Abstract
Background
Chronic kidney failure (CKF) is often treated with dialysis, which is invasive and costly and carries major medical risks. The existing studies of patients with CKF requiring dialysis that are based on claims data from German statutory health insurance (SHI) carriers employ varying definitions of this entity, with unclear consequences for the resulting statistical estimates.
Methods
We carried out a cohort study on four random samples, each consisting of 62 200 persons aged 70 or above, from among the insurees of the SHI AOK Nordost, with one sample for each of the years 2012, 2014, 2016, and 2018. The prevalence, incidence, mortality, and direct health care costs of CKF requiring dialysis were estimated and compared on the basis of four different definitions from literature and a new definition developed by the authors in reference to billing data.
Results
The different definitions led to variation in 12-month prevalences (range: 0.33–0.61%) and 6-month incidences (0.058–0.100%). The percentage of patients with prior acute kidney injury (AKI) ranged from 27.6% to 61.8%. Among incident patients, three-month survival ranged from 70.2% to 88.1%, and six-month survival from 60.5% to 81.3%. In CKF patients without prior AKI, the survival curves differed less across definitions (80.2–91.8% at three months, 70.7–84.4% at six months). The monthly health care costs ranged from €6010 to €9606, with marked variability across definitions in the costs of inpatient and outpatient care.
Conclusion
The lack of a standardized definition of CKF requiring dialysis in German SHI claims data leads to variability in the estimated case numbers, mortality, and health care costs. These differences are most probably in part due to the variable inclusion of inpatients who received short-term dialysis after AKI.
Chronic kidney failure (CKF) requiring dialysis places a significant burden not only on patients but also on the health care system. Patients with CKF suffer considerable loss of quality of life and functionality (1, 2), complex multimorbid health impairments (3), and are at high risk for cardiovascular events (4), treatment complications, and (early) mortality (3, 5–8). Moreover, dialysis treatment itself is highly invasive, burdensome, and time-consuming. In the case of chronic hemodialysis (HD), the commonest method of renal replacement therapy in CKF (3), patients are treated 3–4 × per week for 4–5 h in dialysis centers and practices. From a care and health system perspective, CKF requiring dialysis takes up manifold medical, nursing, and monetary resources, particularly in old age (9). The direct health costs for dialysis patients in Germany are estimated to be more than 3 billion Euros per year (10).
In view of demographic aging, it is expected that the prevalence of patients with CKF requiring dialysis will continue to rise (11). Despite the relevance of CKF on both an individual and a structural level, its high risk profile, and the high costs involved, there is no nationwide dialysis registry in Germany. Although annual quality reports on dialysis are drawn up on behalf of the German Joint Federal Committee (Gemeinsamer Bundesausschuss, G-BA) (12), these have a number of limitations: The reports are based solely on data for outpatients with CKF requiring dialysis who have statutory health insurance and have survived on dialysis for at least 3 months. Since Germany does not have a central death registry, mortality data are incomplete. In view of the high premature mortality rate within the first 3 months of starting dialysis as well as different treatment and insurance modalities (day-care patients, privately insured patients), this suggests a relevant underestimation of the group of patients with CKF requiring dialysis in older age.
Routinely recorded health care data are becoming ever more important for clinical, epidemiological, and health care systems research (e1–e3) and represent a valuable alternative data basis for patients with CKF requiring dialysis. Likewise in Germany, claims data from statutory health insurers (SHI) were employed to analyze mortality, hospitalizations, and health care costs for CKF requiring dialysis (3, 10, 13–19). However, what is striking here is that the definitions used vary between the studies. At present, there is no standardized definition in Germany to identify patients with CKF requiring dialysis in claims data, also to differentiate those requiring short-term reversible dialysis treatment following, for example, acute kidney injury (AKI). However, a correct distinction between chronic dialysis treatment for CKF and acute short-term dialysis treatment following AKI is extremely important, since the two treatment modalities differ qualitatively and quantitatively and have different treatment goals.
The aim of this study was to analyze the variation between different definitions of CKF requiring dialysis in SHI claims data in the literature, as well as a new definition, in order to investigate the identifiability of patients with CKF requiring dialysis and their differentiation from those receiving dialysis treatment following AKI. To this end, we compared the definitions based on prevalence, incidence, early mortality, and direct health care costs following the initiation of dialysis in the billing data for patients aged ≥ 70 years insured by the SHI AOK Nordost.
Methods
Data basis
This secondary data-based cohort study was conducted on the basis of billing data from the AOK Nordost that were gathered as part of the GUIDAGE-CKD Innovation Fund project. Data from separate random samples from each of 4 years (2012, 2014, 2016, and 2018, with data from 1 January to 31 December of each year) were analyzed, each with n = 62 200 insurees aged at least 70 years without previous kidney transplant (see eSupplement for details on case number estimation and sampling).
Definition of CKF requiring dialysis
There is no uniform definition of CKF requiring dialysis based on diagnosis or treatment codes in SHI claims data. To identify insurees with CKF requiring dialysis, we applied criteria using diagnoses according to ICD-10-German Modification (GM) (e4) and fee schedule items (Gebührenordnungspositionen, GOP) in the German Uniform Value Scale (Einheitlicher Bewertungsmaßstab) for outpatient treatment as well as operation and procedure codes (Operationen- und Prozedurenschlüssel, OPS) for inpatient treatment. Only “confirmed” outpatient and “main” and “secondary” inpatient diagnoses were used. For the literature-based definitions, a literature search was conducted for relevant keywords and to identify the described criteria. A number of studies were excluded because that the criteria for the operationalization of CKF requiring dialysis were identical to other studies (15), were not described (16–18), or only a GOP code was given as a criterion (19). Table 1 summarizes the criteria used for the literature-based definitions (a)–(d) of CKF requiring dialysis (3, 10, 13, 14). The selection of the criteria for a new definition (e), based on a billing rationale, that are required for reimbursement by the SHI was carried out following research in the relevant treatment catalogs, consultation with office-based and inpatient nephrologists with coding and billing expertise, and in coordination with the AOK Nordost. In contrast to the literature-based definitions, we used only OPS and ICD-10-GM codes billed for day-care treatment in definition (e), since acute short-term dialysis treatment, such as after AKI or sepsis, is predominantly performed in the hospital and patients are transferred to outpatient or day-care treatment if they become chronic.
Table 1. Case numbers for patients with CKF requiring dialysis together with sociodemographic and clinical characteristics according to different definitions in claims data.
| Definition | Criteria*1 | 12-Month prevalence | 6-Month incidence *2 |
Women, n (%) |
Age, M (SD) |
Prior AKI*4, n (%) |
|||||
|
Cases, n |
Standardized*3, % [95% CI] |
Cases, n |
Standardized*3, % [95% CI] |
||||||||
| a) | Gandjour et al., 2020 (10) |
1. | ICD-10-GM N18.5 “and” | 1466 | 0.58 [0.55; 0.61] | 190 | 0.081 [0.071; 0.093] | 67 (35.3) | 80.8 (6.2) | 86 (45.3) | |
| 2. | ICD-10-GM Z49*, Z99.2 | ||||||||||
| b) | Kolbrink et al., 2023 (3) |
1. | ICD-10-GM N18.5 “and” | 1447 | 0.57 [0.54; 0.60] | 178 | 0.073 [0.063; 0.084] | 60 (33.7) | 81.2 (6.5) | 110 (61.8) | |
| 2. | Either | ||||||||||
| 2.1. | OPS 8-854.2–5, 8-855, 8-857 “or” | ||||||||||
| 2.2. | GOP 13610, 13611 | ||||||||||
| c) | Lonnemann et al., 2017 (13) |
1. | ICD-10-GM N18.5 “and” | 1547 | 0.61 [0.58; 0.64] | 213 | 0.089 [0.078; 0.101] | 79 (37.1) | 81.2 (6.2) | 104 (48.8) | |
| 2. | Either | ||||||||||
| 2.1. | ICD-10-GM Z49*, Z99.2 “or” | ||||||||||
| 2.2. | GOP 40800–8, 40812–3, 40820–3 “or” | ||||||||||
| 2.3. | OPS 8-854 | ||||||||||
| d) | Schellartz et al., 2021 (14) |
1. | ICD-10-GM N18.5 “and” | 1262 | 0.50 [0.47; 0.53] | 241 | 0.100 [0.088; 0.113] | 81 (33.6) | 81.0 (6.5) | 120 (49.8) | |
| 2. | Either | ||||||||||
| 2.1. | GOP 13611, 40823–7, 40837–8 “or” | ||||||||||
| 2.2. | OPS 8-853, 8-854, 8-855, 8-857 | ||||||||||
| e) | Billing rationale | 1. | Outpatient dialysis: | 832 | 0.33 [0.31; 0.35] | 134 | 0.058 [0.050; 0.069] | 40 (29.9) | 80.3 (6.0) | 37 (27.6) | |
| 1.1. | ICD-10-GM Z49.1–2 “and” | ||||||||||
| 1.2. | ICD-10-GM Z99.2 “and“ | ||||||||||
| 1.3. | GOP 13610, 13611 | ||||||||||
| “or” | |||||||||||
| 2. | Day-care dialysis: | ||||||||||
| 2.1. | ICD-10-GM Z49.1–2 “and” | ||||||||||
| 2.2. | OPS 8-853, 8-854, 8-855, 8-857 | ||||||||||
*1 Only individuals fulfilling all criteria with the Boolean operator “and” were included in the analyses. The first date on which all criteria were fulfilled was defined as the index date. Commas and hyphens represent an “or” condition. Unless otherwise stated, both outpatient and inpatient codes were used.
*2 Incidence was determined as the proportion of all prevalent cases in the 2nd or 3rd quarter of an individual year out of all persons at risk (neither prevalent nor deceased in the respective 1st quarter).
*3 Prevalence and incidence are standardized for individuals aged ≥ 70 years. We used year-, age-, and gender-specific DESTASTIS weights for individuals aged ≥ 70 years from Berlin, Brandenburg, and Mecklenburg-Western Pomerania.
*4 Number of cases with acute kidney injury (AKI; ICD-10-GM N17*) within 3 months before or on the index date.
CKF, chronic kidney failure; GOP, fee schedule items (Gebührenordnungspositionen) in the German Uniform Value Scale (Einheitlicher Bewertungsmaßstab, EBM); ICD-10-GM, International statistical classification of diseases and related health problems, 10th revision, German Modification. Only “confirmed” outpatient and “main” and “secondary” (partial) inpatient diagnoses were used; M, mean; 95% CI, 95% confidence interval; OPS, German “Operations and Procedures Code”; a list and description of all codes used can be found in the eSupplement.
The first exact date on which all criteria of a definition were fulfilled was defined as the index date of dialysis. To analyze the endpoints mortality and health care costs, incident cases in the 2nd and 3rd quarters of a year were identified. To this end, all cases that were already prevalent or deceased in the 1st quarter of a year or only became incident in the 4th quarter were excluded. Follow-up was carried out for up to 6 months following the index date, with censoring on the date of death or on December 31 of the respective year.
Endpoints and statistical analyses
The different definitions (a)–(e) were compared with regard to 12-month prevalence, 6-month incidence, age, and gender. Prevalence and incidence were standardized by year-, age-, and gender-specific weights from the German Federal Statistical Office (DESTATIS) for individuals aged 70 years and over in the German federal states falling under the insurance area of the AOK Nordost (Berlin, Brandenburg, Mecklenburg-Western Pomerania). Mortality within 6 months following the index date was calculated using the Kaplan–Meier method. Furthermore, the proportion of cases diagnosed with AKI (ICD-10-GM N17*) in the period from 3 months before up to the index date of dialysis was determined.
For the analysis of direct health care costs from an SHI perspective, all outpatient, inpatient, and drug costs within 6 months after the index date were analyzed overall and separately by care sector and adjusted to the cost level in 2018 according to purchasing power parity. The rates per person-month were calculated by dividing total costs by total person-time in months. Bootstrapping with 1000 replications was used to determine 95% confidence intervals [CI] for health care costs (20). Mortality analyses were stratified by the presence of prior AKI, age, sex, and the respective year.
Results
The varying definitions used led to significantly different case numbers, clinical characteristics, mortality data, and health care costs. Overall, the case numbers for prevalent patients varied between 832 and 1547 across all years, and for incident patients between 134 and 241 (Table 1). If the definition according to billing rationale (e) was used, both the standardized 12-month prevalence and the standardized 6-month incidence were at their lowest (prevalence: 0.33% [95% confidence interval: 0.31; 0.35]; incidence: 0.058% [0.050; 0.069]). In contrast, definition (c) resulted in the highest prevalence at 0.61% [0.58; 0.64] and definition (d) in the highest incidence at 0.100 % [0.088; 0.113]. The percentage of female patients with CKF requiring dialysis varied between 29.9% in definition (e) and 37.1% in definition (c), whereas the average age barely differed between 80.3 and 81.2 years.
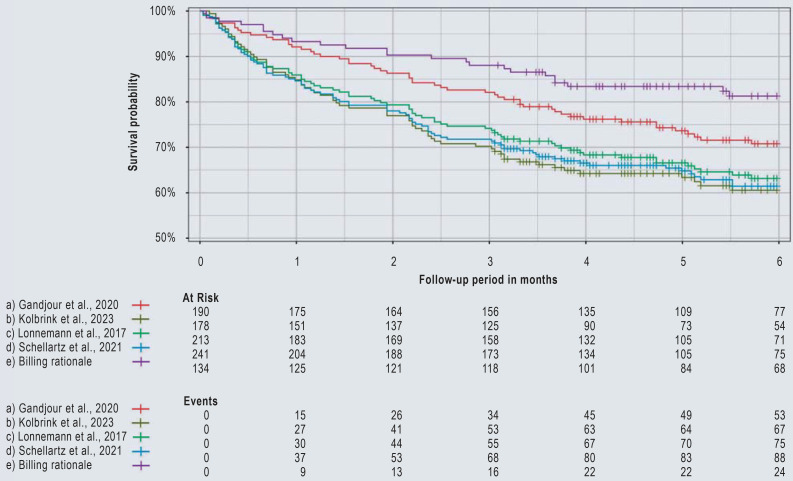
The percentage of insurees diagnosed with AKI within the 3 months before or on the index date of dialysis was lowest when using definition (e) at 27.6%, significantly higher when using definitions (a), (c), and (d) at 45.3–49.8%, and highest with definition (b) at 61.8%. Survival probability was highest in definition (e) (88.1% and 81.3% at 3 and 6 months, respectively) and lowest in definition (b) (70.2% and 60.5% at 3 and 6 months, respectively; Table 2, Figure).
Table 2. Survival probability and direct health care costs for patients with incident CKF requiring dialysis according to different definitions.
| Definition | Incident cases, n |
Survival probability*1, % [95% CI] |
Direct health care costs*2 (in €) within 6 months following incident CKF requiring dialysis, rate per person-month [95% CI]*3 | ||||
| At 3 months | At 6 months | Outpatient | Inpatient | Drugs | Total | ||
| a) Gandjour et al., 2020 (10) | 190 | 82.1 [76.8; 87.7] | 70.8 [64.4; 77.8] | 1864 [1653; 2053] | 4067 [3127; 5242] | 661 [520; 825] | 6797 [5824; 8010] |
| b) Kolbrink et al., 2023 (3) | 178 | 70.2 [63.8; 77.3] | 60.5 [53.4; 68.6] | 1906 [1680; 2118] | 6556 [4969; 8509] | 606 [466; 767] | 9606 [8055; 11527] |
| c) Lonnemann et al., 2017 (13) | 213 | 74.2 [68.5; 80.3] | 63.1 [56.7; 70.3] | 1554 [1358; 1760] | 5503 [4333; 6908] | 564 [438; 713] | 8020 [6820; 9377] |
| d) Schellartz et al., 2021 (14) | 241 | 71.8 [66.3; 77.7] | 61.4 [55.3; 68.3] | 2057 [1847; 2246] | 6227 [5019; 7626] | 600 [494; 737] | 9445 [8267; 10,808] |
| e) Billing rationale | 134 | 88.1 [82.7; 93.7] | 81.3 [74.7; 88.4] | 2930 [2762; 3121] | 2061 [1582; 2611] | 807 [639; 1.012] | 6010 [5471; 6632] |
*1 The survival probability was calculated using the Kaplan–Meier method taking censoring into account (31st December or death within an individual year).
*2 The direct health care costs from an SHI perspective was determined as all outpatient and inpatient case costs as well as drug costs within 6 months following incident dialysis (index date) with censoring on death or on 31st December of the respective year. All drugs costs with a dispensing date and case costs with a final billing date within the follow-up period were included. Costs were adjusted to the cost level in 2018 according to purchasing power parity (OECD).
*3 The rate per person-month was calculated as the sum of costs divided by the sum of person-time in months in the follow-up period. Bootstrapping with 1000 replications was used to determine 95% confidence intervals.
CKF, chronic renal failure; 95% CI, 95% confidence interval.
Figure.
Kaplan–Meier survival curves within 6 months after incident chronic kidney failure (CKF) requiring dialysis for different definitions contained in claims data. The number of events is cumulative.
The total direct health care costs per person-month within 6 months following the dialysis index date varied between the different definitions from €6022 [5482; 6646] in definition (e) to €9635 [8081; 11,556] in definition (b) (Table 2). The differences in individual care sectors were most evident in outpatient and inpatient costs: Outpatient costs were highest with definition (e) at €2935 [2766; 3126], while inpatient costs were highest with definition (b) at €6578 [4991; 8532]. Drug costs varied less markedly, ranging from €566 to €809.
Following stratification between patients with and those without a documented diagnosis of AKI before the start of dialysis, the survival probabilities were significantly higher in patients without prior AKI and varied less between the definitions (80.2–91.8% at 3 months, 70.7–84.4% at 6 months). In patients with prior AKI, the probability of survival at 6 months was by far the highest if definition (e) was used (75.4 % [62.6; 90.8]), whereas it was similar if definitions (a)–(d) were used (47.0–53.6%) (eFigure 1). The differences between the definitions in terms of survival probability in the main analysis were similar in the subgroup analyses stratified by age, sex, and year (eFigures 2–4).
Discussion
Our analysis shows that the use of different definitions of CKF requiring dialysis in SHI claims data leads to variability in the estimated prevalence, incidence, mortality, and health care costs. The definition of CKF requiring dialysis, based on an SHI billing rationale that takes into account outpatient and only day-care dialysis treatments led to lower case numbers and significantly higher survival probabilities following the initiation of dialysis. These divergences appear to be due in part to the presence of prior AKI and the inclusion of inpatients and intensive care patients receiving dialysis treatment following acute events alongside CKF patients requiring dialysis. The proportion of cases with prior AKI was around half to two-thirds when using the literature-based definitions compared to one-third when using the billing rationale-based definition. This is borne out by significantly higher inpatient health care costs for the literature-based definitions, although there was also significant variation between these, while outpatient care costs were highest for the billing rationale-based definition. The inclusion of patients treated in an inpatient setting, who likely have complex multimorbidity and receive short-term dialysis treatments, can result in an overestimation of case numbers as well as bias in the endpoints under consideration.
The estimates for early mortality following initiation of dialysis differ in international studies (8). Due to methodological differences, it is sometimes difficult to compare these estimates, not least since registry data often systematically exclude patients who died early on within the first 90 days after starting dialysis (21). Early mortality within 6 months after starting dialysis was estimated to be 19% based on French registry data (22), which is consistent with the results from definition (e). In order to precisely determine the mortality risks due to dialysis, further analyses based on SHI claims data or randomized controlled trials are required (23).
Although dialysis treatment for CKF is a highly invasive and costly treatment method, Germany does not have a national dialysis registry. As a result, there are no precise figures on prevalence or even on mean survival times of dialysis patients. Therefore, risk stratifications or predictions about adverse outcomes, as well as statements on the specific application of dialysis procedures in routine care, are currently not possible, thereby hampering tailored advice and decision-making for patients. The quality assurance data from which the GBA reports feed are subject to structural limitations in that they only collect data on people with statutory health insurance (meaning that around 13% of insurees go completely unrecorded [e5]); include no information on mortality; systematically underestimate prevalence due to the 3-month criterion; and do not yet show longitudinal trends. In view of this, SHI claims data currently represent the most valid data available in Germany for the analysis of mortality and other hard endpoints (for example, cardiovascular events) in CKF requiring dialysis.
However, there are challenges associated with the scientific use and correct interpretation of SHI claims data: For a multitude of clinical pictures and treatments, there is a lack of standardized procedures and uniform definitions, meaning that results can vary depending on the criteria selected. Moreover, there are only a handful of studies that validate claims data using other data sources. For clearly identifiable diseases such as hypertension and breast cancer, as well as for rarer diseases such as Campylobacter enteritis, claims data show good usability (24–26), whereas the ability to differentiate between type-1 and type-2 diabetes as well as specific stages of chronic kidney disease and the identification of emergency department treatments must be deemed limited (27–30). Likewise in the case of chronic obstructive pulmonary disease, the use of claims data alone is not optimal; here, a combination of claims and self-reported data from patients appears to be more expedient (31). Furthermore, there are relevant differences in the structure of insurees and clinical characteristics within the large number of health insurance carriers in Germany (32). To improve the scientific usability of SHI claims data, efforts should be made towards the linkage of claims data with other data sources, and comparative studies should be further promoted (33, 34).
For CKF requiring dialysis, there is—over and above the general limitations of claims data—the difficulty that there is no clear and valid coding based on diagnoses or treatment codes and, thus, no way of differentiating it from dialysis treatments for other indications. Also, in the interests of good scientific practice and reproducibility of study results (35, 36), it should be mandatory to have a precise definition and provide the respective criteria (codes used, time period criteria) when using SHI claims data in scientific articles (37).
When using claims data on CKF requiring dialysis, limitations such as potential misclassification, flexibility in operationalization, and difficulties in the differentiation between short-term and chronic dialysis treatment must be taken into account. The determination of dialysis-related risks for mortality, other clinical events such as hospitalizations, and costs all play a central role in the appropriate allocation of resources, since not all patients benefit from dialysis (38). In the first instance, this involves informed decision-making by patients regarding the choice of appropriate CKF treatment pathway (39, 40) and evidence-based management of high-quality health care.
Limitations
The results of this study relate solely to insurees of the AOK Nordost aged ≥ 70 years. In order to make these statements generalizable, analyses based on other SHI claims data and other age groups should be undertaken. To achieve better comparability, estimates on prevalence and incidence were standardized in these analyses. Furthermore, only data for 4 separate years could be used. Therefore, the effects of different definitions on endpoints over longer follow-up periods should be further investigated. However, since in particular early mortality following the initiation of dialysis is of great clinical interest, the analyses presented here can nevertheless make a valuable contribution. For the analysis of health care costs, only inpatient and outpatient treatment costs as well as redeemed drug prescriptions were taken into consideration. Other aspects such as dialysis-related travel costs, remedies and medical aids, rehabilitative treatments, and non-prescription drugs should be analyzed for a more comprehensive view of health costs associated with CKF requiring dialysis.
Conclusion
Different definitions of CKF requiring dialysis in SHI claims data lead to variation in estimates for epidemiological, clinical, and health-economic endpoints. Our newly developed definition, which is based on plausible mortality figures and health care costs, makes it possible to more precisely identify patients with CKF requiring dialysis as distinct from patients receiving short-term dialysis treatment following AKI. The aspects and hurdles identified in this study in terms of the operationalization of CKF requiring dialysis in SHI claims data may also be of great relevance for other diseases and target populations beyond dialysis.
Acknowledgments
Acknowledgments
We would like to thank Dr. Thomas Weinreich, Prof. Dr. Martin Kuhlmann, and PD Dr. Wolfram Jabs for their helpful support in this study.
Funding
This study was funded in part by the Innovationsfonds – Versorgungsforschung des Gemeinsamen Bundesausschusses (G-BA; GUIDAGE-CKD project, FKZ 01VSF20020).
Data sharing statement
The data used in this study cannot be made available in the manuscript, the supplemental files, or in a public repository due to German data protection laws (Bundesdatenschutzgesetz). To ensure the replicability of the results, the data used are stored on a secure data device at the Charité – Universitätsmedizin Berlin. Access to the raw data used in this study can only be provided to external parties under the conditions of a cooperation contract and upon written request (bis@charite.de).
Translated from the original German by Christine Rye.
Footnotes
Conflict of interest statement
ES and NE receive funding from Bayer AG. ES receives funding from the National Kidney Foundation (USA).
JF is employed by the AOK Nordost.
The remaining authors declare that no conflict of interests exists.
References
- 1.Abdel-Kader K, Unruh ML, Weisbord SD. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4:1057–1064. doi: 10.2215/CJN.00430109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolbrink B, Schussel K, von Samson-Himmelstjerna FA, et al. Patient-focused outcomes after initiation of dialysis for ESRD: mortality, hospitalization, and functional impairment. Nephrol Dial Transplant. 2023;38:2528–2536. doi: 10.1093/ndt/gfad099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckardt KU, Gillespie IA, Kronenberg F, et al. High cardiovascular event rates occur within the first weeks of starting hemodialysis. Kidney Int. 2015;88:1117–1125. doi: 10.1038/ki.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haapio M, Helve J, Gronhagen-Riska C, Finne P. One- and 2-year mortality prediction for patients starting chronic dialysis. Kidney Int Rep. 2017;2:1176–1185. doi: 10.1016/j.ekir.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floege J, Gillespie IA, Kronenberg F, et al. Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int. 2015;87:996–1008. doi: 10.1038/ki.2014.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan KE, Maddux FW, Tolkoff-Rubin N, Karumanchi SA, Thadhani R, Hakim RM. Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol. 2011;6:2642–2649. doi: 10.2215/CJN.03680411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson BM, Zhang J, Morgenstern H, et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85:158–165. doi: 10.1038/ki.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blankestijn PJ, Bruchfeld A, Cozzolino M, et al. Nephrology: achieving sustainability. Nephrol Dial Transplant. 2020;35:2030–2033. doi: 10.1093/ndt/gfaa193. [DOI] [PubMed] [Google Scholar]
- 10.Gandjour A, Armsen W, Wehmeyer W, Multmeier J, Tschulena U. Costs of patients with chronic kidney disease in Germany. PLoS One. 2020;15 doi: 10.1371/journal.pone.0231375. e0231375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liyanage T, Ninomiya T, Jha V, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 12.IQTIG - Institut für Qualitätssicherung und Transparenz im Gesundheitswesen. Jahresbericht 2019 zur Qualität in der Dialyse. www.g-ba.de/downloads/39-261-4568/2020-11-20_QSD-RL_IQTIG-Jahresbericht-2019.pdf (last accessed on 10 January 2024) [Google Scholar]
- 13.Lonnemann G, Duttlinger J, Hohmann D, Hickstein L, Reichel H. Timely referral to outpatient nephrology care slows progression and reduces treatment costs of chronic kidney diseases. Kidney Int Rep. 2017;2:142–151. doi: 10.1016/j.ekir.2016.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schellartz I, Mettang S, Shukri A, Scholten N, Pfaff H, Mettang T. Early referral to nephrological care and the uptake of peritoneal dialysis. An analysis of German claims data. Int J Environ Res Public Health. 2021;18 doi: 10.3390/ijerph18168359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shukri A, Mettang T, Scheckel B, et al. Hemodialysis and peritoneal dialysis in Germany from a health economic view—a propensity score matched analysis. Int J Environ Res Public Health. 2022;19 doi: 10.3390/ijerph192114007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann F, Haastert B, Koch M, Giani G, Glaeske G, Icks A. The effect of diabetes on incidence and mortality in end-stage renal disease in Germany. Nephrol Dial Transplant. 2011;26:1634–1640. doi: 10.1093/ndt/gfq609. [DOI] [PubMed] [Google Scholar]
- 17.Claessen H, Narres M, Kvitkina T, et al. Renal replacement therapy in people with and without diabetes in Germany, 2010-2016: an analysis of more than 25 million inhabitants. Diabetes Care. 2021;44:1291–1299. doi: 10.2337/dc20-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reichel H, Seibert E, Tillmann FP, et al. Economic burden of secondary hyperparathyroidism in Germany: a matched comparison. Int Urol Nephrol. 2023;55:1291–1300. doi: 10.1007/s11255-022-03425-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hackl D, Kossack N, Schoenfelder T. Prävalenz, Kosten der Versorgung und Formen des dialysepflichtigen chronischen Nierenversagens in Deutschland: Vergleich der Dialyseversorgung innerhalb und außerhalb stationärer Pflegeeinrichtungen. Gesundheitswesen. 2021;83:818–828. doi: 10.1055/a-1330-7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Efron B, Tibshirani R. Boca Raton, FL: Chapman & Hall/CRC; 1993. An introduction to the bootstrap. [Google Scholar]
- 21.Foley RN, Chen SC, Solid CA, Gilbertson DT, Collins AJ. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int. 2014;86:392–398. doi: 10.1038/ki.2014.15. [DOI] [PubMed] [Google Scholar]
- 22.Couchoud C, Labeeuw M, Moranne O, et al. A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant. 2009;24:1553–1561. doi: 10.1093/ndt/gfn698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy E, Burns A, Murtagh FEM, Rooshenas L, Caskey FJ. The Prepare for Kidney Care Study: prepare for renal dialysis versus responsive management in advanced chronic kidney disease. Nephrol Dial Transplant. 2021;36:975–982. doi: 10.1093/ndt/gfaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frank J. Comparing nationwide prevalences of hypertension and depression based on claims data and survey data: an example from Germany. Health Policy. 2016;120:1061–1069. doi: 10.1016/j.healthpol.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Langner I, Ohlmeier C, Haug U, Hense HW, Czwikla J, Zeeb H. Implementation of an algorithm for the identification of breast cancer deaths in German health insurance claims data: a validation study based on a record linkage with administrative mortality data. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-026834. e026834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schorling E, Lick S, Steinberg P, Bruggemann DA. Health care utilizations and costs of Campylobacter enteritis in Germany: a claims data analysis. PLoS One. 2023;18 doi: 10.1371/journal.pone.0283865. e0283865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt C, Reitzle L, Dress J, Rommel A, Ziese T, Heidemann C. Prävalenz und Inzidenz des dokumentierten Diabetes mellitus - Referenzauswertung für die Diabetes-Surveillance auf Basis von Daten aller gesetzlich Krankenversicherten. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2020;63:93–102. doi: 10.1007/s00103-019-03068-9. [DOI] [PubMed] [Google Scholar]
- 28.Brinks R, Tönnies T, Hoyer A. Importance of diagnostic accuracy in big data: false-positive diagnoses of type 2 diabetes in health insurance claims data of 70 million Germans. Front Epidemiol. 2022;2 doi: 10.3389/fepid.2022.887335. 887335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bothe T, Fietz AK, Schäffner E, et al. Diagnostic validity of chronic kidney disease in health claims data over time: results from a cohort of community-dwelling older adults in Germany. Clin Epidemiol (in press) doi: 10.2147/CLEP.S438096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greiner F, Slagman A, Stallmann C, et al. Routinedaten aus Notaufnahmen: Unterschiedliche Dokumentationsanforderungen, Abrechnungsmodalitäten und Datenhalter bei identischem Ort der Leistungserbringung. Gesundheitswesen. 2020;82:72–82. doi: 10.1055/a-0996-8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller S, Gottschalk F, Groth A, et al. Primary data, claims data, and linked data in observational research: the case of COPD in Germany. Respir Res. 2018;19 doi: 10.1186/s12931-018-0865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann F, Icks A. Unterschiede in der Versichertenstruktur von Krankenkassen und deren Auswirkungen für die Versorgungsforschung: Ergebnisse des Bertelsmann-Gesundheitsmonitors. Gesundheitswesen. 2012;74:291–297. doi: 10.1055/s-0031-1275711. [DOI] [PubMed] [Google Scholar]
- 33.March S. Individual data linkage of survey data with claims data in Germany—an overview based on a cohort study. Int J Environ Res Public Health. 2017;14 doi: 10.3390/ijerph14121543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pigeot I, Bongaerts B, Eberle A, et al. Verknüpfung von Abrechnungsdaten gesetzlicher Krankenkassen mit Daten epidemiologischer Krebsregister: länderspezifische Möglichkeiten und Limitationen. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2022;65:615–623. doi: 10.1007/s00103-021-03475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Munafo MR, Nosek BA, Bishop DVM, et al. A manifesto for reproducible science. Nat Hum Behav. 2017;1 doi: 10.1038/s41562-016-0021. 0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swart E, Bitzer EM, Gothe H, et al. STandardisierte BerichtsROutine für Sekundärdaten Analysen (STROSA) - ein konsentierter Berichtsstandard für Deutschland, Version 2. Gesundheitswesen. 2016;78:e145–e160. doi: 10.1055/s-0042-112008. [DOI] [PubMed] [Google Scholar]
- 37.Slagman A, Hoffmann F, Horenkamp-Sonntag D, Swart E, Vogt V, Herrmann WJ. Analyse von Routinedaten in der Gesundheitsforschung: Validität, Generalisierbarkeit und Herausforderungen. Zeitschrift für Allgemeinmedizin. 2023;99:86–92. [Google Scholar]
- 38.Wong SPY, Rubenzik T, Zelnick L, et al. Long-term outcomes among patients with advanced kidney disease who forgo maintenance dialysis: a systematic review. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.2255. e222255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schellartz I, Ohnhaeuser T, Mettang T, Scholten N. Information about different treatment options and shared decision making in dialysis care—a retrospective survey among hemodialysis patients. BMC Health Serv Res. 2021;21 doi: 10.1186/s12913-021-06599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tonkin-Crine S, Okamoto I, Leydon GM, et al. Understanding by older patients of dialysis and conservative management for chronic kidney failure. Am J Kidney Dis. 2015;65:443–450. doi: 10.1053/j.ajkd.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E1.Thompson AM, Southworth MR. Real world data and evidence: support for drug approval: applications to kidney diseases. Clin J Am Soc Nephrol. 2019;14:1531–1532. doi: 10.2215/CJN.02790319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Corrigan-Curay J, Sacks L, Woodcock J. Real-world evidence and real-world data for evaluating drug safety and effectiveness. JAMA. 2018;320:867–868. doi: 10.1001/jama.2018.10136. [DOI] [PubMed] [Google Scholar]
- E3.Mues KE, Liede A, Liu J, et al. Use of the medicare database in epidemiologic and health services research: a valuable source of real-world evidence on the older and disabled populations in the US. Clin Epidemiol. 2017;9:267–277. doi: 10.2147/CLEP.S105613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Graubner B, editor. Köln: Deutscher Ärzteverlag; 2013. ICD-10-GM 2014 Systematisches Verzeichnis: Internationale statistische Klassifikation der Krankheiten und verwandter Gesundheitsprobleme 10. Revision - German Modification, Version 2014. [Google Scholar]
- E5.Busse R, Blumel M, Knieps F, Barnighausen T. Statutory health insurance in Germany: a health system shaped by 135 years of solidarity, self-governance, and competition. Lancet. 2017;390:882–897. doi: 10.1016/S0140-6736(17)31280-1. [DOI] [PubMed] [Google Scholar]