Abstract
Background
Branched-chain amino acid (BCAA) has been reported to be associated with obesity, the association of BCAA with visceral fat area (VFA) and subcutaneous fat area (SFA) remained unclear in patients with type 2 diabetes.
Methods
This cross-sectional study was conducted in 284 patients with type 2 diabetes mellitus. Enzyme-linked immunospecific assay was used to measure levels of serum BCAA and branched-chain keto acid (BCKA). VFA and SFA were measured with bio-impedance analysis method. The association between BCAA and VFA was calculated using Pearson correlation and multivariable linear regression analysis.
Results
There were significant differences in the means of body mass index, waist circumstance, SFA and VFA among the three groups divided by total BCAA tertiles (all p < 0.05). Compared to patients with lower levels of serum BCAA (the lower tertile group), the means of VFA and SFA were significantly larger in the middle and upper tertile groups (all p < 0.05). However, the differences in above obesity parameters were nonsignificant according to various BCKA tertiles. Pearson correlation analysis also demonstrated that BCAA levels were positive associated with each obesity parameter (p < 0.05). Nevertheless, multivariable linear regression analysis showed that levels of serum BCAA were correlated with VFA, BMI and WC (all p < 0.05) rather than SFA after adjusted for other confounders.
Conclusions
levels of serum BCAA were more closely correlated with VFA than SFA, prospective studies should be warranted to further explore the mechanism mediating BCAA and visceral fat accumulation in Human beings.
Clinical trial number
Not applicable.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12902-024-01768-1.
Keywords: Branched-chain amino acids, Visceral fat area, Subcutaneous fat area, Obesity, Type 2 diabetes
Background
Globally, 39% of adults were overweight and 13% were obese according to the World Health Organization report released 2021 [1]. Obesity is associated with the development or aggravation of cardiometabolic diseases, such as insulin resistance, dyslipidemia, hypertension cardiovascular disease and particularly type 2 diabetes [2]. Obesity-related comorbidities caused serious socioeconomic burden worldwide [3]. Poor lifestyle habits, including smoking, excessive alcohol consumption, physical inactivity and unbalanced diets, have been established to account for the booming of obesity and obesity-associated diseases [4]. In the past, it was believed that obesity resulted from high-fat and high-calorie diet [5]. Recently, researchers found that excessive intake or malmetabolism of branched-chain amino acid (BCAA) was also associated with many morbidities including obesity, insulin resistance, dyslipidemia, diabetes and even pancreatic cancer [6–8].
A majority of studies demonstrated that BCAA intake as well serum BCAA levels were positively associated with general and abdominal obesity [8–10], while restriction of BCAA intake in diet has been reported to ameliorate obesity in Humans and rodents [11]. However, there were some concerns. Firstly, the association between BCAA and obesity was controversial as an inverse association between dietary BCAA and prevalence of obesity has also been reported [12, 13]. Secondly, previous studies focused on obesity indices such body mass index (BMI) and waist circumstance (WC) [8, 10], a few studies explored the relationship between BCAA and visceral fat area (VFA), an obesity parameter closely associated with insulin resistance and onset of diabetes [14, 15]. Furthermore, previous studies mainly concentrated on obese adolescents, middle-aged and elderly population, or patients with polycystic ovary syndrome [8, 16, 17], the association between BCAA and obesity was seldomly studied in patients with type 2 diabetes, though elevated levels of serum BCAA has been reported in these individuals [18].
Therefore, we explored the relationship of circulating BCAA and its primary metabolite branched-chain keto acid (BCKA) with subcutaneous fat area (SFA) and VFA in patients with type 2 diabetes.
Methods
Study design and patients
Patients were recruited from the Department of Endocrinology, Putuo Hospital, Shanghai University of Traditional Chinese Medicine from January 1st, 2021 to August 30th, 2023. Type 2 diabetes was defined in accordance with the criteria of The American Diabetes Association [19]. We excluded patients as following: severe hepatic or renal insufficiency; type 1 diabetes mellitus or gestational diabetes; malignant tumors; severe infections or traumatic diseases; cognitive dysfunction or psychiatric disorders. Finally, 284 patients (105 females and 179 males) with type 2 diabetes mellitus were enrolled in this cross-sectional study.
The study was approved by the Ethics Committee of Department of Endocrinology, Putuo Hospital, Shanghai University of Traditional Chinese Medicine with the Declaration of Helsinki (Number of approval form of ethics committee: PTEC-A-2024-40(S)-1). Informed consents were acquired from all subjects.
Anthropometric and biochemical measurements
Anthropometric measurements including height, weight, WC and hip circumferences (HC) were measured by a trained nurse. Body mass index (BMI, kg/m2) was calculated as dividing the weight by height squared. VFA and SFA was determined using bioimpedance analysis method (OMRON, Tokyo).
Venous blood samples were drawn after an overnight fast of at least 10 h. Biochemical Indexes including fasting blood glucose, fasting insulin, postprandial blood glucose, triglycerides, total cholesterol, high- density lipoprotein - cholesterol (HDL-C), and low- density lipoprotein -cholesterol (LDL-C) were assayed by Roche cobas 8000 fully automated biochemical analyzer. Enzyme-linked immunospecific assay was used to measure serum BCAA (abcam, ab83374, USA) and BCKA (Fu sheng Biologicals, A137748, Shanghai, China). Participants were classified into three groups according to total BCAA levels (including leucine, isoleucine and valine): lower tertile ≤ 2.20 mmol/L (n = 94), 2.20 mmol/L < middle tertile < 2.88 mmol/L (n = 95), upper tertile ≥ 2.88 mmol/L (n = 95). Patients were also divided into three groups based on BCKA levels: lower tertile ≤ 121.4 umol/L (n = 94), 121.4 umol/L < middle tertile < 130.6 umol/L (n = 95), upper tertile ≥ 130.6 umol/L (n = 95).
Statistical analysis
Continuous variables were expressed as median (interquartile range), and categorical variables as number (percentage). Kruskal–Wallis test examined differences in medians among three groups (Table 1, supplementary Table 1). Differences in proportions were tested using the chi-square test. The general linear model was utilized to compare obesity indices according to tertiles of serum BCKA and BCAA, and LSD method was applied for post-hoc between-group comparison (Fig. 1). Pearson correlation analysis was used to evaluate the association of BCAA with obesity parameters (Fig. 2). Multiple linear regression analysis was used to examine the association of BCAA and BCKA with obesity parameters considering other clinical parameters (Table 2). All the analyses were performed using SPSS 26.0 (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.); two - sided p-values < 0.05 were considered statistically significant.
Table 1.
Characteristics of study participants with different serum BCAA levels
| Variables | BCAAs tertiles | P value | ||
|---|---|---|---|---|
| Lower tertile (n = 94) |
Middle tertile (n = 95) |
Upper tertile (n = 95) |
||
| Gender, male | 55 (58.5) | 55 (57.9) | 69 (72.4) | 0.044 |
| Age, years | 63.0 (55.8–67.3) | 63.0 (58.0–69.0) | 60.0 (48.0–66.0) | 0.040 |
| Duration of diabetes, years | 7.0 (0.2–17.5) | 7.0 (1.0–20.0) | 6.0 (0.2–16.0) | 0.65 |
| Current smoker, % | 18 (19.1) | 22 (23.2) | 15 (15.8) | 0.56 |
| Current drinkers, % | 12 (12.8) | 12 (12.6) | 15 (15.8) | 0.54 |
| BMI, kg/m2 | 24.2 (21.5–26.0) | 25.0 (23.0–27.2) | 25.3 (23.5–28.0) | 0.003 |
| WC, cm | 90.0 (84.0–95.1) | 93.0 (87.0–98.9) | 92.6 (88.8–101) | 0.001 |
| HTN, % | 65 (69.4) | 69 (72.6) | 62 (65.3) | 0.56 |
| SBP, mmHg | 133 (125–147) | 130 (123–144) | 136 (127–147) | 0.42 |
| DBP, mmHg | 81 (79–90) | 80 (75–89) | 82 (78–89) | 0.38 |
| FPG, mmol/L | 7.37 (5.70–10.0) | 8.00 (6.10–10.5) | 7.90 (6.20–10.6) | 0.30 |
| HbA1c, % | 9.9 (8.3–12.3) | 9.5 (7.9–11.5) | 9.2 (7.6–10.9) | 0.17 |
| F-INS, pmol/L | 61.9 (34.6–110) | 74.9 (43.3–125) | 67.7 (41.1–103) | 0.48 |
| HOMA-IR, mmol/L*mU/L | 3.11 (1.52–5.77) | 4.18 (2.12–6.05) | 3.44 (2.20–5.32) | 0.35 |
| TC, mmol/L | 4.67 (3.80–5.61) | 4.63 (3.57–5.68) | 4.56 (3.79–5.41) | 0.92 |
| TG, mmol/L | 1.30 (0.97–1.93) | 1.59 (1.16–2.25) | 1.61 (1.28–2.40) | 0.006 |
| HDL-C, mmol/L | 1.11 (0.91–1.34) | 1.07 (0.92–1.27) | 1.02 (0.85–1.24) | 0.17 |
| LDL-C, mmol/L | 3.12 (2.38–3.72) | 3.06 (2.19–3.88) | 2.94 (2.25–3.90) | 0.99 |
| BCAA, mmol/L | 1.86 (1.30–2.09) | 2.51 (2.40–2.67) | 3.34 (3.08–3.95) | < 0.001 |
| BCKA, umol/L | 128 (106–155) | 129 (116–137) | 127 (119–131) | 0.49 |
Data are presented as median (IQR) for continuous variables and number (proportion) for category variables. Differences in medians were examined using Kruskal–Wallis test among three groups; Differences in proportions were tested using the Chi-square test
BMI Body mass index, WC waist circumference, FPG fasting plasma glucose, TC total cholesterol, HTN hypertension, TG triglyceride, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, FCP fasting C-peptide, SBP systolic blood pressure, DBP diastolic blood pressure, VFA visceral fat area, SFA subcutaneous fat area, BCAA branched-chain amino acids, BCKA branched-chain keto acids
Fig. 1.
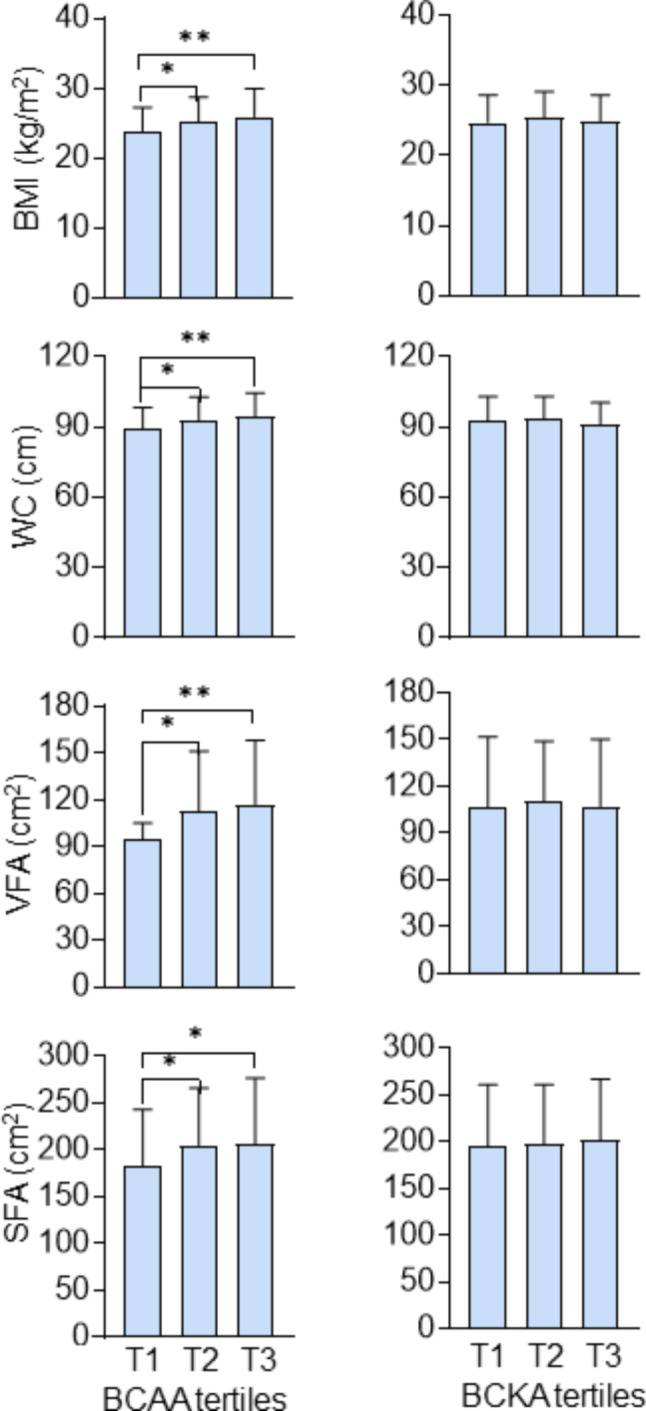
Levels of obesity parameters according to tertiles of BCAA and BCKA. *p < 0.05, **p < 0.001. BMI Body mass index, WC waist circumference, VFA visceral fat area, SFA subcutaneous fat area, BCAA branched-chain amino acid, BCKA branched-chain keto acid
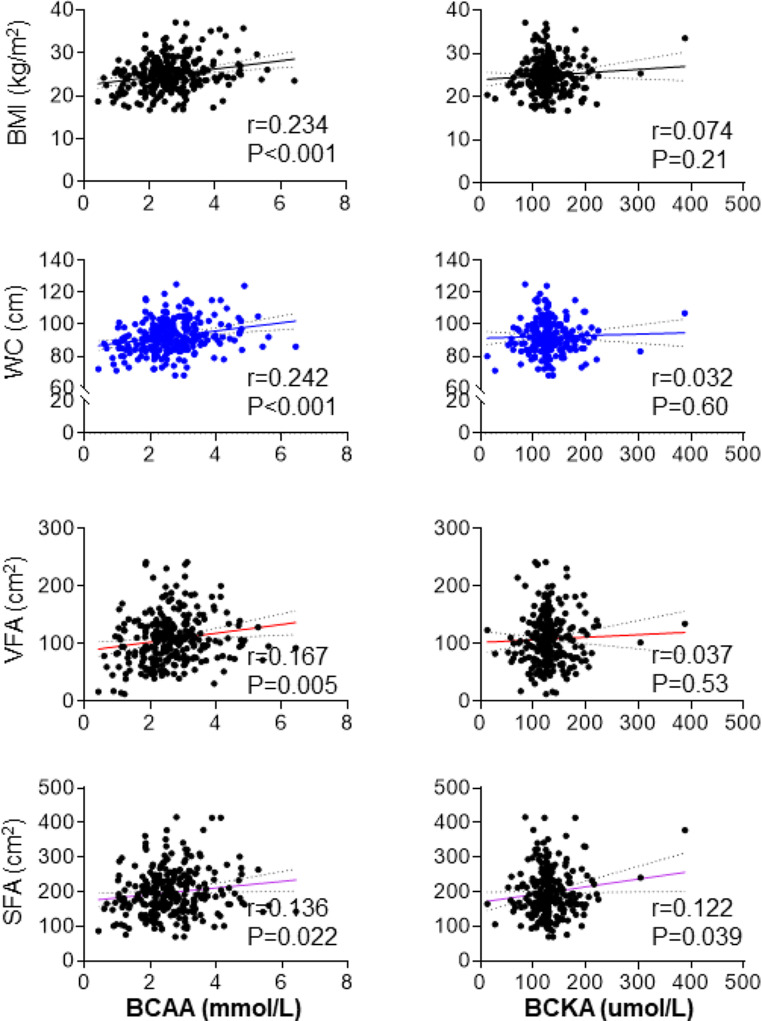
Fig. 2.
Association of BCAA and BCKA with obesity parameters
Table 2.
Correlation of BCAA and BCKA with each obesity parameter
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| β | P value | β | P value | β | P value | |
| BMI | ||||||
| BCAA | 0.953 | < 0.001 | 0.794 | 0.001 | 0.682 | 0.009 |
| BCKA | 0.008 | 0.214 | 0.007 | 0.284 | -0.002 | 0.813 |
| WC | ||||||
| BCAA | 2.553 | < 0.001 | 1.988 | 0.002 | 1.643 | 0.018 |
| BCKA | 0.009 | 0.604 | 0.006 | 0.712 | -0.016 | 0.378 |
| VFA | ||||||
| BCAA | 7.670 | 0.005 | 6.649 | 0.016 | 6.120 | 0.039 |
| BCKA | 0.045 | 0.531 | 0.043 | 0.545 | -0.012 | 0.881 |
| SFA | ||||||
| BCAA | 9.502 | 0.022 | 7.563 | 0.068 | 5.741 | 0.198 |
| BCKA | 0.224 | 0.039 | 0.203 | 0.055 | 0.102 | 0.399 |
Data were analyzed using multiple linear regression analysis
In Model 1, independent variables included BCAA or BCKA; In Model 2, independent variables included age, gender and BCAA (or BCKA); In Model 3, independent variables included variables in Model 2 plus duration of diabetes, systolic blood pressure, HbA1c, TG, HDL-C, smoking status, drinking status, and HOMA-IR
BMI Body mass index, WC waist circumference, TG triglyceride, HDL-C high-density lipoprotein cholesterol, VFA visceral fat area, SFA subcutaneous fat area, BCAA branched-chain amino acids, BCKA branched-chain keto acids
Results
Clinical characteristics of the participants
As shown in Table 1, there were differences in the proportion of male and levels of triglyceride (TG), BMI and waist circumstance among groups divided by BCAA tertiles. No statistical differences were observed among three groups in duration of diabetes, blood pressure (systolic and diastolic), fasting C-peptide, fasting plasma glucose, glycosylated hemoglobin, fasting insulin, insulin resistance index, total cholesterol, low density lipoprotein cholesterol (LDL-C), high density lipoprotein cholesterol (HDL-C), and proportion of current smokers, current alcohol drinkers. However, there were no difference in levels of BMI and waist circumstance according to BCKA tertiles.
Obesity indexes according to various levels of BCAA or BCKA
As shown in Fig. 1, there were significant differences in the means of VFA among the three groups divided by BCAA tertiles (p = 0.001). The mean (cm2) of VFA in the lower tertile group was significantly smaller than that in the middle tertile and upper tertile groups (94.7 versus 112.2, 94.7 versus 117.1, all p < 0.05). In ascending order, the means of SFA (cm2) were 183.0, 204.8 and 206.4 among above three groups (p for trend = 0.02). Similar trends were also observed in the levels of BMI and WC according to the BCAA tertiles (all p < 0.01). However, the differences in VFA SFA BMI and WC were nonsignificant among groups classified by BCKA tertiles.
Association of BCAA with VFA
As shown in Fig. 2, Pearson correlation analysis indicated that BCAA was associated with common obesity parameters including BMI (r = 0.234, p < 0.001) and WC (r = 0.242, p < 0.001). Further analysis showed that BCAA was more closely associated with VFA (r = 0.167, p = 0.005) in comparison to SFA (r = 0.136, p = 0.022). However, BCKA was just correlated with SFA (r = 0.122, p = 0.039).
As shown Table 2, univariable linear regression analysis indicated BCAA was positively associated with BMI, WC, VFA and SFA (all p < 0.05), whereas, BCKA was only correlated with SFA (β = 0.224, p = 0.039). The association of BCAA and BMI, WC and VFA remained significant after adjusted for age and gender (Model 2) and further adjusted for duration of diabetes, systolic blood pressure, HbA1c, TG, HDL-C, smoking status, drinking status, and Homeostasis model assessment for insulin resistance (HOMA-IR) (Model 3) (all p < 0.05). Nevertheless, the correlation of BCAA and SFA became non-significant after adjustment for other confounders (Model 2, Model 3 in Table 2). Furthermore, the correlation of BCKA with obesity indices was of no statistical significance in multivariable regression analysis.
Discussion
Several interesting conclusions could be drawn in this study: (1) levels of serum BCAA were positively correlated with obesity parameters including BMI, WC, VFA and SFA; (2) BCAA was associated with VFA rather than SFA after adjustment for other confounders; (3) No correlation was observed between levels of serum BCKA and obesity indices considering other confounders.
Previous studies have pointed out BCAA was closely associated with obesity. A majority of studies demonstrated that elevated levels of BCAA in diet and serum were correlated with BMI and WC [8, 17, 20–22]. In animal studies, BCAA supplementation in diet increased perirenal white fat mass [23], whereas restriction of BCAA intake could rapidly reduce the abdominal fat mass [9, 24]. Our previous study also found that elevated BCAA intake deteriorated pancreatic steatosis in high fat diet (HFD) - fed mice [25]. In this study, our data also showed that serum BCAA was positively with BMI and WC as previously reported. However, there were few studies concerning the relationship between BCAA and VFA in patients with type 2 diabetes, although researchers pointed out that BCAA was associated with VFA in adolescents [14]. On the other hand, elevated VFA was associated with increased risk of diabetes and its complications including diabetic macro- and micro-vascular complications [26–29]. In this study, our data also demonstrated that BCAA was independently linked to VFA rather than SFA, indicating that reducing serum levels of BCAA (such as by restriction of its intake or accelerate BCAA catabolism) might reduce visceral fat accumulation, and further prevent patients from diabetic complications.
The mechanisms mediating BCAA and VFA are complicated. Firstly, acetyl-CoA from BCAA catabolism was an important source of de novo lipogenesis, and about 30% acetyl-CoA came from BCAA catabolism in adipogenesis [30]. Secondly, BCAA catabolism could promote adipocytes differentiation by amplifying the expression of peroxisome proliferator-activated receptor gamma (PPARγ) in a Sirtuin 4 (SIRT4) dependent pattern [31]. Thirdly, BCAA was closely associated with insulin resistance. Research pointed out that BCAA supplementation could activated mammalian target of rapamycin complex 1 (mTORC1) and subsequent ribosomal protein S6 kinase, thus resulting in decreased insulin-induced phosphoinositide 3-kinase activity and subsequently impaired insulin signaling [32], on the other hand, insulin resistance could further accelerate lipogenesis [33]. Finally, visceral adipocytes might modulate levels of serum BCAA. Tan et al. point out that the levels of serum BCAA was dependent on the reduction of VFA after bariatric surgery [34].
However, there were some limitations in our study. First, this was a cross-sectional study and more prospective studies should be warranted to explored their causal relationship between BCAA and VFA. Also, the mechanisms mediating the association between BCAA and VFA had not been investigated in this study. Moreover, the study subjects were restricted to patients with type 2 diabetes, and a majority of subjects were male, and the extrapolation of the conclusion to the whole population demands more large-scale studies.
Conclusions
In conclusion, levels of serum BCAA were more closely correlated with VFA than SFA, reduced levels of serum BCAA may help prevent visceral fat deposition and further diabetic complications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Thanks to X C, W L, and J L for conducting statistical analysis and writing the manuscript, J G, Y S, and H W for assisting in data collection, L W and T L for participating in the design of this study and reviewing the manuscript. Thanks for obtaining permission from all those mentioned.
Abbreviations
- BMI
Body mass index
- WC
waist circumference
- FPG
fasting plasma glucose
- TC
total cholesterol
- HTN
hypertension
- TG
triglyceride
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- FCP
fasting C-peptide
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- VFA
visceral fat area
- SFA
subcutaneous fat area
- BCAA
branched-chain amino acids
- BCKA
branched-chain keto acids
Author contributions
X C, W L and J L performed the statistical analysis and wrote the manuscript, J G, Y S and H W contributed to data collection, L Wand T L participated in the design of this study and reviewed the manuscript.
Funding
This work was supported by Clinical Characteristic of Health System in Putuo District, Shanghai (2020tszk01), Shanghai Medical Key Specialties (ZK2019B16), Research Project of Shanghai Municipal Health Care Commission (202240309), Technology Innovation Project of Putuo District Health System (ptkwws202003, ptkwws202302).
Data availability
The datasets used and/or analyzed during the current study were available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Ethics Committee of Shanghai Putuo District Central Hospital (Shanghai University of Traditional Chinese Medicine Affiliated Putuo Hospital) has approved the research in accordance with the Declaration of Helsinki, with approval number: PTEC-A-2024-40(S)-1. Informed consents were acquired from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xinghua Cai and Wenmin Li contributed equally to this work.
Contributor Information
Tao Lei, Email: taolei_12@sina.com.
Jun Lu, Email: lujundoctor@163.com.
References
- 1.World Health organization. obesity-and-overweight (accessed on 9 June 2021). https://www.who.int/news-room/fact-sheets/detail/
- 2.(WHO) WHO. Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8–11 December 2008. Geneva: World Health Organization; 2021. [Google Scholar]
- 3.Divino V, Ramasamy A, Anupindi VR, et al. Complication-specific direct medical costs by body mass index for 13 obesity-related complications: a retrospective database study. J Manag Care Spec Pharm. 2021;27(2):210–22. 10.18553/jmcp.2020.20272. [published Online First: 20201214]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim C, Costello FJ, Lee KC, et al. Predicting factors affecting adolescent obesity using general bayesian network and What-If analysis. Int J Environ Res Public Health. 2019;16(23). 10.3390/ijerph16234684. [DOI] [PMC free article] [PubMed]
- 5.Paik J, Fierce Y, Treuting PM, et al. High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible Mdr1a-/- male mice. J Nutr. 2013;143(8):1240–7. 10.3945/jn.113.174615. [published Online First: 20130612]. [DOI] [PubMed] [Google Scholar]
- 6.Shah SH, Crosslin DR, Haynes CS, et al. Branched-chain amino acid levels are associated with improvement in insulin resistance with weight loss. Diabetologia. 2012;55(2):321–30. 10.1007/s00125-011-2356-5. [published Online First: 2011/11/09]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katagiri R, Song M, Zhang X, et al. Dietary intake of branched-chain amino acids and risk of Colorectal Cancer. Signal Transduct Target Therapy. 2020;13(1):65–72. 10.1038/s41392-020-0168-0. [published Online First: 2020/05/30]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paczkowska K, Rachoń D, Berg A, et al. Specific alteration of branched-chain amino Acid Profile in Polycystic Ovary Syndrome. Biomedicines. 2023;11(1). 10.3390/biomedicines11010108. [DOI] [PMC free article] [PubMed]
- 9.Xiao F, Du Y, Lv Z, et al. Effects of essential amino acids on lipid metabolism in mice and humans. J Mol Endocrinol. 2016;57(4):223–31. [DOI] [PubMed] [Google Scholar]
- 10.Okekunle AP, Lee H, Provido SMP, et al. Dietary branched-chain amino acids and odds of obesity among immigrant Filipino women: the Filipino women’s diet and health study (FiLWHEL). BMC Public Health. 2022;22(1):654. 10.1186/s12889-022-12863-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fontana L, Cummings NE, Arriola Apelo SI, et al. Decreased consumption of branched-chain amino acids improves Metabolic Health. Cell Rep. 2016;16(2):520–30. 10.1016/j.celrep.2016.05.092. [published Online First: 20160623]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y-C, Li Y, Liu L-Y, et al. The ratio of Dietary branched-chain amino acids is Associated with a lower prevalence of obesity in Young Northern Chinese adults: an internet-based cross-sectional study. Nutrients. 2015;7(11):9573–89. 10.3390/nu7115486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cogate PG, Natali AJ, de Oliveira A, et al. Consumption of branched-chain amino acids is inversely Associated with Central Obesity and Cardiometabolic Features in a Population of Brazilian middle-aged men: potential role of leucine intake. J Nutr Health Aging. 2015;19(7):771–7. 10.1007/s12603-015-0522-z. [published Online First: 2015/07/22]. [DOI] [PubMed] [Google Scholar]
- 14.Rietman A, Stanley TL, Clish C, et al. Associations between plasma branched-chain amino acids, β-aminoisobutyric acid and body composition. J Nutr Sci. 2016;5:e6. 10.1017/jns.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi T, Boyko EJ, McNeely MJ, et al. Visceral adiposity, not abdominal subcutaneous fat area, is associated with an increase in future insulin resistance in Japanese americans. Diabetes. 2008;57(5):1269–75. 10.2337/db07-1378. [published Online First: 20080225]. [DOI] [PubMed] [Google Scholar]
- 16.Segovia-Siapco G, Khayef G, Pribis P, et al. Animal protein intake is Associated with General Adiposity in adolescents: the Teen Food and Development Study. Nutrients. 2019;12(1). 10.3390/nu12010110. [DOI] [PMC free article] [PubMed]
- 17.Asoudeh F, Salari-Moghaddam A, Keshteli AH, et al. Dietary intake of branched-chain amino acids in relation to general and abdominal obesity. Eat Weight Disord. 2022;27(4):1303–11. 10.1007/s40519-021-01266-6. [DOI] [PubMed] [Google Scholar]
- 18.Ramzan I, Ardavani A, Vanweert F, et al. The Association between circulating branched chain amino acids and the temporal risk of developing type 2 diabetes Mellitus: a systematic Review & Meta-Analysis. Nutrients. 2022;14(20). 10.3390/nu14204411. [published Online First: 20221020]. [DOI] [PMC free article] [PubMed]
- 19.2. Classification and diagnosis of diabetes: standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S13–27. 10.2337/dc18-S002. [DOI] [PubMed] [Google Scholar]
- 20.Bogl LH, Kaye SM, Rämö JT, et al. Abdominal obesity and circulating metabolites: a twin study approach. Metab Clin Exp. 2016;65(3):111–21. 10.1016/j.metabol.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 21.McCormack SE, Shaham O, McCarthy MA, et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr Obes. 2013;8(1):52–61. 10.1111/j.2047-6310.2012.00087.x. [published Online First: 2012/09/11]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J, Gu Y, Liu H, et al. Daily branched-chain amino acid intake and risks of obesity and Insulin Resistance in children: a cross-sectional study. Obes (Silver Spring Md). 2020;28(7):1310–16. 10.1002/oby.22834. [published Online First: 2020/06/09]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li X, Wang X, Liu R, et al. Chronic leucine supplementation increases body weight and insulin sensitivity in rats on high-fat diet likely by promoting insulin signaling in insulin-target tissues. Mol Nutr Food Res. 2013;57(6):1067–79. 10.1002/mnfr.201200311. [DOI] [PubMed] [Google Scholar]
- 24.Cummings NE, Williams EM, Kasza I, et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J Physiol. 2018;596(4):623–45. 10.1113/JP275075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu J, Pan T, Gao J, et al. Reduced branched-chain amino acid Intake Improved High-Fat Diet-Induced nonalcoholic fatty pancreas disease in mice. Pancreas. 2024;53(2):e157–63. 10.1097/mpa.0000000000002281. [published Online First: 2024/01/16]. [DOI] [PubMed] [Google Scholar]
- 26.Chiba I, Lee S, Bae S, et al. Visceral fat accumulation is associated with risk of diabetes in community-dwelling Japanese older adults. Geriatr Gerontol Int. 2021;21(3):306–12. 10.1111/ggi.14131. [published Online First: 20210208]. [DOI] [PubMed] [Google Scholar]
- 27.Bouchi R, Ohara N, Asakawa M, et al. Is visceral adiposity a modifier for the impact of blood pressure on arterial stiffness and albuminuria in patients with type 2 diabetes? Cardiovasc Diabetol. 2016;15:10. 10.1186/s12933-016-0335-3. [published Online First: 20160121]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anan F, Masaki T, Ito Y, et al. Diabetic retinopathy is associated with visceral fat accumulation in Japanese type 2 diabetes mellitus patients. Metab Clin Exp. 2010;59(3):314–9. 10.1016/j.metabol.2009.06.001. [published Online First: 20091209]. [DOI] [PubMed] [Google Scholar]
- 29.Anan F, Masaki T, Umeno Y, et al. Correlations of visceral fat accumulation and atherosclerosis in Japanese patients with type 2 diabetes mellitus. Metab Clin Exp. 2008;57(2):280–4. 10.1016/j.metabol.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 30.Green CR, Wallace M, Divakaruni AS, et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat Chem Biol. 2016;12(1):15–21. 10.1038/nchembio.1961. [published Online First: 20151116]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zaganjor E, Yoon H, Spinelli JB, et al. SIRT4 is an early regulator of branched-chain amino acid catabolism that promotes adipogenesis. Cell Rep. 2021;36(2):109345. 10.1016/j.celrep.2021.109345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metabol. 2009;9(4):311–26. 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith GI, Shankaran M, Yoshino M, et al. Insulin resistance drives hepatic de novo lipogenesis in nonalcoholic fatty liver disease. J Clin Invest. 2020;130(3):1453–60. 10.1172/JCI134165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan HC, Khoo CM, Tan MZ-W, et al. The effects of Sleeve Gastrectomy and gastric bypass on branched-chain amino acid metabolism 1 year after bariatric surgery. Obes Surg. 2016;26(8):1830–35. 10.1007/s11695-015-2023-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study were available from the corresponding author on reasonable request.