, Scopus, web of Science, Ovid, and Cochrane Central from inception until 30 March, 2023. We included randomized controlled trials (RCTs) that compared DCB versus DES in ISR patients. Our primary endpoints were major adverse cardiac events (MACE) and late lumen loss (LLL). Secondary clinical endpoints were all-cause death, cardiac death, MI, TLR, TVR, and stent thrombosis, and angiographic outcomes were MLD, and in-stent binary restenosis.
Results
Ten RCTs comprising 1977 patients were included in this meta-analysis. The incidence of MACE was 15.57% in the DCB group compared to 14.13% in the DES group, with no significant difference in the risk of MACE following DCB (odds ratio [OR] 1.04, 95% confidence interval [CI]: 0.87 to 1.44). Compared with the DES intervention, the risk of LLL was comparable to the DCB intervention (mean difference [MD] -0.08, 95% CI: -0.18 to 0.02), while the incidence of TLR was increased in the DCB intervention (OR: 1.54, 95% CI: 1.2 to 1.99).
Conclusion
DCB was comparable to DES implantation is ISR patients regarding clinical outcomes, however it showed an increase in TLR events. Moreover, a RCT with large sample size and longer follow-up duration is warrened to validate these results.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13019-024-03046-6.
Keywords: DCB, DES, In-stent restenosis, PCI, Meta-analysis
Introduction
In-stent re-stenosis (ISR) commonly occurs in clinical settings subsequent to the implantation of bare metal stents (BMS) due to the augmented neointimal proliferation induced by these devices. Furthermore, drug-eluting stents (DES) may also exhibit the occurrence of ISR, particularly when implemented in unfavorable clinical circumstances and anatomical conditions [1–3]. The fundamental aim of percutaneous coronary intervention (PCI) is to optimize hemodynamic perfusion through the amelioration of narrowed coronary arteries. Nevertheless, the clinical efficacy of this minimally invasive technique has encountered prolonged impediments in the form of re-stenosis, an occurrence defined by the subsequent re-narrowing of previously treated arteries, which has persisted since its inception in 1977 [4].
Compared to bare-metal stents (BMS) and conventional balloon angioplasty (BA), DES has demonstrated a substantial decrease in re-stenosis likelihood [5–7]. Nevertheless, because of the ongoing rise in DES implantation, the rate of DES re-stenosis is still significant [8]. Despite the widespread use of new-generation DES, DES-ISR continues to occur in 5–10% of patients after DES deployment and has established itself as a common clinical problem [9, 10].
Drug-coated balloon (DCB) is a novel therapeutic approach for BMS-ISR and DES-ISR; studies have shown that it is associated with better outcomes when compared to other traditional treatment techniques [9]. Previous randomized studies have examined the application of DCB in patient populations exclusively comprised of either BMS-ISR or DES-ISR individuals. However, no study has included patients with any form of in-stent re-stenosis (i.e., both BMS-ISR and DES-ISR [4].
Notably, recent clinical practice guidelines imply that these 2 therapy methods (DES and DES) presently constitute the best treatments available (both with the level of recommendation IA) for patients with ISR DCB are at least as effective as first-generation DES in these patients as first-generation DES and are more effective than conventional therapy approaches [1, 11]. The comparative efficacy of DCB versus “new generation” DES in patients with ISR is largely unestablished.
DCB is an attractive strategy for managing recurrent DES-ISR due to its potential to obviate the need for additional metallic layers and prolonged dual anti-platelet treatment (DAPT). Nonetheless, there is a scarcity of research investigating the utilization of DCB for the treatment of recurrent DES-ISR. The outcomes obtained thus far are subject to debate [9]. The objective of our study was to conduct a comparative analysis of the efficacy and safety of DCB and DES in the management of ISR.
Methods
In this meta-analysis, we adhered to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement, which outlines guidelines for transparently reporting systematic reviews and meta-analyses. Additionally, we adhered to the guidelines outlined in the Cochrane Handbook of Systematic Reviews and Meta-Analysis of Interventions (version 5.1.0) [12].
Eligibility criteria
In our analysis, we incorporated all randomized controlled trials that focused on patients with in-stent re-stenosis undergoing PCI and treated with drug-coated balloons as the intervention group and drug-eluting stents as the control group. Our study reported outcomes of interest using intention-to-treat analysis and excluded animal studies, conference abstracts, and unpublished data. We did not limit our search to English language studies and considered foreign language studies as part of our inclusion criteria.
Primary and secondary outcomes
Our primary investigation focused on evaluating the incidence of Major Adverse Cardiovascular Events (MACE) and late lumen loss (LLL), utilizing the definitions provided by each respective author. Additionally, we conducted an analysis of several secondary outcomes of interest, including all-cause death, cardiac death, myocardial infarction (MI), target lesion revascularization (TLR), target vessel revascularization (TVR), in-segment binary re-stenosis, stent thrombosis, and minimum lumen diameter (MLD). The definitions for these outcomes were based on the definitions explicitly reported by the authors of the individual studies incorporated in our analysis.
Literature search
A comprehensive electronic search was conducted across multiple databases including PubMed, Scopus, Web of Science, Ovid, and the Cochrane central library. The search encompassed articles published from the inception of these databases up until March 30th, 2023. The search strategy employed in this study was as follows:
Scopus: (“drug-coated balloon” OR “DESs”) AND (“Drug-Eluting Stent” OR “DES” OR “PCI”) AND (“in-stent re-stenosis” OR “re-stenosis” OR “coronary Stent re-stenosis”) AND (“coronary artery disease*” OR “myocardial infarction”) AND (“PCI” OR “Percutaneous Coronary Intervention”).
Others: (drug-coated balloon OR DESs) AND (Drug-Eluting Stent OR DES OR PCI) AND (in-stent re-stenosis OR re-stenosis OR coronary Stent re-stenosis) AND (coronary artery disease* OR myocardial infarction) AND (PCI OR Percutaneous Coronary Intervention).
The duplicates were identified and removed using EndNote. We screened all relevant included studies and prior meta-analyses for additional citations.
Screening of the literature search results
The results were screened in a two-step way; the first was a title and abstract screening, then a full-text screening of the relevant studies.
Data extraction
A designated data extraction sheet was employed for the purpose of acquiring essential information. The data extracted encompassed four primary domains, namely: (1) the distinctive attributes of the studies incorporated in the analysis, (2) the characteristics of the population under study, and (3) the domains associated with the evaluation of potential bias, and (4) Outcome measures, which included MACE, all-cause death, cardiac death, MI, TLR, TVR, in-segment binary re-stenosis, stent thrombosis, LLL, and MLD. Data was extracted independently, in duplicate, compared for discrepancies, and that any disputes were resolved by consensus.
Synthesis of results
We pooled the reported outcomes based on the follow-up duration, in which short-term follow-up was defined as a follow-up less than or equal to 1 year, while long-term follow-up was defined as a follow-up longer than 1 year. In cases where studies provided data of long-term follow-up at multiple time points, we focused on the outcomes reported at the final follow-up point. We used the DerSimonian-Laird random-effect model to pool the frequency of events and the total number of patients for dichotomous outcomes, reporting the results as odds ratios (OR). We used the DerSimonian-Laird random-effect model for continuous outcomes to pool the mean difference (MD) and its 95% confidence interval (CI). We used Stata MP Version 17 for Mac to conduct all analyses.
Assessment of heterogeneity
The Chi-square test (specifically the Cochrane Q test) was utilized to assess the statistical heterogeneity across the studies. The evaluation was conducted by employing the following equation:  A P-value of less than 0.1 for the Chi-square test was considered significant heterogeneity. We defined high heterogeneity as I-square values of 50% or greater. In cases of significant heterogeneity, we utilized the leave-one-out sensitivity analysis model to address the reported heterogeneity. Additionally, we used the Galbraith plot to detect any heterogeneity across studies.
A P-value of less than 0.1 for the Chi-square test was considered significant heterogeneity. We defined high heterogeneity as I-square values of 50% or greater. In cases of significant heterogeneity, we utilized the leave-one-out sensitivity analysis model to address the reported heterogeneity. Additionally, we used the Galbraith plot to detect any heterogeneity across studies.
Quality assessment
We evaluated the quality of the clinical trials included in our analysis using the Cochrane Risk of Bias 2 (ROB-2) tool for randomized controlled trials (RCTs). This tool assesses the risk of bias in five domains: selection bias, performance bias, detection bias, attrition bias, and reporting bias [13]. The authors’ determinations were categorized into three levels of bias assessment: ‘High risk of bias’, ‘Some concerns’, or ‘Low risk of bias’. To investigate the potential existence of publication bias across the included studies, we employed the funnel plot model to assess the relationship between effect size and standard error. In order to evaluate the presence of publication bias more comprehensively, we conducted Egger’s regression test.
Due to the limited amount of data reported and the cumulative pooling of trials, there is an increased risk of type 1 and type 2 errors. To address this, we used Trial Sequential Analysis (TSA) to determine if the evidence from the pooled trials is conclusive and reliable. The intervention’s confidence level is considered conclusive and sufficient when the z-line on the curve crosses both the conventional boundary and the boundary of sequence monitoring, indicating that no further studies are necessary. Conversely, if the z-line on the curve does not cross any boundary, the evidence is insufficient, and further studies are necessary. In this meta-analysis, we used an alpha error of 0.05 and a beta error of 80% power. We calculated the mean difference in the meta-analysis to determine the sample size required for TSA.
Results
Literature search results
A total of 563 articles were retrieved through a comprehensive electronic search. After title and abstract screening, 60 studies were eligible, and following full-text screening, 10 studies and further 6 follow-up studies were included in this systematic review and meta-analysis. The PRISMA flow chart is shown in Fig. 1.
Fig. 1.
PRISMA flow chart of our included studies
Included studies characteristics
Our meta-analysis included 10 studies [3, 4, 6, 10, 11, 14–18]. with 6 follow-up extension studies [1, 7, 19–22]. comprising 1977 patients comparing DCB with the DES intervention. All studies assessed our primary clinical outcome, MACE, and our angiographic outcome, LLL. Baseline characteristics and a summary of included studies are shown in Table 1.
Table 1.
Baseline and summary characteristics of included studies
| Characteristics of All included Studies | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, year | Country | Patients (n) | Age (mean) | male, n | angio follow up (months) | clinical follow up (months) | DCB type | DES type | RVD, mean | MLD, mean | Medical Conditions, n | Target vessel, n | DS, mean | ISR Mehran classification, n | |||||||||
| History of CABG | History of MI | DM | HTN | Hyperlipidaemia | Current smoker | LAD | LCX | RCA | I | II | III-IV | ||||||||||||
| Pleva 2016 | Czechia | 68/68 | 65.6/65.5 | 43/46 | 12 | 12 | Paclitaxel-Eluting Balloon | Everolimus-Eluting Stents | 2.64/2.66 | 0.92/0.79 | 3\6 | 43/41 | 17/18 | NA | NA | 31/29 | 35/40 | NA | 22/22 | 71.8/78.0 | 30/21 | 34/35 | 10\18 |
| Pleva 2018 (follow-up) | Czechia | 68/68 | 65.6/65.5 | 43/46 | NA | 36 | Paclitaxel-Eluting Balloon | Everolimus-Eluting Stents | 2.64/2.66 | 0.92/0.79 | 3\6 | 43/41 | 17/18 | NA | NA | 31/29 | 35/40 | NA | 22/22 | 71.8/78.0 | 30/21 | 34/35 | 10\18 |
| Unverdorben 2009 | Germany | 66/65 | 64.6/ 65.1 | 48/50 | 6 | 12 | Paclitaxel-Coated Balloon | paclitaxel-eluting stent | 2.85/2.83 | 0.74 / 0.77 | NA | NA | 22/17 | 53/54 | 52/46 | 16/15 | 20/28 | 24/19 | 22/17 | 73.9/72.8 | 31/25 | 20/26 | 15/14 |
| Unverdorben 2015 (follow up) | Germany | 66/65 | 64.6/ 65.1 | 48/50 | 6 | 36 | Paclitaxel-coated balloon |
paclitaxel-coated stent |
2.85/2.83 | 0.74/ 0.77 | NA | NA | 22/17 | 53/54 | 52/46 | 16/15 | 20/28 | 24/19 | 22/17 | 73.9/72.8 | 31/25 | 20/26 | 15/14 |
| Wong 2018 | China | 86/86 | 67/ 66 | 61/62 | 14 | 12 | Paclitaxel-Coated Balloon | everolimus-eluting stents | 2.85/ 3.06 | 0.63/ 0.63 | NA | 26/22 | 43/38 | 60/65 | 49/53 | 40/37 | 48/52 | 13\11 | 24/21 | 77\79 | 45/45 | 1\10 | 12-Oct |
| Xu 2014 | China | 109/106 | 61.8/ 62.1 | 88/86 | 9 | 12 | Paclitaxel-Coated Balloon | Paclitaxel-Eluting Stent | 2.66/ 2.72 | 2.63/ 2.45 | 3/0 | 53/37 | 44/35 | 78/69 | 38/35 | 23/27 | 47/61 | 21/13 | 45/34 | 68.26/68.43 | 67\54 | 21/22 | 15/23 |
| Xu 2016 (follow-up) | China | 109/106 | 61.8/ 62.1 | 88/86 | NA | 24 | Paclitaxel-Coated Balloon | Paclitaxel-Eluting Stent | 2.66/ 2.72 | 2.63/ 2.45 | 3/0 | 53/37 | 44/35 | 78/69 | 38/35 | 23/27 | 47/61 | 21/13 | 45/34 | 68.26/68.43 | 67\54 | 21/22 | 15/23 |
| Adriaenssens 2014 | Belgium | 25/25 | 67/64 | 18/25 | 9 | 12 | Paclitaxel-Coated Balloon | everolimus-eluting stent | 3/2.847 | 0.98/0.57 | NA | 12\10 | 6\1 | 16/15 | 24/24 | 5\3 | 6\11 | 5\7 | 13\6 | 67.7/79.4 | 8\9 | 13\10 | 4\6 |
| Alfonso 2014 | Spain | 95/94 | 67/64 | 82/82 | 9 | 12 | Paclitaxel-Coated Balloon | everolimus-eluting stent | 2.64/2.64 | 1.02/0.93 | 4\7 | 57/56 | 30/19 | 68/68 | 69/62 | NA | 35/37 | 21/22 | 37/32 | 61/65 | 38/34 | 45/42 | 12\18 |
| Alfonso 2015 | Spain | 154/155 | 66/66 | 127/130 | 9 | 12 | Paclitaxel-Coated Balloon | everolimus-eluting stents | 2.59/2.67 | 0.79/0.75 | 16/17 | 73/77 | 75/66 | 110/121 | 110/121 | NA | 77/71 | 27/34 | 43/45 | 69/72 | 97/99 | 53/44 | 4\12 |
| Alfonso 2016 (follow-up) | Spain | 95/94 | 67/64 | 82/82 | NA | 36 | NA | Everolimus-Eluting Stent | 2.64/2.64 | 1.02/0.93 | NA | NA | 30/19 | 68/68 | 69/62 | NA | 35/37 | 21/22 | 37/32 | 61/65 | 38/34 | 45/42 | 12\18 |
| Alfonso 2018 (follow-up) | Spain | 154/155 | 66/66 | 127/130 | NA | 36 | NA | Everolimus-Eluting Stent | 2.59/2.67 | 0.79/0.75 | NA | NA | 75/66 | 110/121 | 110/121 | NA | 77/71 | 27/34 | 43/45 | 69/72 | 97/99 | 53/44 | 4\12 |
| Baan 2018 | Netherlands | 137/141 | 66/65 | 72/84 | 6 | 12 | Paclitaxel-Coated Balloon | everolimus-eluting stents | 2.56/2.59 | 0.77/0.79 | 14/16 | 53/52 | 31/33 | 64/67 | 59/60 | 17/13 | 41/39 | 20/24 | 37/35 | 69.7/69.3 | 51/53 | 32/34 | NA |
| Byrne 2013 | Germany | 137/131 | 67.7/68.8 | 105/88 | 8 | 12 | Paclitaxel-Coated Balloon | paclitaxel-eluting stents | NA | 0·97/0·93 | 15/32 | 53/50 | 56/61 | 105/101 | 108/103 | 19/15 | 59/50 | 54/61 | 59/56 | 64.4/66.7 | 119/110 | 44/49 | 9\9 |
| Kufner 2015 (follow-up) | Germany | 137/131 | 67.7/68.8 | 105/88 | NA | 36 | Paclitaxel-Coated Balloon | paclitaxel-eluting stents | NA | 0·97/0·93 | 15/32 | 53/50 | 56/61 | 105/101 | 108/103 | 19/15 | 59/50 | 54/61 | 59/56 | 64.4/66.7 | 119/110 | 44/49 | 9\9 |
| Jensen 2018 | Germany | 157/72 | 67.2/69.4 | 122/49 | 6 | 18 | Paclitaxel-Coated Balloon | sirolimus-eluting stent (SES) | 3/2.9 | 1/0.9 | NA | 93/35 | 48/24 | 144/70 | 134/62 | NA | NA | NA | NA | 67.2/68.9 | 105\53 | 46/17 | NA |
The data are presented as DCB/DES. Angio: Angiographic; DCB: drug-coated balloons; DES: drug-eluting stents; RVD: reference vessel diameter; MLD: mean lumen diameter; DS: diameter stenosis; ISR: in-stent restenosis
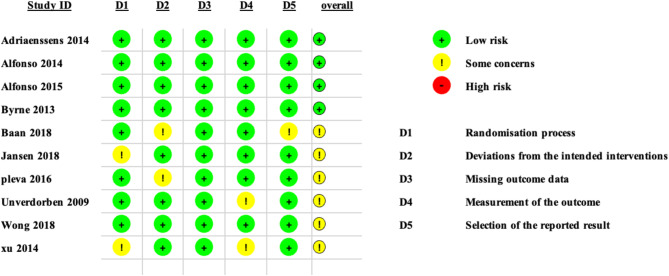
Risk of bias assessment
A summary and graph of the risk of bias assessment of our included studies are shown in Fig. 2. Most studies showed an overall unclear bias; however, seven studies showed an overall low risk of bias. The authors’ judgement was made according to the Cochrane risk of bias assessment tool.
Fig. 2.
Risk of bias assessment tool-2 (ROB-2)
MACE
All included studies assessed MACE with an overall incidence rate of 15.57% (159 of 1021) in the DCB group compared to 14.13% (133 of 941) in the DES group. We ran the analysis in two scenarios; the pooled OR based on the follow-up duration showed no significant difference between the DCB intervention and the DES intervention to reduce the incidence of MACE (OR = 1.04, 95% CI [0.87, 1.24], p = 0.68). Subgroup analysis revealed no significant difference between the two studies interventions. On the other hand, the pooled analysis of 6 studies that assessed long-term incidence revealed no significant difference between the two interventions used (OR = 0.99, 95% CI [0.77, 1.27], p = 0.95). The pooled studies were not heterogenous (I2 = 0.00%, p = 0.39), as shown in Fig. 3.
Fig. 3.
Pooled estimates from RCTs evaluating the effect of DCB on the incidence of MACE with a random-effects model. DCB: Drug-coated balloons; DES: drug-eluting stents; CI: confidence interval
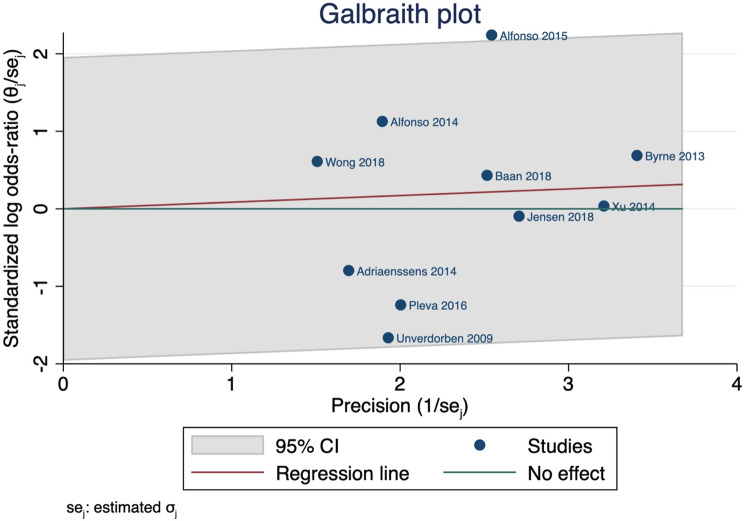
We also used the Galbraith plot to detect the heterogeneity, and by inspection, only one study was considered outliers, indicating its heterogeneity from other studies, as shown in Fig. 4. Insufficient literature search and clinical heterogeneity may attribute to the current evidence.
Fig. 4.
Galbraith plot indicating the heterogeneity across studies assessing MACE. CI: confidence interval
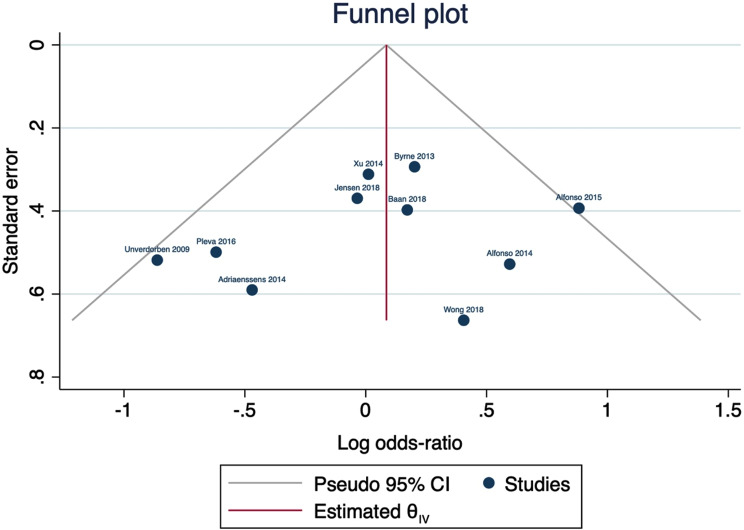
We used the funnel plot to detect possible publication bias, and by inspection, only one study was located outside the 95% CI of precision area, indicating that there was a possible publication bias; however, when we used the trim and fill method, no studies were imputed assuming possible stability across studies, as shown in Fig. 5.
Fig. 5.
Funnel plot for possible publication bias regarding MACE
We performed another analysis based on the type of ISR studies, and the pooled analysis showed that DCB was comparable to DES in either BMS ISR or DES ISR with the following values (OR: 0.78, 95% CI: 0.56 to 1.09, p = 0.15 in BMS ISR, and 1.16, 95% CI: 0.94 to 1.44, p = 0.16 in DES ISR), as shown in Supplementary Fig. S1.
We performed a trial sequential analysis (TSA) on 10 studies that assessed MACE excluding studies of follow-up of which the cumulative Z-line on the curve crossed the conventional boundary of benefit only; and did not cross the trial sequential monitoring boundary, suggesting that DCB is comparable to DES to decrease the incidence of MACE, and further large volume RCTs should be carried out to validate our results, as shown in Fig. 6.
Fig. 6.
A trial sequential analysis (TSA) for 10 RCTs illustrating that the cumulative Z-curve crossed the conventional boundary for benefit only and did not cross the trial sequential monitoring boundary for benefit, establishing sufficient but not conclusive evidence and suggesting further trials are still needed to validate our results. A diversity-adjusted required information size of 2731 patients was calculated using an alpha error of 0.05, a beta error of 0.20 (power 80%), an anticipated RR reduction of 10% in DCB, and a control event proportion of 14.13%, as calculated from the control group in this meta-analysis. RCT: randomised controlled trial; RR: risk ratio
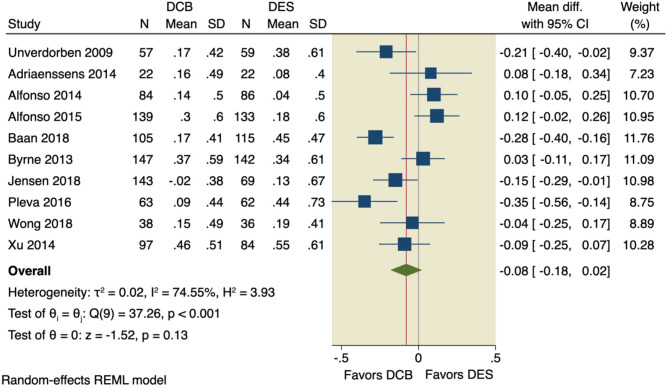
Late lumen loss (LLL)
LLL was assessed in all included studies, of which the pooled MD showed no significant difference between the DCB intervention and the DES intervention (MD = -0.08, 95% CI [-0.18, 0.02], p = 0.13); the pooled studies were significantly heterogenous (I2 = 74.55%, p < 0.001), as shown in Fig. 7. Leave-one-out model analysis was performed, and no single study had a disproportional effect on the overall MD, as shown in Fig. 8.
Fig. 7.
Pooled estimates from RCTs evaluating the effect of DCB on the LLL with a random-effects model. DCB: Drug-coated balloons; DES: drug-eluting stents; CI: confidence interval
Fig. 8.
Leave-one-out analysis of LLL
Four studies were inspected as outliers of the 95% CI of the precision area, using the Galbraith plot, indicating their heterogeneity from other studies; however, no other studies were computed in the funnel plot model to achieve stability, as shown in Supplementary Figs. S2–S3.
Secondary outcomes
Our analysis did not detect any significant difference between DCB intervention and DES intervention regarding in-stent binary restenosis (OR: 1.02, 95% CI [0.72 to 1.44], p = 0.92), all-cause death (OR = 0.79, 95% CI [0.52, 1.19], p = 0.26), cardiac death (OR = 0.83, 95% CI [0.47, 1.44], p = 0.50), the incidence of MI (OR = 0.89, 95% CI [0.6, 1.31], p = 0.54), the incidence of TVR (OR = 1.24, 95% CI [0.94, 1.63], p = 0.13), stent thrombosis (OR = 1.08, 95% CI [0.54, 2.16], p = 0.84), or MLD (MD = -0.08, 95% CI [-0.18, 0.02], p = 0.12), as shown in Supplementary Figs. S4–S10.
The pooled studies assessing all-cause death, cardiac death, MI, TVR, and stent thrombosis were homogenous with the following values, respectively: (I2 = 5.42%, p = 0.46; I2 = 0.00%, p = 0.95; and I2 = 0.00%, p = 0.97; I2 = 14.36%, p = 0.24; I2 = 0.00%, p = 0.97). However, the pooled studies assessing MLD were heterogenous (I2 = 60.6%, p = 0.01). Leave-one-out sensitivity analysis for MLD is shown in Supplementary Fig. S11.
On the other hand, DCB was associated with higher incidence of TLR compared to DES (OR: 1.54, 95% CI [1.2 to 1.99], p = 0.00), the pooled studies were homogenous (I2 = 0.00%, p = 0.26), as shown in Supplementary Fig. S12.
Discussion
In this meta-analysis, we aimed to evaluate the efficacy of DCBs and DESs in patients with Coronary ISR. A total of 10 RCTs and six follow-up extensions were included in the analysis. Subgroup analyses were conducted based on intervention indications and follow-up periods to provide a more comprehensive assessment of the interventions’ effectiveness. In the short-term, DCBs showed better outcomes in reducing MACE than DESs, attributed to the absence of permanent foreign material reducing the risk of stent thrombosis and late re-stenosis [23]. DCBs also minimized the inflammatory response caused by DES implantation [24]. Previous studies without subgroup analysis reported no significant difference in MACE reduction between DCBs and DESs [25, 26]. However, DCBs were significantly more effective than DESs in reducing LLL and binary re-stenosis, such as all-cause death, cardiac death, myocardial infarction, target lesion revascularization, target vessel revascularization, stent thrombosis, and minimum lumen diameter. These latter findings were consistent with the previous meta-analyses [25–27].
ISR is a condition in which the artery narrows again after stent placement, leading to recurrent symptoms and potentially compromising the procedure’s outcomes. ISR is a common complication of PCI, with an incidence ranging from 5 to 50%, depending on the patient and procedural factors and the type of stent used [28, 29]. The pathophysiology of ISR is complex and multifactorial, involving inflammation, neointimal hyperplasia, and other factors contributing to tissue overgrowth within the stent [30]. Therefore, ISR prevention and management remain a significant challenge in clinical practice, and several strategies, including DES, non-invasive imaging, and pharmacotherapy, have been proposed to reduce the incidence and improve the outcomes of this condition [31, 32].
A considerable literature, comprising both observational studies and randomized trials, has been carried out to explore the efficacy of DCB and DES in addressing re-stenosis in both BMS and DES [6, 33–35]. However, few randomized clinical trials have been published that directly compare DCB with current-generation DES [14, 15]. These trials consistently employed a comparative approach, evaluating the SeQuent Please paclitaxel-eluting balloon (DEB) against the XIENCE everolimus-eluting stent (EES), which is widely regarded as the reference device within its category. The XIENCE EES has undergone extensive investigation as a DES, while the SeQuent Please paclitaxel-eluting balloon has been extensively studied as a DCB [36–38]. The Alfonso et al. trial (2015), comprising 189 patients, demonstrated that using EES led to superior late angiographic outcomes, as evidenced by a larger MLD at the 9-month follow-up compared to DCB [11]. In the study by Baan et al., the requirement for TVR was found to be statistically comparable between the two groups, but there was a numerical tendency for a higher incidence in the DCB arm [4]. Conversely, the study by Pleva et al., which included 136 patients, reported more favorable angiographic outcomes with DCB than EES, indicated by a smaller late lumen loss at the 12-month follow-up [16].
The Drug-eluting stent and drug-coated balloon AngiopLasty for the occUrrence of coronary in-Stent restenosis (DAEDALUS) study was a comprehensive meta-analysis that compared the effectiveness of DES and DCB angioplasty in treating coronary ISR. The study included individual patient data from 10 randomized clinical trials, with a total of 1,976 participants. The results showed that at 3 years, DCB angioplasty was associated with a moderately higher risk of target lesion revascularization compared to repeat DES implantation, with a significant interaction between treatment effect and the type of restenosed stent. The safety endpoint of all-cause death, myocardial infarction, or target lesion thrombosis was similar between the two groups. However, a subgroup analysis suggested a lower incidence of adverse events with paclitaxel-coated balloons compared to first-generation DES. The study also found that DCB and DES had comparable efficacy and safety profiles in treating bare-metal stent ISR, while repeat DES implantation was more effective in treating DES-ISR, albeit with a numerical excess in the composite safety endpoint. The different efficacy of DCB between BMS-ISR and DES-ISR may be due to variations in individual susceptibility to antiproliferative drug-mediated suppression and the occurrence of neo atherosclerosis [39].
Several meta-analyses also have been published comparing different treatments for ISR. In a study by Lee et al., it was found that both paclitaxel-coated balloons and DES were associated with a significant reduction in TLR and MACE compared to plain old balloon angioplasty (POBA). The OR for TLR was 0.28, and for MACE was 0.84 when comparing DCB or DES to POBA [40]. However, there was no significant difference between DCB and DES groups. Similarly, in a study by Mamuti et al., no significant difference in MACE was observed in the treatment of BMS or DES-ISR when comparing DCB to paclitaxel-eluting stents (PES) or EES. The RR for MACE was 1.04, indicating no significant difference between DCB and PES/EES (P = 0.80) [41]. These meta-analyses provide evidence that DCB and DES are effective in reducing TLR and MACE compared to POBA, while there is no significant difference between DCB and DES regarding their efficacy.
Additionally, DCB has shown comparable effectiveness to PES/EES in the treatment of BMS/DES-ISR, and both DES and DCB appear to be superior to POBA in reducing the risk of TLR and TVR in DES-ISR However, previous meta-analyses have major drawbacks: Geo et al. included only four RCTs, most of their studies were single-arm studies, and Maumti et al. included only four trials. Additionally, many studies have been conducted after these meta-analyses [4, 9, 10, 21, 22].
Multilayer ISR
The management of multi-layered ISR presents a compelling and often challenging clinical scenario. To address this, a non-randomized study examined the outcomes of recurrent ISR lesions treated with at least three prior stents, comparing the efficacy of drug-coated balloons DCBs and second-generation DES. The study analyzed 171 lesions, with 82 lesions treated with second-generation DES and the remaining lesions treated with DCBs. Upon two-year follow-up, the study revealed no significant difference in outcomes between the DES and DCB treatment groups. However, it is noteworthy that the DCB group exhibited a noticeably higher rate of major adverse cardiac events (43.5% vs. 28.8%; P = 0.21) [42]. This finding underscores the importance of careful patient selection and consideration of various factors when deciding between DES and DCB for the treatment of complex ISR lesions. While DCB offer an elegant solution, their long-term efficacy in patients with three or more layers is unsatisfactory [43]. Alternative “leave-nothing-behind” approaches have not demonstrated superiority over DCB in this complex patient population [44]. Further research is imperative to break this therapeutic deadlock. In carefully selected patients with recalcitrant ISR and extensive areas of myocardium at risk, coronary surgery should be regarded not as a concession or failure but as the most prudent strategy to prevent recurrent restenosis and its adverse consequences.
The Tokyo Registry study analyzed 304 patients with 333 ISR lesions who underwent treatment with DEB. The patients were categorized into three groups based on the number of previous metal stent layers: 166 (54%) with one stent layer, 87 (29%) with two layers, and 51 (17%) with three or more layers. The study found that the one-year MACE rate was significantly higher in the group with three or more metal layers (43.1%) compared to the groups with fewer layers [45].
Imaging role
The utilization of coronary physiology and intravascular imaging as adjunctive tools during percutaneous coronary interventions has been extensively studied, with a preponderance of evidence signifying enhanced outcomes when compared to sole angiographic guidance. Although limited in scope, existing reports examining the efficacy of these techniques in conjunction with drug-coated balloon interventions do not diverge in their conclusions from those observed with drug-eluting stents. Fractional flow reserve and pressure gradient measurements, as examples of physiological metrics, have been validated in the context of drug-coated balloon interventions, as evidenced by extant data [46].
A study employing serial optical coherence tomography revealed intriguing morphologic transformations within lesions undergoing ISR treatment with DCB angioplasty [47]. Notably, postprocedural dissections were observed specifically in segments where ISR presented with greater severity [47]. These dissections, elusive to angiographic imaging, were left untreated, yet exhibited complete resolution upon invasive follow-up examination. The integration of intravascular imaging modalities, such as intravascular ultrasound and optical coherence tomography, offers valuable insights into the underlying mechanisms of ISR [48]. By elucidating lesion-specific mechanical and histopathological factors, these imaging techniques inform the decision-making process between DCB and DES interventions, as well as the necessity for lesion preparation strategies prior to DCB application, including aggressive predilation with specialized balloons and high-pressure inflation [48].
Our study’s strengths are noteworthy, as it represents a comprehensive analysis of 16 RCTs with a total sample size exceeding 2,500 patients. Subgroup analyses were conducted based on follow-up duration and an indication of the intervention, providing a comprehensive understanding of the effectiveness of DCBs compared to other treatments. Additionally, the study employed various statistical methods, such as funnel plots, leave-one-out analysis, and Galbraith plots, to detect publication bias and outliers, thereby improving the robustness of the findings. Also, we ran a TSA analysis to test the robustness of the evidence pooled from the included studies which was not applied in the past meta-analyses.
The study’s limitations encompass several aspects. First, significant heterogeneity was observed in certain pooled analyses, which can be attributed to clinical heterogeneity stemming from the pooling of different generations of DES with DCB. Additionally, there was a lack of stratification of patients based on the number of metal-layered DES-ISR, methodological differences arising from variations in patient populations. Moreover, some of the studies included in the analysis had small sample sizes, potentially reducing the statistical power to detect significant differences between interventions. Lastly, despite the rigorous inclusion of only RCTs to ensure the highest level of evidence, the quality of the study’s findings may still be influenced by the potential risk of bias inherent in the individual studies incorporated in the analysis.
Conclusion
Our meta-analysis provides compelling evidence for the superior efficacy of DCBs over DES in treating coronary ISR. The analysis included six follow-up extensions, demonstrating that DCBs reduce MACE in the short term due to reduced stent thrombosis and late restenosis. DCBs also minimize inflammatory responses associated with DES. These findings directly impact daily practice, especially with the prevalent use of DES in ISR treatment, highlighting DCBs as the possible preferred intervention for improved ISR outcomes. Furthermore, to substantiate these findings, RCTs with a larger sample size and extended follow-up period is warranted to enhance the robustness of the current results.
Daily practice summary
The European Society of Cardiology’s guidelines on myocardial revascularization advocate the utilization of DCBs specifically for the treatment of ISR, with no extensions to other indications [49]. This cautious recommendation reflects the state of clinical knowledge at the time of guideline development and stands in contrast to the broader contemporary usage of DCBs in Europe. However, in recent years, there has been a significant expansion of data and clinical trials evaluating the efficacy of DCBs in treating de novo coronary lesions. The treatment paradigm for de novo lesions with DCBs offers a unique advantage by circumventing the need for permanent implants, thereby reducing the long-term risks associated with such devices [50]. The success of DCB angioplasty hinges on meticulous lesion preparation, and in cases of diffuse coronary artery disease, it can serve as a valuable adjunct to DES, minimizing the number and length of stents required. This hybrid approach underscores the importance of proper training and education for interventional cardiologists to ensure its effective implementation. Despite the accumulating clinical data and experience with DCBs in Europe, their usage for coronary artery disease treatment has not been approved by the United States Food and Drug Administration, warranting the need for large-scale randomized clinical trials powered for clinical endpoints to bridge this regulatory gap.
Electronic supplementary material
Acknowledgements
Nothing to declare.
Abbreviations
- DCB
Drug-coated balloons
- DES
Drug-eluting stents
- ISR
In-stent restenosis
- CAD
Coronary artery disease
- PCI
Percutaneous coronary intervention
- PRISMA
Systematic Reviews and Meta-analysis
- TSA
Trial sequential analysis
- OR
Odds ratio
- NA
Not Assigned
Author contributions
Ahmed Abdelaziz: Conceptualization, Supervision, data collection revision, statistical analysis, writing – original draft and editing.Karim Atta: Full-text screening, data collection, data validation.Abdelrahman Hafez: summary and characteristics, quality assessment, writing – revision.Hanaa Elsayed: screening, data collection, original draft writing Ahmed A. Ibrahim: quality assessment, writing – revision.Mohamed Abdelaziz: screening, data collection, revision of data collection, quality assessment.Hallas Kadhim: data collection, quality assessment, draft writing.Ahmed Mechi: data collection, data validation, Final version.Ahmed Elaraby: screening, data collection, data revision for analysis.Mahmoud Ezzat: screening, data collection, data revision for analysis.Ahmed Fadel: summary and characteristics, quality assessment, writing – revision.Abdullah Nouh: quality assessment, data collection, writing – revision.Rahma AbdElfattah Ibrahim: summary and characteristics, quality assessment, writing – revision.Mohamed Hatem Ellabban: summary and characteristics, quality assessment, writing – revision.Ali Bakr: summary and characteristics, quality assessment, writing – revision.Ahmed Nasr: quality assessment, manuscript drafting and writing.Mustafa Suppah: Conceptualization, screening, writing – original draft.
Funding
This work has no fund.
Data availability
All data generated during this study are illustrated in this article and in supplementary data.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alfonso F, Pérez-Vizcayno MJ, García del Blanco B, Otaegui I, Masotti M, Zueco J, et al. Long-term results of Everolimus-Eluting stents Versus Drug-Eluting balloons in patients with Bare-Metal In-Stent restenosis. JACC Cardiovasc Interv. 2016;9:1246–55. 10.1016/j.jcin.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 2.Habara S, Iwabuchi M, Inoue N, Nakamura S, Asano R, Nanto S, et al. A multicenter randomized comparison of paclitaxel-coated balloon catheter with conventional balloon angioplasty in patients with bare-metal stent restenosis and drug-eluting stent restenosis. Am Heart J. 2013;166:527–e5332. 10.1016/j.ahj.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Jensen CJ, Richardt G, Tölg R, Erglis A, Skurk C, Jung W, et al. Angiographic and clinical performance of a paclitaxel-coated balloon compared to a second-generation sirolimus-eluting stent in patients with in-stent restenosis: the BIOLUX randomised controlled trial. EuroIntervention. 2018;14:1096–103. 10.4244/EIJ-D-17-01079. [DOI] [PubMed] [Google Scholar]
- 4.Baan J, Claessen BE, Dijk KB, Vendrik J, van der Schaaf RJ, Meuwissen M, et al. A Randomized comparison of Paclitaxel-Eluting Balloon Versus Everolimus-Eluting Stent for the treatment of any In-Stent restenosis. JACC Cardiovasc Interv. 2018;11:275–83. 10.1016/j.jcin.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 5.Habara S, Mitsudo K, Kadota K, Goto T, Fujii S, Yamamoto H, et al. Effectiveness of Paclitaxel-Eluting Balloon catheter in patients with Sirolimus-Eluting Stent Restenosis. JACC Cardiovasc Interv. 2011;4:149–54. 10.1016/j.jcin.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 6.Byrne RA, Neumann F-J, Mehilli J, Pinieck S, Wolff B, Tiroch K, et al. Paclitaxel-eluting balloons, paclitaxel-eluting stents, and balloon angioplasty in patients with restenosis after implantation of a drug-eluting stent (ISAR-DESIRE 3): a randomised, open-label trial. Lancet. 2013;381:461–7. 10.1016/S0140-6736(12)61964-3. [DOI] [PubMed] [Google Scholar]
- 7.Kufner S, Cassese S, Valeskini M, Neumann F-J, Schulz-Schüpke S, Hoppmann P, et al. Long-term efficacy and safety of Paclitaxel-Eluting balloon for the treatment of drug-eluting stent restenosis. JACC Cardiovasc Interv. 2015;8:877–84. 10.1016/j.jcin.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 8.Rittger H, Brachmann J, Sinha A-M, Waliszewski M, Ohlow M, Brugger A, et al. A Randomized, Multicenter, single-blinded trial comparing paclitaxel-coated balloon angioplasty with Plain Balloon Angioplasty in Drug-Eluting Stent Restenosis. J Am Coll Cardiol. 2012;59:1377–82. 10.1016/j.jacc.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 9.Abdelaziz A, Hafez A, Atta K, Elsayed H, Abdelaziz M, Elaraby A, et al. Drug-coated balloons versus drug-eluting stents in patients with acute myocardial infarction undergoing percutaneous coronary intervention: an updated meta-analysis with trial sequential analysis. BMC Cardiovasc Disord. 2023;23(1):605. 10.1186/s12872-023-03633-w. [DOI] [PMC free article] [PubMed]
- 10.Wong YTA, Kang D-Y, Lee JB, Rha S-W, Hong YJ, Shin E-S, et al. Comparison of drug-eluting stents and drug-coated balloon for the treatment of drug-eluting coronary stent restenosis: a randomized RESTORE trial. Am Heart J. 2018;197:35–42. 10.1016/j.ahj.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, García del Blanco B, García-Touchard A, López-Minguéz JR, et al. A prospective Randomized Trial of Drug-Eluting balloons Versus Everolimus-Eluting stents in patients with In-Stent restenosis of drug-eluting stents. J Am Coll Cardiol. 2015;66:23–33. 10.1016/j.jacc.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700–2700. 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;l4898. 10.1136/bmj.l4898. [DOI] [PubMed]
- 14.Adriaenssens T, Dens J, Ughi G, Bennett J, Dubois C, Sinnaeve P, et al. Optical coherence tomography study of healing characteristics of paclitaxel-eluting balloons vs. everolimus-eluting stents for in-stent restenosis: the SEDUCE (Safety and Efficacy of a drug elUting balloon in coronary artery rEstenosis) randomised clinical trial. EuroIntervention. 2014;10:439–48. 10.4244/EIJV10I4A77. [DOI] [PubMed] [Google Scholar]
- 15.Alfonso F, Pérez-Vizcayno MJ, Cárdenas A, García del Blanco B, Seidelberger B, Iñiguez A, et al. A Randomized comparison of drug-eluting balloon Versus Everolimus-Eluting stent in patients with Bare-Metal Stent–In-Stent restenosis. J Am Coll Cardiol. 2014;63:1378–86. 10.1016/j.jacc.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Pleva L, Kukla P, Kusnierova P, Zapletalova J, Hlinomaz O. Comparison of the efficacy of Paclitaxel-Eluting Balloon catheters and Everolimus-Eluting stents in the treatment of coronary In-Stent restenosis. Circ Cardiovasc Interv. 2016;9. 10.1161/CIRCINTERVENTIONS.115.003316. [DOI] [PubMed]
- 17.Unverdorben M, Vallbracht C, Cremers B, Heuer H, Hengstenberg C, Maikowski C, et al. Paclitaxel-Coated Balloon Catheter Versus Paclitaxel-Coated Stent for the treatment of coronary In-Stent restenosis. Circulation. 2009;119:2986–94. 10.1161/CIRCULATIONAHA.108.839282. [DOI] [PubMed] [Google Scholar]
- 18.Xu B, Gao R, Wang J, Yang Y, Chen S, Liu B, et al. A prospective, Multicenter, Randomized Trial of Paclitaxel-Coated Balloon Versus Paclitaxel-Eluting Stent for the treatment of drug-eluting stent In-Stent restenosis. JACC Cardiovasc Interv. 2014;7:204–11. 10.1016/j.jcin.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Alfonso F, Pérez-Vizcayno MJ, Cuesta J, García del Blanco B, García-Touchard A, López-Mínguez JR, et al. 3-Year clinical Follow-Up of the RIBS IV Clinical Trial. JACC Cardiovasc Interv. 2018;11:981–91. 10.1016/j.jcin.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 20.Pleva L, Kukla P, Zapletalova J, Hlinomaz O. Long-term outcomes after treatment of bare-metal stent restenosis with paclitaxel-coated balloon catheters or everolimus-eluting stents: 3-year follow-up of the TIS clinical study. Catheter Cardiovasc Interv. 2018;92:E416–24. 10.1002/ccd.27688. [DOI] [PubMed] [Google Scholar]
- 21.Unverdorben M, Vallbracht C, Cremers B, Heuer H, Hengstenberg C, Maikowski C, et al. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis: the three-year results of the PEPCAD II ISR study. EuroIntervention. 2015;11:926–34. 10.4244/EIJY14M08_12. [DOI] [PubMed] [Google Scholar]
- 22.Xu B, Qian J, Ge J, Wang J, Chen F, Chen J, et al. Two-year results and subgroup analyses of the PEPCAD China in-stent restenosis trial: a prospective, multicenter, randomized trial for the treatment of drug-eluting stent in-stent restenosis. Catheter Cardiovasc Interv. 2016;87:624–9. 10.1002/ccd.26401. [DOI] [PubMed] [Google Scholar]
- 23.Muramatsu T, Kozuma K, Tanabe K, Morino Y, Ako J, Nakamura S, et al. Clinical expert consensus document on drug-coated balloon for coronary artery disease from the Japanese Association of Cardiovascular Intervention and therapeutics. Cardiovasc Interv Ther. 2023;38:166–76. 10.1007/s12928-023-00921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buccheri D, Piraino D, Andolina G, Cortese B. Understanding and managing in-stent restenosis: a review of clinical data, from pathogenesis to treatment. J Thorac Dis. 2016;8:E1150–62. 10.21037/jtd.2016.10.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui K-Y, Lyu S-Z, Zhang M, Song X-T, Yuan F, Xu F. Drug-eluting balloon versus New-Generation Drug-Eluting Stent for the treatment of In-Stent restenosis. Chin Med J (Engl). 2018;131:600–7. 10.4103/0366-6999.226073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao S, Shen J, Mukku VK, Wang MJ, Akhtar M, Liu W. Efficacy of drug-eluting balloons for patients with In-Stent restenosis. Angiology. 2016;67:612–21. 10.1177/0003319715611826. [DOI] [PubMed] [Google Scholar]
- 27.Peng N, Liu W, Li Z, Wei J, Chen X, Wang W, et al. Drug-coated balloons versus Everolimus-Eluting stents in patients with In-Stent restenosis: a pair-wise Meta-analysis of Randomized trials. Cardiovasc Ther. 2020;2020:1–11. 10.1155/2020/1042329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byrne RA, Joner M, Alfonso F, Kastrati A. Drug-coated balloon therapy in coronary and peripheral artery disease. Nat Rev Cardiol. 2014;11:13–23. 10.1038/nrcardio.2013.165. [DOI] [PubMed] [Google Scholar]
- 29.Ullrich H, Olschewski M, Münzel T, Gori T. Coronary In-Stent restenosis: predictors and treatment. Dtsch Arztebl Int. 2021. 10.3238/arztebl.m2021.0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shlofmitz E, Iantorno M, Waksman R. Restenosis of drug-eluting stents. Circ Cardiovasc Interv. 2019;12. 10.1161/CIRCINTERVENTIONS.118.007023. [DOI] [PubMed]
- 31.Pleva L, Kukla P, Hlinomaz O. Treatment of coronary in-stent restenosis: a systematic review. J Geriatr Cardiol. 2018;15:173–84. 10.11909/j.issn.1671-5411.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briguori C, Sarais C, Pagnotta P, Liistro F, Montorfano M, Chieffo A, et al. In-stent restenosis in small coronary arteries. J Am Coll Cardiol. 2002;40:403–9. 10.1016/S0735-1097(02)01989-7. [DOI] [PubMed] [Google Scholar]
- 33.Kubo S, Kadota K, Otsuru S, Hasegawa D, Habara S, Tada T, et al. Everolimus-eluting stent implantation versus repeat paclitaxel-coated balloon angioplasty for recurrent in-stent restenosis lesion caused by paclitaxel-coated balloon failure. EuroIntervention. 2015;10:e1–8. 10.4244/EIJV10I9A180. [DOI] [PubMed] [Google Scholar]
- 34.Naganuma T, Latib A, Costopoulos C, Oreglia J, Testa L, De Marco F, et al. Drug-eluting balloon versus second-generation drug-eluting stent for the treatment of restenotic lesions involving coronary bifurcations. EuroIntervention. 2016;11:989–95. 10.4244/EIJY14M11_01. [DOI] [PubMed] [Google Scholar]
- 35.Almalla M, Schröder J, Pross V, Marx N, Hoffmann R. Paclitaxel-eluting balloon versus everolimus-eluting stent for treatment of drug-eluting stent restenosis. Catheter Cardiovasc Interv. 2014;83:881–7. 10.1002/ccd.25072. [DOI] [PubMed] [Google Scholar]
- 36.Siontis GCM, Stefanini GG, Mavridis D, Siontis KC, Alfonso F, Pérez-Vizcayno MJ, et al. Percutaneous coronary interventional strategies for treatment of in-stent restenosis: a network meta-analysis. Lancet. 2015;386:655–64. 10.1016/S0140-6736(15)60657-2. [DOI] [PubMed] [Google Scholar]
- 37.Richelsen RKB, Overvad TF, Jensen SE. Drug-eluting balloons in the treatment of coronary De Novo lesions: a Comprehensive Review. Cardiol Ther. 2016;5:133–60. 10.1007/s40119-016-0064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Navarese EP, Kowalewski M, Kandzari D, Lansky A, Górny B, Kołtowski Ł, et al. First-generation versus second-generation drug-eluting stents in current clinical practice: updated evidence from a comprehensive meta-analysis of randomised clinical trials comprising 31 379 patients. Open Heart. 2014;1:e000064. 10.1136/openhrt-2014-000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giacoppo D, Alfonso F, Xu B, Claessen BEPM, Adriaenssens T, Jensen C, et al. Paclitaxel-coated balloon angioplasty vs. drug-eluting stenting for the treatment of coronary in-stent restenosis: a comprehensive, collaborative, individual patient data meta-analysis of 10 randomized clinical trials (DAEDALUS study). Eur Heart J. 2020;41(38):3715–28. 10.1093/eurheartj/ehz594. PMID: 31511862; PMCID: PMC7706792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JM, Park J, Kang J, Jeon K-H, Jung J, Lee SE, et al. Comparison among drug-eluting balloon, drug-eluting stent, and Plain Balloon Angioplasty for the treatment of In-Stent restenosis. JACC Cardiovasc Interv. 2015;8:382–94. 10.1016/j.jcin.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 41.Mamuti W, Ablimit A, Kelimu W, Rao F, Jiamali A, Zhang W, et al. Comparison of drug-eluting balloon versus drug-eluting stent in patients with in-stent restenosis: insight from randomized controlled trials. Int J Cardiol. 2015;179:424–9. 10.1016/j.ijcard.2014.11.114. [DOI] [PubMed] [Google Scholar]
- 42.Kawamoto H, Ruparelia N, Latib A, Miyazaki T, Sato K, Mangieri A, et al. Drug-coated balloons Versus Second-Generation Drug-Eluting stents for the management of recurrent multimetal-layered In-Stent restenosis. JACC Cardiovasc Interv. 2015;8(12):1586–94. Epub 2015 Sep 17. PMID: 26386758. [DOI] [PubMed] [Google Scholar]
- 43.Alfonso F, Cuesta J. The Therapeutic Dilemma of Recurrent In-Stent Restenosis. Circ Cardiovasc Interv. 2018;11(8):e007109. 10.1161/CIRCINTERVENTIONS.118.007109. PMID: 30354787. [DOI] [PubMed]
- 44.Alfonso F, Cuesta J, Pérez-Vizcayno MJ, García D, Blanco B, Rumoroso JR, Bosa F, et al. Interventional Cardiology Working Group of the Spanish Society of Cardiology. Bioresorbable vascular scaffolds for patients with In-Stent restenosis: the RIBS VI study. JACC Cardiovasc Interv. 2017;10(18):1841–51. Epub 2017 Aug 30. PMID: 28866036. [DOI] [PubMed] [Google Scholar]
- 45.Yabushita H, Kawamoto H, Fujino Y, Tahara S, Horikoshi T, Tada M et al. Clinical Outcomes of Drug-Eluting Balloon for In-Stent Restenosis Based on the Number of Metallic Layers. Circ Cardiovasc Interv. 2018;11(8):e005935. 10.1161/CIRCINTERVENTIONS.117.005935. PMID: 30354780. [DOI] [PubMed]
- 46.Leone PP, Mangieri A, Regazzoli D, Laricchia A, Khokhar A, Rossi ML et al. Drug-Coated Balloon Angioplasty Guided by Postpercutaneous Coronary Intervention Pressure Gradient: The REDUCE-STENT Retrospective Registry. JACC Cardiovasc Interv. 2023;16(3):363–365. doi: 10.1016/j.jcin.2022.09.054. Epub 2023 Jan 4. PMID: 36609047. [DOI] [PubMed]
- 47.Agostoni P, Belkacemi A, Voskuil M, Nathoe HM, Doevendans PA, Stella PR. Serial morphological and functional assessment of drug-eluting balloon for in-stent restenotic lesions: mechanisms of action evaluated with angiography, optical coherence tomography, and fractional flow reserve. JACC Cardiovasc Interv. 2013;6(6):569–76. Epub 2013 May 15. PMID: 23683736. [DOI] [PubMed] [Google Scholar]
- 48.Stefanini GG, Alfonso F, Barbato E, Byrne RA, Capodanno D, Colleran R et al. Management of myocardial revascularisation failure: an expert consensus document of the EAPCI. EuroIntervention. 2020;16(11):e875-e890. 10.4244/EIJ-D-20-00487. PMID: 32597391. [DOI] [PubMed]
- 49.Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. 10.1093/eurheartj/ehy394. Erratum in: Eur Heart J. 2019;40(37):3096. doi: 10.1093/eurheartj/ehz507. PMID: 30165437.
- 50.Madhavan MV, Kirtane AJ, Redfors B, Généreux P, Ben-Yehuda O, Palmerini T et al. Stent-Related Adverse Events > 1 Year After Percutaneous Coronary Intervention. J Am Coll Cardiol. 2020;75(6):590–604. doi: 10.1016/j.jacc.2019.11.058. PMID: 32057373. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated during this study are illustrated in this article and in supplementary data.