Abstract
Background
Brazil has the highest number of HTLV-1 infection in Latin America, with around one million cases spread unevenly across regions. However, there is a limited number of studies on this infection in the general population. This cross-sectional study aimed to estimate the prevalence of HTLV as well as identify types, and subtypes of HTLV among the urban population of Campo Grande, capital of Mato Grosso do Sul state (MS).
Results
Between July 2023 and March 2024, all information was obtained from self-reported interviews, and blood samples were collected and screened for anti-HTLV-1/2 by immunoassay and confirmed using the immunoblot method. The proviral DNA of HTLV-1/2 in positive samples was quantified by real-time PCR (qPCR) and genotyped by nucleotide sequencing (Sanger’s method). The study enrolled 611 participants, with the majority being women (90.54%), mixed race (46.32%), heterosexual (87.64%), and with a median age of 39 years. The prevalence rate of anti-HTLV-1 infection was 0.82% (CI 95% 0.34–1.96). All positive samples (n = 5) were identified as belonging to the Cosmopolitan subtype, one belonging to Japanese and four to Transcontinental subgroups. Among the five positive individuals, two presented symptoms associated with HTLV-1 infection.
Conclusion
This study highlights an intermediate prevalence of HTLV-1 in the urban population of Campo Grande, Mato Grosso do Sul, and provides epidemiological information that could help bridge the gaps in the distribution of HTLV in the general population. Also, medical care was provided for individuals presenting clinical manifestations who were previously unaware of their serological status.
Keywords: HTLV, Prevalence, Central Brazil
Introduction
Human T-lymphotropic virus (HTLV) is a retrovirus that mainly targets T lymphocytes. This includes HTLV-1 and HTLV-2, which were identified in the 1980s, and later, HTLV-3 and HTLV-4 [1, 2]. HTLV-1 is categorized into seven distinct subtypes: 1-a to 1-g. The 1-a or Cosmopolitan subtype (HTLV-1a), is divided into six subgroups [3]. Of these, the Japanese (HTLV-1aJpn) and Transcontinental (HTLV-1aTC) subgroups have already been reported in Brazil [4–8].
The routes of transmission include contact with contaminated blood products, vertical transmission, and condomless sexual intercourse [9]. HTLV is associated with the development of several diseases such as HTLV-1-associated myelopathy (HAM), adult T-cell leukemia/lymphoma (ATLL), infectious dermatitis, uveitis, arthritis, and other “minor” neurological signs that do not meet diagnostic criteria for HAM [10].
HTLV-1 is prevalent globally, notably in regions such as South Japan, the Caribbean Basin, and parts of South America. Brazil has the largest number of people living with HTLV-1 in Latin America, with approximately one million people infected heterogeneously distributed throughout all regions [2, 11].
Several studies have reported the presence and prevalence of HTLV infection among specific groups in Brazil [7, 8, 12–18]. Furthermore, the introduction of mandatory HTLV-1/2 screening in potential blood donors in 1993 allowed for a better understanding of the distribution of the virus in all regions of the country [19, 20]. However, blood donors and vulnerable groups in Brazil may not represent the general population due to various factors such as age restrictions, gender disparities, risk behaviors, and health conditions.
This cross-sectional study aimed to describe the prevalence of HTLV infection in the urban population of Campo Grande, the capital of Mato Grosso do Sul state, and the seroepidemiological and molecular profiles of HTLV-1/2.
Materials and methods
Ethics statement
This study was approved by the Ethical Committee on Human Research of the Universidade Federal de Mato Grosso do Sul (CEP/UFMS) by protocol number CAAE: 63756922.00000.0021. All research was performed according to relevant guidelines and regulations. The consent form elucidated the study's objectives, voluntary nature of participation, and right to withdraw at any time without repercussions. Participants were informed about the procedures, including blood sample collection and the confidentiality of their data. It was explicitly stated that if they tested positive for HTLV-1/2, they would receive their results privately via email or telephone, accompanied by appropriate medical referrals, if warranted. Furthermore, participants were informed of potential health implications and the importance of early detection, and were provided with information regarding prevention and treatment of symptoms options. This protocol ensured that all participants were aware of their rights, nature of the study, and consequences of a positive result.
Sample calculation
The calculation of the sample size for the number of individuals to be investigated was defined by the calculation of prevalence in infinite populations, as there was no assumption of prevalence estimated for HTLV. A maximum frequency of 50% was used to obtain the largest sample size with a confidence level of 95%, sampling error of 2%, and non-response rate of 20%. This cross-sectional study was a part of a population-based study conducted in the northern, northeastern, and central-western regions of Brazil. Therefore, for the study in Mato Grosso do Sul State, a sample size of 319 individuals was considered the minimum number of individuals participating in the study.
Study population
A cross-sectional study was conducted among the urban population of Campo Grande, capital of Mato Grosso do Sul state (MS)—Central Brazil, between July 2023 and March 2024. The general profile of residents from the capital is, in the majority, mixed race, median age of 34 years, and women [21]. The inclusion criteria were residents of the capital city with a minimum age of 9 years. Prior to enrollment, all study participants or, in the case of individuals under the age of 18, their legal guardians provided written informed consent. Participants underwent an interview with a standardized questionnaire containing sociodemographic and risky behavior information and provided blood samples.
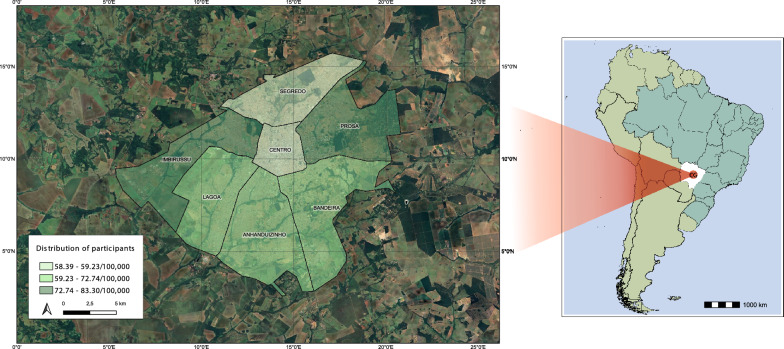
This study was conducted using a convenience method at the Universidade Federal de Mato Grosso do Sul, and 21 basic family health units were distributed in all seven sanitary districts in the capital (Fig. 1). These health units carry out health promotion, protection, and recovery activities, ranging from administering vaccines and dispensing medications to dental care.
Fig. 1.
Distribution of participants from all seven sanitary districts in Campo Grande (CG), the capital of MS State. To calculate the distribution, the total number of participants in the district/total number of residents in district X 100,000. The map was generated using QGIS (version 3.26.2) with 3 classes precision, and the Jenks natural breaks model was selected.
Serological and molecular tests
All samples were screened using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Murex HTLV I + II—DiaSorin) following the manufacturer’s instructions to detect anti-HTLV-1/2 antibodies. Positive samples were repeatedly tested and confirmed using an HTLV-1/2 Western Blot (WB) assay (MP Diagnostics HTLV BLOT 2.4, Singapore). HTLV infection was defined as positive in both ELISA screening and confirmatory WB tests.
The anti-HTLV-positive samples were subjected to molecular analysis. To determine proviral load, peripheral blood mononuclear cells (PBMCs) were isolated from EDTA blood samples of HTLV-1-infected individuals using density gradient centrifugation. DNA was extracted using the QIAamp DNA Blood Mini Kit (Qiagen) and subjected to amplification by multiplex real-time PCR (mqPCR) with an amplicons size of 100 bp of the HTLV-1/2 tax region to determine proviral load, as previously reported [22].
For sequencing, DNA was extracted from whole blood samples of anti-HTLV-positive participants using the QIAamp DNA Blood Mini Kit (Qiagen), according to the manufacturer’s instructions. A 646 bp fragment of the HTLV-1 5’LTR region was amplified by nested PCR [23]. The amplicons were purified using a QIA Quick Purification kit (Qiagen Inc., Maryland, USA) according to the manufacturer’s instructions. The fragments were sequenced by Sanger's method using the BigDye Terminator Cycle Sequencing Ready Reaction Kit and ABI 3500XL (Applied Biosystems, Foster City, CA, United States).
HTLV-1 isolates were subjected to analysis using the Basic Local Alignment Search Tool (BLAST) after nucleotide sequencing to identify subtypes and subgroups. Nucleotide sequences were aligned and compared to 55 published HTLV-1 sequences available from GenBank, with reference sequences used as outgroups, and sequences were searched with filter HTLV-1 and Transcontinental and Brazil, using MAFFT plugin, version 7.308, available in Geneious software, version 7.1.3, employing the L-INS-i algorithm [24].
Phylogenetic tree inference analysis was reconstructed using Maximum Likelihood analysis and performed using RAxML, utilizing the GTRGAMMA model. Support values were estimated from 1000 bootstrap pseudoreplicates on the CIPRES Science Gateway portal (https://www.phylo.org) [25]. The phylogenetic tree was edited using the Interactive Tree Of Life (iTOL) software v6 (https://itol.embl.de) [26].
GenBank nucleotide sequence accession number included in the phylogenetic analysis: DQ235700; L36905; MK936943; KM023768; KM023765; KM023769; KM023757; AF054627; Y16481; DQ235698; DQ235699; J02029; JF271837; JF271838; AF033817; DQ070891; DQ070892; DQ471196; FJ853491; KY490575; FJ853490; DQ471194; DQ471193; OM863789; OM863790; OK247616; OK247617; KY510690; KY490581; JF271836; GQ443755; KM023763; KM023762; GQ443757; JF271840; JF271842; JF271841; KY510691; GQ443756; DQ471187; DQ471195; DQ471190; DQ471192; DQ471191; DQ471189; DQ471188; EU392160; AY499185; EU392159; Y17014; AY920503; Z32527; L76310; DQ471197; L02534.
Data analysis
The variables were analyzed using the Stata software (version 13.0; Stata Corporation, College Station, TX, USA). Categorical variables were presented as absolute and percentage frequencies. Continuous variables were expressed as medians and ranges. The prevalence of anti-HTLV-1/2 was calculated with a 95% confidence interval (95% CI). Fisher’s exact test was used to evaluate the sociodemographic and behavioral characteristics of HTLV-1/2 (p < 0.05 were considered statistically significant).
Results
A total of 611 individuals agreed to participate in the study between July 2023 and March 2024 (Table 1) from all seven sanitary districts from the Capital, with participation coverage ranging from 58.39 to 83.30/100,000 from each district (Fig. 1). The general profile of the group based on self-report was a majority of women (90.54%), with a median age of 39 years (1IQ 28 to 3IQ 54 years old). Most of them considered themselves to be mixed race (46.32%), 87.64% heterosexual, and 99.67% cisgender. In addition, most of them (49.67%) had a high educational level (> 12 years of study), and a low family income (42.76%), with one up to two minimum wages ($660). Furthermore, 62.14% were born by normal delivery, and 90.76% were breastfed, with a majority for more than 6 months (76.54%) by their biological mother (93.78%). Most of the study participants declared that they were not undergoing any health monitoring during the recruitment period (56.07%). Of those who underwent any medical follow-up, eight individuals were related to Sexually Transmitted Infections (STIs).
Table 1.
Sociodemographic and behavioral characteristics of urban population, Campo Grande-MS (n = 611)
| Variable | Negative (n = 606) | Positive (n = 5) | p value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Age (years) | |||||
| < 45 | 366 | 99.46 | 2 | 0.54 | 0.861 |
| ≥ 45 | 240 | 98.77 | 3 | 1.23 | |
| Biological sex | |||||
| Male | 179 | 99.44 | 1 | 0.56 | 0.539 |
| Female | 427 | 99.07 | 4 | 0.93 | |
| Sexual orientation | |||||
| Heterosexual | 528 | 99.25 | 4 | 0.75 | 0.484 |
| Homosexual | 43 | 97.73 | 1 | 2.27 | |
| Others | 31 | 100 | 0 | 0 | |
| Marital status | |||||
| Steady partner | 290 | 98.98 | 3 | 1.02 | 0.462 |
| No steady partner | 316 | 99.18 | 2 | 0.63 | |
| State of birth in Brazil | |||||
| Mato Grosso do Sul | 436 | 99.32 | 3 | 0.68 | 0.624 |
| Other states | 170 | 98.80 | 2 | 1.16 | |
| Ethnicity | |||||
| White | 239 | 99.17 | 2 | 0.83 | 0.086 |
| Mixed race | 282 | 99.65 | 1 | 0.35 | |
| Black | 70 | 98.59 | 1 | 1.41 | |
| Japanese descendant | 9 | 90.00 | 1 | 10.00 | |
| Others | 6 | 100 | 0 | 0 | |
| Years of study | |||||
| > 12 | 302 | 100 | 0 | 0 | 0.064 |
| 10–12 | 191 | 98.45 | 3 | 1.55 | |
| 1–9 | 99 | 98.02 | 2 | 1.98 | |
| lecture | 11 | 100 | 0 | 0 | |
| History of tattoo | |||||
| No | 401 | 99.26 | 3 | 0.74 | 0.550 |
| Yes | 205 | 99.03 | 2 | 0.97 | |
| History of blood transfusion before 1993 | |||||
| No | 555 | 99.46 | 3 | 0.54 | 0.067 |
| Yes | 53 | 96.36 | 2 | 3.64 | |
| Ever exposed to blood in accidents | |||||
| No | 521 | 99.24 | 4 | 0.76 | 0.480 |
| Yes | 72 | 98.63 | 1 | 1.37 | |
| History of condom use (lifetime) | |||||
| Regular | 122 | 100 | 0 | 0 | 0.317 |
| Irregular | 470 | 98.95 | 5 | 1.05 | |
| History of STI | |||||
| No | 477 | 99.17 | 4 | 0.83 | 0.592 |
| Yes | 93 | 98.94 | 1 | 1.06 | |
| Familiar PLwHTLV | |||||
| No | 320 | 99.38 | 2 | 0.62 | 0.924 |
| Yes | 13 | 100 | 0 | 0 | |
| Breastfeeding historical | |||||
| No | 51 | 100 | 0 | 0 | 0.747 |
| Yes | 498 | 99.40 | 3 | 0.60 | |
| Breastfed by | |||||
| Biological mother | 449 | 99.34 | 3 | 0.66 | 0.824 |
| Cross-nursing | 30 | 100 | 0 | 0 | |
STI: sexually transmitted infection; PLwHTLV: People living with HTLV
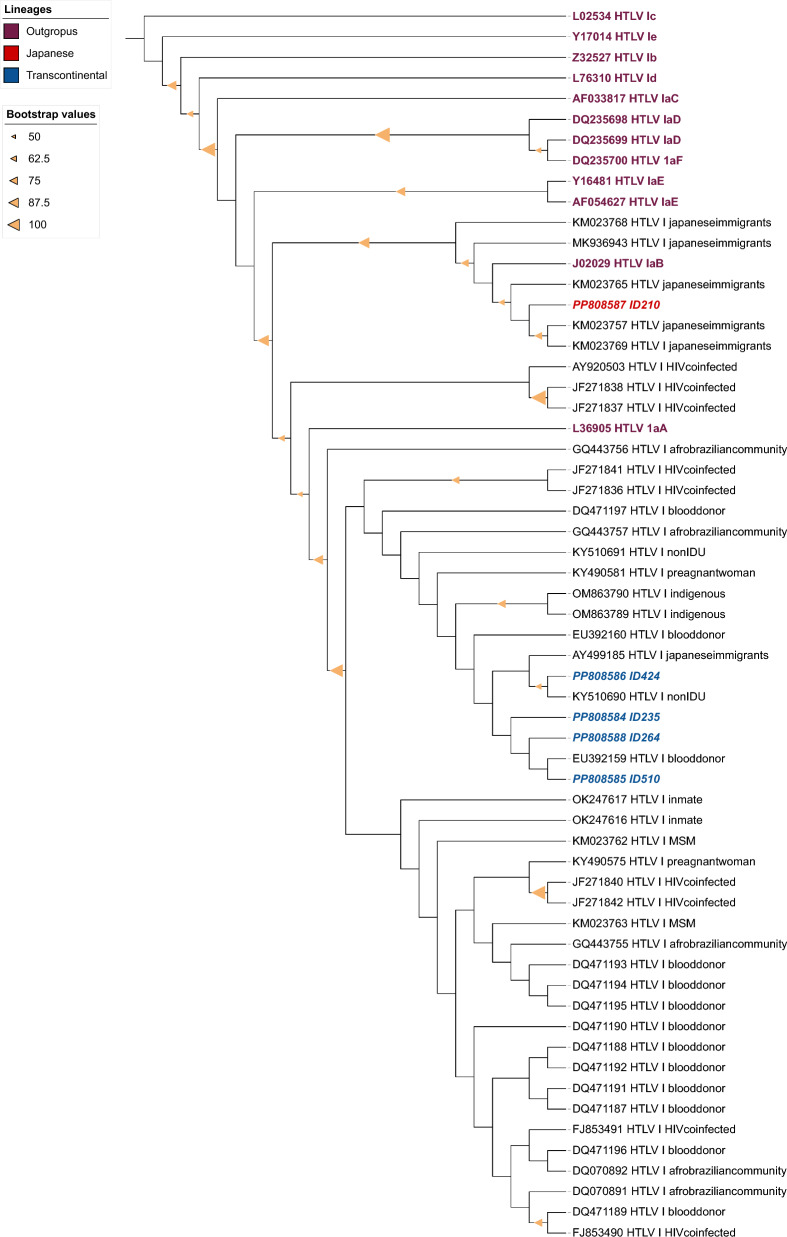
Five individuals were screened and confirmed as anti-HTLV-1 seropositive. We did not identify any familial connections among positive individuals. Furthermore, the prevalence of anti-HTLV-1 found was 0.82 (CI 95% 0.34–1.96). All five positive samples were successfully sequenced. The phylogenetic analysis revealed that isolates were classified as Cosmopolitan subtype (HTLV-1a), one Japanese (HTLV-1aJpn) and four Transcontinental (HTLV-1aTc) subgroups (Fig. 2), and the GenBank accession numbers for these sequences were: PP808584 (ID-235), PP808585 (ID-510), PP808586 (ID-424), PP808587 (ID-210), and PP808588 (ID-264). Four positive samples were amplified by mqPCR to determine HTLV DNA proviral load (PVL), and the results varied from 253 to 21,876 copies per 106 cells (Table 2).
Fig. 2.
Phylogenetic tree analysis. Phylogenetic tree of HTLV-1 subtypes from different groups. Phylogenetic tree constructed based on Maximum likelihood. Support for branching was determined by 1000 bootstrap replicates, and only values of 50% or higher were shown for Maximum likelihood. PP808584 (ID-235), PP808585 (ID-510), PP808586 (ID-424), PP808587 (ID-210), PP808588 (ID-264) clustered with different subgroups (Japanese and Transcontinental) and population groups.
Table 2.
Sociodemographic, risk behavior, and virological aspects of 5 individuals anti-HTLV-1 positive
| Characteristic | ID-210 | ID-235 | ID-264 | ID-424 | ID-510 |
|---|---|---|---|---|---|
| Sanitary district | Imbirussu | Anhanduizinho | Anhanduizinho | Bandeira | Imbirussu |
| Type of HTLV | HTLV-1 | HTLV-1 | HTLV-1 | HTLV-1 | HTLV-1 |
| Subtype | Cosmopolitan | Cosmopolitan | Cosmopolitan | Cosmopolitan | Cosmopolitan |
| Subgroup | Japanese | Transcontinental | Transcontinental | Transcontinental | Transcontinental |
| Presence of symptoms related to HTLV-1 infection | Asymptomatic | Asymptomatic | Symptomatic | Symptomatic | Asymptomatic |
| HTLV DNA Proviral load (copies/106 cells) | 2255 | 253 | 21,876 | 404 | Sample not provided |
| Age (years) | 67 | 65 | 42 | 77 | 29 |
| Biological sex | Male | Female | Female | Female | Female |
| Ethnicity | Japanese descendant | Black | White | Mixed race | White |
| History of STI | No | No | Yes | No | No |
| History of condom use (lifetime) | Irregular | Irregular | Irregular | Irregular | Irregular |
| Blood transfusion before 1993 | No | Yes | No | No | No |
| History of IDU | No | No | No | No | No |
| Family history of PLwHTLV | Do not know | Do not know | No | No | Do not know |
STI: sexually transmitted infection; IDU: Injecting Drug Use; PLwHTLV: People living with HTLV
All individuals who were anti-HTLV-1 positive were referred to an infectious disease specialist for clinical evaluation at the University Hospital, but only three participants agreed, while the other two did not attend the clinical evaluation due to a lack of interest, even in our effort to refer them to clinical follow-up. During the interview, ID-210 reported that he had been monitored for herpes zoster and did not have any risk behavior for HTLV, except for a history of irregular condom use. Upon clinical evaluation, a characteristic scar in the left cervical region and left hemithorax region was observed. No signs or symptoms of HAM were identified through physical examination (grade 2 patellar reflex, no clonus, and no muscle spasticity). ID-264 self-reported history of HPV infection, urinary incontinence, and muscle and joint pain. During the medical evaluation, no signs or symptoms of HAM were observed. However, tendonitis and bursitis in the left shoulder, mixed depression, and anxiety disorder with cognitive impairment were also present. ID-424 reported experiencing dry eyes and mouth, as well as urinary incontinence, muscle pain, numbness in her lower limbs, joint pain, and difficulty walking following a car accident in 2009, which resulted in her use of a wheelchair. Although no signs of uveitis were present in medical care, neurogenic bladder and bilateral spastic paraparesis were identified, according to Castro Costa [27]. After clinical evaluation, it was determined that the traffic accident in which the participant was involved was not the cause of her difficulty walking, but rather an HTLV-1-associated disease. The ID-235 reported a history of blood transfusion before 1993 and irregular condom use. Meanwhile, ID-510 informs have piercing and irregular condom use. Neither ID-235 nor ID-510 attended the medical evaluation and did not report any signs or symptoms related to HTLV. Among all HTLV-1-positive individuals, two had symptoms related to infection.
Discussion
Investigations into the prevalence of HTLV infection in the state of Mato Grosso do Sul were performed specifically in groups of the Japanese community, pregnant women, blood donors, the indigenous population, prisoners, men who have sex with men, and remnant quilombos, with rates varying from 0.1% to 10.0% [7, 13–15, 28–31]. Therefore, this is the first HTLV study to be carried out in the state of Mato Grosso do Sul in an urban area, covering the general population and, to the best of our knowledge, in Central Brazil. The high coverage of participation shows a strong commitment and interest from the community in contributing to the research, bolstering the validity and representativeness of the findings (Fig. 1). The sociodemographic profile of all study group corroborates with the scenario of the residents from Campo Grande, majority of habitants are mixed race, median age of 34 years old, and women [21]. Regarding the sample containing a higher proportion of women, this reflects the actual demographic composition of the city, as indicated by the Brazilian Institute of Geography and Statistics (IBGE) [21], which reports that there are more women than men in the population of Campo Grande. Furthermore, in addition to the significant increase in the number of women in the study, it is important to note the Brazilian cultural context in which women tend to assume a more proactive role in healthcare than men. Social and cultural norms attribute to women the responsibility of caring for the family, which often extends to their own health.
The prevalence of HTLV-1 infection was 0.82% (CI 95% 0.34–1.96). It can be stratified as low to medium [32] if the CI interval is considered. A study conducted in a metropolitan area in Belém found a prevalence of 1.40%, considering only HTLV-1 [33]. More recently (2022), another study conducted in the same geographic area found a prevalence of 0.38% (CI 0.07–0.69%) for HTLV-1/2 and 0.19% for HTLV-1 [34]. Accordingly, this indicates a decrease in the prevalence in Belém area, which may be attributed to the sample selection process and, also the alteration in the sensitivity of diagnostic techniques. The results of our study are consistent with those of the first investigation, which revealed an intermediate prevalence rate. Additionally, our findings indicate a higher prevalence of HTLV-1 in Belém compared to the current scenario.
Prevalence studies among blood donors have often led to extrapolation of the findings to the general population due to the lack of studies in this one. Studies varied in their rates, ranging from 0.17 to 4.8/1000 donations [31, 35–37], with an estimated global prevalence in the country of 0.4% [38]. However, owing to the risk-free behavioral selection process for candidate blood donors, this could potentially reduce the global prevalence of PLwHTLV in the general population.
All five HTLV-positive individuals were tested for type 1 of the virus, which is consistent due to South America being an endemic region for HTLV-1. It is expected that HTLV-2 would be found in restricted groups such as PWID (Person Who Inject Drugs) and indigenous communities [2]. However, even in indigenous populations of MS State, only the presence of HTLV-1 has been reported [30]. The study found Japanese (HTLV-1aJpn) and Transcontinental (HTLV-1aTc) subgroups belonging to the Cosmopolitan subtype. The Japanese subgroup (HTLV-1aJpn) has previously been identified on both national territory and in MS State [4, 7, 8], as well as within the transcontinental subgroup [15, 30, 39, 40].
HTLV DNA Proviral load (PVL) could be divided into three categories based on the percentage of HTLV-1 DNA copies per 102 PBMC: < 1% as low, 1–10% as medium, and > 10% as high [41]. Several hypotheses can be proposed regarding the PVL results obtained in this study, ranging from 253 to 21,876 copies per 106 cells (PBMC).
First, it should be noted that PVL levels cannot be directly compared between participants, as both symptomatic and asymptomatic individuals living with HTLV may have high or low PVL levels [42, 43]. Therefore, this assessment seems to be more useful as a prognostic factor for patient follow-up than for comparative purposes. Second, the PVL of ID-264, 21,876 (copies/ 106 cells) may be related to the cognitive impairment observed, as there is a correlation between elevated PVL and neuropsychological deviations [44]. Third, the PVL of ID-424, 404 (copies/106 cells) as a symptomatic carrier may be explained by an A125 mutation in tax-responsive elements, which has been reported in Latin American strains of the HTLV-1 Cosmopolitan Transcontinental subtype. This mutation is associated with lower PVL without affecting the progression of HTLV-1-related diseases [45]. These cases suggest that while there is a potential association between proviral load and clinical presentation, the relationship is complex and may involve other factors, such as genetic mutations.
It is worth mentioning here that, in the case of ID-424, it took more than 10 years to receive a correct diagnosis of HTLV-1, during which time the patient's symptomatology was misattributed to a traffic accident by medical professionals. It is crucial to emphasize the need for ongoing education and training of all healthcare professionals. The delay in obtaining an accurate diagnosis may have affected the patient’s current clinical condition. During the data collection phase, health promotion actions were conducted with the general population and healthcare professionals, and informative leaflets on HTLV were distributed, providing clarification about the infection, transmission routes, associated diseases, necessary examinations, and the importance of diagnosis. In addition to knowing her HTLV serological status and the cause of her leg symptoms, patient ID-424 was referred and has been followed up in multidisciplinary medical care and started physiotherapy.
The current study had certain limitations that must be acknowledged. Underreporting of risk behaviors may have occurred due to fear and distrust associated with discrimination and stigma as well as memory bias, which could have resulted in participants forgetting or misremembering relevant events. These factors could lead to an underestimation of the potential risk factors associated with HTLV infection. Additionally, over-representation of certain demographic groups was observed. These limitations are inherent to the design of this study.
This study presents a comprehensive view of the urban population in the capital city, as it employed a significant sample size that was representative of the population. Additionally, this study provides epidemiological information that could help bridge gaps in the distribution of HTLV infection in the general population, as the results indicate the presence of HTLV-1 infection in Campo Grande. The data obtained can be used to develop effective public health programs aimed at reducing viral transmission. Overall, these findings underscore the importance of the epidemiological scenario of HTLV infection in the general population residing in urban areas.
We propose expanding investigations to additional regions, particularly those with limited healthcare infrastructure or restricted access to medical services, where a lack of awareness regarding infection may be of concern. Furthermore, it emphasizes the significance of continuous monitoring of HTLV-1 epidemiology in diverse settings to enhance control and prevention strategies.
Acknowledgements
We are grateful to the SESAU institution that helped in some way in this work. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES)- Finance Code 001 and the National Council for Scientific and Technological Development - CNPq (#442522/2019-3, #302935/2021-5, and #314908/2021-8). This work was carried out with the support of the Federal University of Mato Grosso do Sul – UFMS/MEC – Brazil.
Author contributions
Conceptualization: C.A, L.M.B, I.M.V.C.V, A.C.R.V, A.R.C.M.C; methodology: C.A, L.M.B, W.M.C, A.S.P.S, R.I.T, M.S.J, F.B.F, S.N.O.U; analyzed the data: C.A, A.R.C.M.C; contributed reagents/materials/analysis tools: A.R.C.M.C, A.C.R.V; writing—original draft preparation, and editing: C.A, L.M.B, A.R.C.M.C; writing—review: C.A, L.M.B, W.M.C, A.S.P.S, R.I.T, M.S.J, F.B.F, S.N.O.U, I.M.V.C.V, A.C.R.V, A.R.C.M.C; supervision: A.R.C.M.C. All authors have read and agreed to this manuscript version.
Funding
This research was supported in part by funding from the National Council for Scientific and Technological Development – CNPq #442522/2019-3.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethical Committee on Human Research of the Universidade Federal de Mato Grosso do Sul (CEP/UFMS) by protocol number CAAE: 63756922.00000.0021. All subjects or their legal guardians, in the case of individuals under age 18, gave their written informed consent to participate in the study at the time of sampling. All research was performed following relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mahieux R, Gessain A. The human HTLV-3 and HTLV-4 retroviruses: new members of the HTLV family. Pathol Biol. 2009;57(2):161–6. 10.1016/j.patbio.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol. 2012;15(3):388. 10.3389/fmicb.2012.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afonso PV, Cassar O, Gessain A. Molecular epidemiology, genetic variability and evolution of HTLV-1 with special emphasis on African genotypes. Retrovirology. 2019;16(1):39. 10.1186/s12977-019-0504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vallinoto AC, Muto NA, Pontes GS, Machado LF, Azevedo VN, dos Santos SE, et al. Serological and molecular evidence of HTLV-I infection among Japanese immigrants living in the Amazon region of Brazil. Jpn J Infect Dis. 2004;57(4):156–9. [PubMed] [Google Scholar]
- 5.Souza LA, Lopes IG, Maia EL, Azevedo VN, Machado LF, Ishak MO, et al. Molecular characterization of HTLV-I among patients with tropical spastic paraparesis/HTLV-I associated myelopathy in Belém. Pará Rev Soc Bras Med Trop. 2006;39(5):504–6. 10.1590/s0037-86822006000500017. [DOI] [PubMed] [Google Scholar]
- 6.Galvão-Castro B, Alcântara LCJ, Grassi MFR, Mota-Miranda ACA, Queiroz ATL, Rego FFA, et al. Epidemiologia e origem do HTLV-1 em Salvador Estado da Bahia: a cidade com a mais elevada prevalência desta infecção no Brasil. Gaz Med Bahia. 2009;79:3–10. [Google Scholar]
- 7.Bandeira LM, Uehara SN, Asato MA, Aguena GS, Maedo CM, Benites NH, et al. High prevalence of HTLV-1 infection among Japanese immigrants in non-endemic area of Brazil. PLoS Negl Trop Dis. 2015;9(4): e0003691. 10.1371/journal.pntd.0003691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bandeira LM, Puga MAM, Weis-Torres SMS, Rezende GR, Domingos JA, Tanaka TSO, et al. Human T-cell leukemia virus type 1 infection among Japanese immigrants and their descendants living in Southeast Brazil: a call for preventive and control responses. PLoS Negl Trop Dis. 2021;15(2): e0009066. 10.1371/journal.pntd.0009066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Novoa P, Penalva de Oliveira AC, Posada Vergara MP, da Silva Duarte AJ, Casseb J. Molecular characterization of human T-cell lymphotropic virus type 2 (HTLV-II) from people living in urban areas of Sao Paulo city: evidence of multiple subtypes circulation. J Med Virol. 2007;79(2):182–7. 10.1002/jmv.20775. [DOI] [PubMed] [Google Scholar]
- 10.Ferreira QR, Novaes AF, Santana CS, Umeda AS, de Souza Nascimento JO, de Freitas Santos JPM, et al. Neurological aspects of HTLV-1 infection: symptoms in apparently asymptomatic carriers. J Neurovirol. 2024. 10.1007/s13365-024-01197-9. [DOI] [PubMed] [Google Scholar]
- 11.Assone T, Casseb J. HTLV-1 in Brazil: epidemiological scenario in the highest endemic country in the world. AIDS Rev. 2023;25(4):181–3. 10.24875/AIDSRev.M23000067. [DOI] [PubMed] [Google Scholar]
- 12.Vallinoto A, Pontes G, Muto N, Lopes I, Machado L, Azevedo V. Identification of human T-cell lymphotropic virus infection in a semi-isolated Afro-Brazilian quilombo located in the Marajó Island (Pará, Brazil). Mem Inst Oswaldo Cruz. 2006;101(1):103–5. 10.1590/s0074-02762006000100020. [DOI] [PubMed] [Google Scholar]
- 13.Dal Fabbro MMFJ, da Cunha RV, Bóia MN, Portela P, Botelho CA, de Freitas GMB, et al. Infecção pelo HTLV 1/2: atuação no pré-natal como estratégia de controle da doença no Estado de Mato Grosso do Sul. Rev Soc Bras Med Trop. 2008;41:148–51. 10.1590/S0037-86822008000200003. [DOI] [PubMed] [Google Scholar]
- 14.Castro LS, Rezende GR, Fernandes FRP, Bandeira LM, Puga MAM, Tanaka TSO, et al. Human T cell lymphotropic virus type 1 infection among men who have sex with men in Central Brazil. Braz J Infect Dis. 2018;22(6):472–6. 10.1016/j.bjid.2018.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bandeira LM, Moreira Puga MA, Croda J, Pompílio MA, Amianti C, RochadeRezende G, et al. Human T-lymphotropic virus-1/2 infection in central Brazil prisons: a multicenter study. Front Microbiol. 2022;21(12): 740245. 10.3389/fmicb.2021.740245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abreu IN, Lopes FT, Lima CNC, Barbosa ADN, de Oliveira LR, Fujishima MA, et al. HTLV-1 and HTLV-2 infection among Warao indigenous refugees in the Brazilian Amazon: challenges for public health in times of increasing migration. Front Public Health. 2022;11(10): 833169. 10.3389/fpubh.2022.833169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brito WRDS, Cardoso-Costa GL, Roland Junior LM, Pereira KAS, Lopes FT, Dos Santos BC, et al. Prevalence and risk factors for HTLV-1/2 infection in Quilombo remnant communities living in the Brazilian Amazon. Front Public Health. 2022;30(10): 871865. 10.3389/fpubh.2022.871865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abreu IN, Lima CNC, Sacuena ERP, Lopes FT, da Silva Torres MK, Santos BCD, et al. HTLV-1/2 in indigenous peoples of the Brazilian Amazon: seroprevalence, molecular characterization and sociobehavioral factors related to risk of infection. Viruses. 2023;15(1):22. 10.3390/v15010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.BRASIL. Ministério da Saúde do Brasil. Portaria 1376 de 19/11/1993 - Ministério da Saúde (Ministério da Saúde, 1993).
- 20.Miranda C, Utsch-Gonçalves D, Piassi FCC, Loureiro P, Gomes I, Ribeiro MA, et al. Prevalence and risk factors for human T-Cell Lymphotropic Virus (HTLV) in blood donors in Brazil-A 10-year study (2007–2016). Front Med. 2022;9(9): 844265. 10.3389/fmed.2022.844265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.IBGE – Instituto Brasileiro De Geografia E Estatística. Censo Brasileiro de 2022. Rio de Janeiro: IBGE, 2022. https://censo2022.ibge.gov.br/panorama/mapas.html?localidade=&recorte=N6
- 22.Gonçalves MG, Fukasawa LO, Campos KR, Higa FT, Caterino-de-Araujo A. Development and validation of multiplex quantitative real-time PCR assays for simultaneous detection and differentiation of HTLV-1 and HTLV-2, using different PCR platforms and reagent brands. Front Microbiol. 2022;15(13): 831594. 10.3389/fmicb.2022.831594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Oliveira EH, Oliveira-Filho AB, Souza LA, da Silva LV, Ishak MO, Ishak R, et al. Human T-cell lymphotropic virus in patients infected with HIV-1: molecular epidemiology and risk factors for transmission in Piaui, Northeastern Brazil. Curr HIV Res. 2012;10(8):700–7. 10.2174/1570162x11209080700. [DOI] [PubMed] [Google Scholar]
- 24.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30(14):3059–66. 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller MA, Pfeiffer W, Schwartz T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the gateway computing environments workshop: 1–8. 2010. 10.1109/GCE.2010.5676129.
- 26.Letunic I, Bork P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49(W1):W293–6. 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Castro-Costa CM, Araújo AQ, Barreto MM, Takayanagui OM, Sohler MP, da Silva EL, et al. Proposal for diagnostic criteria of tropical spastic paraparesis/HTLV-I-associated myelopathy (TSP/HAM). AIDS Res Hum Retroviruses. 2006;22(10):931–5. 10.1089/aid.2006.22.931. [DOI] [PubMed] [Google Scholar]
- 28.Kitagawa T, Fujishita M, Taguchi H, Miyoshi I, Tadokoro H. Antibodies to HTLV-I in Japanese immigrants in Brazil. JAMA. 1986;256(17):2342. [PubMed] [Google Scholar]
- 29.Nascimento LB, Carneiro MA, Teles SA, Lopes CL, Reis NR, Silva AM, et al. Prevalência da infecção pelo HTLV-1, em remanescentes de quilombos no Brasil Central [Prevalence of infection due to HTLV-1 in remnant quilombos in Central Brazil]. Rev Soc Bras Med Trop. 2009;42(6):657–60. 10.1590/s0037-86822009000600009. (Portuguese). [DOI] [PubMed] [Google Scholar]
- 30.Amianti C, Bandeira LM, Cesar GA, Weis-Torres S, Tanaka TSO, Machado IR, et al. HTLV infection in Brazil’s second-largest indigenous reserve. Sci Rep. 2022;12(1):16701. 10.1038/s41598-022-21086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amianti C, Bandeira LM, Romeiro JS, Nakao BRO, Vavas MTM, Domingos JA, et al. HTLV infection in blood donors from Mato Grosso do Sul state: a closer look at HTLV screening in Brazilian blood banks. Sci Rep. 2023;13(1):14524. 10.1038/s41598-023-41875-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonçalves DU, Proietti FA, Ribas JG, Araújo MG, Pinheiro SR, Guedes AC, et al. Epidemiology, treatment, and prevention of human T-cell leukemia virus type 1-associated diseases. Clin Microbiol Rev. 2010;23(3):577–89. 10.1128/CMR.00063-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silva IC, Pinheiro BT, Nobre AFS, Coelho JL, Pereira CCC, Ferreira LSC, et al. Moderate endemicity of the human T-lymphotropic virus infection in the metropolitan region of Belém, Pará, Brazil. Rev Bras Epidemiol. 2018;11(21): e180018. 10.1590/1980-549720180018. [DOI] [PubMed] [Google Scholar]
- 34.Lopes FT, de Sousa RS, Carvalho Gomes JL, Vallinoto MC, de Lima ACR, Lima SS, et al. The relevance of a diagnostic and counseling service for people living with HTLV-1/2 in a metropolis of the Brazilian Amazon. Front Public Health. 2022;28(10): 864861. 10.3389/fpubh.2022.864861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mota ACA, Nunes CLX, Melo A, Romeo M, Boa-Sorte NCA, Dourado I, et al. A case-control study of HTLV-infection among blood donors in Salvador, Bahia, Brazil - associated risk factors and trend towards declining prevalence. Rev Bras Hematol Hemoter. 2006;28(2):120–6. 10.1590/S1516-84842006000200011. [Google Scholar]
- 36.Nunes D, Boa-Sorte N, Grassi MFR, Taylor GP, Teixeira MG, Barreto ML, et al. HTLV-1 is predominantly sexually transmitted in Salvador, the city with the highest HTLV-1 prevalence in Brazil. PLoS ONE. 2017;12(2): e0171303. 10.1371/journal.pone.0171303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pereira FM, de Almeida MCC, Santos FLN, Carreiro RP, Regis-Silva CG, Galvão-Castro B, et al. Evidence of new endemic clusters of human T-cell leukemia virus (HTLV) infection in Bahia, Brazil. Front Microbiol. 2019;10:1002. 10.3389/fmicb.2019.01002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galvão-Castro B, Loures L, Rodriques LG, Sereno A, Ferreira Júnior OC, Franco LG, et al. Distribution of human T-lymphotropic virus type I among blood donors: a nationwide Brazilian study. Transfusion. 1997;37(2):242–3. 10.1046/j.1537-2995.1997.37297203532.x. [DOI] [PubMed] [Google Scholar]
- 39.Magri MC, Brigido LF, Rodrigues R, Morimoto HK, Ferreira JL, Caterino-de-Araujo A. Phylogenetic and similarity analysis of HTLV-1 isolates from HIV-coinfected patients from the south and southeast regions of Brazil. AIDS Res Hum Retroviruses. 2012;28(1):110–4. 10.1089/AID.2011.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mota AC, Van Dooren S, Fernandes FM, Pereira SA, Queiroz AT, Gallazzi VO, et al. The close relationship between South African and Latin American HTLV type 1 strains corroborated in a molecular epidemiological study of the HTLV type 1 isolates from a blood donor cohort. AIDS Res Hum Retroviruses. 2007;23(4):503–7. 10.1089/aid.2006.0203. [DOI] [PubMed] [Google Scholar]
- 41.Demontis MA, Hilburn S, Taylor GP. Human T cell lymphotropic virus type 1 viral load variability and long-term trends in asymptomatic carriers and in patients with human T cell lymphotropic virus type 1-related diseases. AIDS Res Hum Retroviruses. 2013;29(2):359–64. 10.1089/AID.2012.0132. [DOI] [PubMed] [Google Scholar]
- 42.Montanheiro PA, Oliveira AC, Posada-Vergara MP, Milagres AC, Tauil C, Marchiori PE, et al. Human T-cell lymphotropic virus type I (HTLV-I) proviral DNA viral load among asymptomatic patients and patients with HTLV-I-associated myelopathy/tropical spastic paraparesis. Braz J Med Biol Res. 2005 Nov;38(11):1643–7. 10.1590/s0100-879x2005001100011. Epub 2005 Oct 26. Erratum in: Braz J Med Biol Res. 2005 Dec;38(12):1890. Montanheito, PA [corrected to Montanheiro, PA]. [DOI] [PubMed]
- 43.Pineda MV, Bouzas MB, Remesar M, Fridman A, Remondegui C, Mammana L, et al. Relevance of HTLV-1 proviral load in asymptomatic and symptomatic patients living in endemic and non-endemic areas of Argentina. PLoS ONE. 2019;14(11): e0225596. 10.1371/journal.pone.0225596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kalil RS, Vasconcellos I, Rosadas C, Cony A, Lima DP, Gonçalves CCA, et al. Association between high proviral load, cognitive impairment, and white matter brain lesions in HTLV-1-infected individuals. J Neurovirol. 2021;27(6):810–9. 10.1007/s13365-021-00944-6. [DOI] [PubMed] [Google Scholar]
- 45.Gomes YCP, Silva MTT, Leite ACCB, Lima MASD, Araújo AQC, Silva Filho IL, et al. Polymorphisms in HTLV-1 Tax-responsive elements in HTLV-1-associated myelopathy/tropical spastic paraparesis patients are associated with reduced proviral load but not with disease progression. J Gen Virol. 2021. 10.1099/jgv.0.001649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.