Abstract
Purpose
People with multiple sclerosis (pwMS) experience autoimmunity-mediated inflammation and neurodegeneration throughout the central nervous system. There remains a need for clinically accessible, reliable functional markers of neurodegeneration in MS. Previous research has described changes to electroretinography (ERG)-derived measures of retinal bipolar cell function in pwMS early in the disease course. We, therefore, investigated ERG as a potential outcome measure in individuals with more advanced disease.
Methods
This cross-sectional observational study included pwMS with Expanded Disability Status Scale (EDSS) scores of ≥3.0 and healthy control (HC) participants who underwent ERG, optical coherence tomography, high- and low-contrast visual acuity measurement, and an ophthalmological examination. ERG findings in MS eyes with and without previous optic neuritis (MS +ON; MS –ON) were compared with those in HC eyes. Effects of EDSS, disease duration, ON, and treatment status on selected ERG outcomes were measured. Additional exploratory analyses assessed potential influences of MS phenotype and disease status (clinically active, radiologically active, and disease progression).
Results
Delays to two ERG peak times (dark-adapted 3.0 b-wave; light-adapted flicker) were recorded in MS +ON and MS –ON eyes. No influences of EDSS score, disease duration, previous ON, or treatment status were observed. Exploratory analyses were consistent with no effects of MS phenotype or disease status.
Conclusions
ERG findings are abnormal in individuals with moderate-severe disability caused by MS; however, these findings are not distinct from those observed earlier in the disease course. Although bipolar dysfunction appears to be common in pwMS throughout the disease course, ERG is likely not useful in monitoring or prognostication of MS.
Keywords: electroretinogram, multiple sclerosis, optical coherence tomography
Multiple sclerosis (MS) is a complex, incurable neurological disease characterized by inflammation, demyelination, and neurodegeneration within the central nervous system and is the leading cause of neurological disability among young adults worldwide.1 It is believed that the disease is driven predominantly by pathogenic autoreactive lymphocytes, with numerous environmental factors interacting against the background of a complex polygenetic trait.2 Relapsing–remitting MS (RRMS) is the most common form of the disease and is characterized by episodes of inflammatory demyelination (relapses) followed by periods of variable recovery and relatively stable neurological status (remissions).3,4 Individuals diagnosed with RRMS subsequently may develop a gradual, progressive accumulation of disability independently of relapses, defined as secondary progressive MS (SPMS); alternatively, a minority of patients exhibit progressive disease activity and disability accumulation in the absence of relapses from disease onset, defined as primary progressive MS (PPMS).3,4 Although these historical phenotype descriptions remain useful and widely used, subsequently accumulated knowledge has driven awareness of their limitations. Consequently, recent recommendations have emphasized the importance of assessing disease status, with MS now also described as clinically or radiologically active, with or without evidence of disease progression, in addition to established phenotypic classification.5
Afferent visual pathway involvement is common in MS, with optic neuritis (ON) being the presenting symptom in approximately 25% of patients and documented during the disease course in approximately 70%.6 Optic nerve damage is a near ubiquitous observation at autopsy,7 suggesting that many cases of MS-related ON may go undocumented. Sequelae of ON include atrophy of the retinal ganglion cells and their axons at the inner retina,8,9 with corresponding functional deficits.10 However, recent years have also seen a number of authors describe alterations to outer retinal function in people with MS (pwMS), as measured with full-field electroretinography (ERG). Results typically show delays to the cone– and/or rod–cone ERG b-waves,11–17 which are believed to be generated by the retinal bipolar cells.18–20 Previous work from our group11 suggests that these delays to the ERG occur independently of ON, a finding reinforced by the documentation of similar results in MS eyes without previous ON,12,13,15 and is thus attributable to MS directly rather than post-ON retrograde degeneration. Longitudinal worsening of Expanded Disability Status Scale (EDSS) score has been associated with increasing delay of ERG b-waves,21 raising the possibility that these electrophysiological outcome measures may be prognostic of MS disease course. Variants of the ERG have also been proposed to be of value in MS diagnosis22–24 and in distinguishing MS from other neuroimmunological diseases25 (although see Hanson et al.26).
ERG studies published to date in MS have been conducted predominantly with participants without significant disability (as evidenced, for example, by median EDSS scores of 1.011,13,17), with relatively short disease duration (up to approximately 5 years)11–13,15,17 and who have never experienced ON.12,13,15 Consequently, the effects of severe and long-established MS are unknown. Additionally, ERG-derived measures of bipolar function are affected by autoimmunity-mediated disease activity (e.g., Weleber et al., Ohta et al., and Zacks et al.27–29) and may be used both in assessing the effectivity of medical treatment29–31 and in diagnosis.32 Finally, potential influences of MS phenotype (relapsing/inflammatory vs. progressive/neurodegenerative) and disease activity and status have not been investigated, with previous studies examining only people with RRMS12,13 or aggregating patients with relapsing and progressive disease.11,15 Disease progression may occur independent of relapse activity (progression independent of relapse activity,2 silent progression33), and markers of this worsening are highly desirable.2 Owing to the known outer retinal functional abnormalities in MS and other immunological diseases discussed above, ERG is a potential candidate for such a marker. Investigations in individuals with more advanced MS are essential to adequately assess the usefulness of ERG in this respect.
Against this background, we examined individuals with moderate-severe MS (as defined by EDSS score) and measured potential effects of disability (EDSS), disease duration, treatment, clinical disease status, and phenotype on preganglionic retinal function as measured with the ERG. Our aim was to assess the usefulness of ERG as a potential objective functional marker in pwMS.
Methods
The research questions were investigated using a cross-sectional observational study design. We recruited pwMS from the neurology outpatient clinics at the University Hospital Zurich. Inclusion criteria were: age ≥18 years, preexisting diagnosis of MS according to the 2017 McDonald criteria,3 and an EDSS score of ≥3.0. Exclusion criteria were refractive errors >6 diopters, coexisting ocular or neurological disease other than MS, and diabetes mellitus. Individuals without known ocular or systemic diseases (e.g., diabetes mellitus, autoimmune disease, neoplasia, vascular disease), as ascertained by history taking, were recruited from hospital staff and friendship and family circles to serve as a healthy control (HC) cohort. All pwMS and HC participants consented in writing to participate in the study, and received a small payment to cover transport costs (including those of a carer, when necessary). The study adhered to the tenets of the Declaration of Helsinki and was approved by the Cantonal Ethics Committee of Zurich (BASEC ID 2018-00047).
Examinations consisted of subjective refraction; best-corrected high-contrast visual acuity (HCVA) and low-contrast VA (LCVA) using Early Treatment Diabetic Retinopathy Study–style and 2.5% contrast Sloan charts, respectively, and recorded as logMAR; optical coherence tomography (OCT), ERG, and anterior segment and mydriatic fundus examination by an experienced ophthalmologist. Although the primary focus of the study was retinal function, with retinal structure in pwMS already extensively studied previously (as reviewed by Petzold and colleagues8), OCT was included to assist in detecting previous subclinical unilateral ON episodes in cases where the clinical history was incomplete or uncertain.34 LCVA was measured for similar reasons35 (as reviewed by Balcer et al.36). Details of the OCT protocols and parameters analyzed are contained in the Supplementary Methods.
Study visits lasted for approximately two hours and took place between March 2018 and February 2023, including an institutionally mandated pause during the global coronavirus disease 2019 pandemic.
ERG
Ganzfeld (full-field) ERG was performed according to contemporary recommendations of the International Society for Clinical Electrophysiology of Vision (ISCEV),37 as described previously,11,21 and in the Supplementary Methods, using an Espion System (Diagnosys LLC, Lowell, MA, USA). Dawson, Trick, and Litzkow (DTL) recording electrodes positioned at the lower lid margin were used, together with gold-plated skin electrodes for reference (positioned at the ipsilateral outer canthi) and ground (positioned at the center of the forehead). From the ERG, peak times and amplitudes were extracted for the dark-adapted (DA) 0.01 b-wave, DA and light-adapted (LA) 3.0 a- and b-waves, and LA flicker stimulus for each eye.37
Statistical Analyses
Our statistical analysis plan was defined a priori. The primary goals of the study were to compare retinal function in participants with moderate to severe MS and HC participants and to examine the influence of EDSS, disease duration, MS treatment status (treated/untreated), and ON history on selected measures of retinal function (DA3.0 and LA3.0 b-wave peak times) in participants with moderate to severe MS.
We also planned additional analyses of the effects of MS phenotype and disease status (clinically active, radiologically active, and evidence of progression5) on DA3.0 and LA3.0 b-wave peak times. These analyses were conceived as exploratory, as we anticipated that some subgroups would be too small to permit sufficient statistical power, and therefore only effect sizes and 95% confidence intervals were calculated to obtain an idea of the relevant effect sizes and if the effects of any of the parameters analyzed were worthy of further study. For completeness, retinal structural (OCT) findings in MS +ON, MS −ON, and HC eyes were also analyzed and compared (see Supplementary Methods).
We selected DA3.0 and LA3.0 b-wave peak times as the most appropriate variables for detailed analysis based on the results of recent studies that documented abnormalities in these outcome measures in pwMS.11–15,17 In many cases, potentially relevant older studies predated recommended standards for ERG recording38 and the widespread adoption of EDSS,39 making comparison with contemporary studies challenging; we, therefore, selected the ERG parameters for more in-depth analysis based primarily on the results of more recent works. Previous studies, both recent and historical, are summarized in Supplementary Table S1.
After imputation of data missing at random,40 results were summarized descriptively. Disease duration was recorded and summarized in months but converted to years for co-analysis with ERG data. Generalized estimating equation (GEE) models adjusted for age and sex were used to compare ERG results in MS eyes with and without previous ON (MS +ON; MS –ON) with HC eyes, and to assess the influence of EDSS, disease duration, and treatment on DA3.0 and LA3.0 b-wave peak times. Both eyes of each participant were analyzed, with the intereye dependency of within-participant measurements accounted for within the GEE models.41 All P values were corrected for multiple comparisons using the method of Benjamini and Hochberg.42 P values of 0.05–0.01, 0.01–0.001, and <0.001 were interpreted as moderate, strong, and very strong evidence, respectively, of a difference between the relevant participant subgroups.43
All analyses were performed, and figures generated in, R version 4.2.244 using supplementary packages for analysis and visualization. Full details of the nonbase packages used can be found in the Supplementary Methods.
Results
Participants and Demographics
A total of 58 pwMS were recruited, of whom two were excluded from the study owing to high refractive error and glaucoma, leaving 56 eligible participants. We examined 38 HCs. There were 17 pwRRMS who had a history of ON over the disease course (10 unilaterally, 7 bilaterally), and 7 pwSPMS had previously had ON (4 unilaterally, 3 bilaterally). No pwMS were documented as having experienced bilateral simultaneous ON. A single participant with PPMS had previously had unilateral ON. Therefore, the number of MS +ON eyes was 24, 10, and 1 in the RRMS, SPMS, and PPMS subgroups, respectively. No pwMS had experienced ON within 3 months of the examination.11,45 A total of 10 MS eyes and 3 HC eyes were excluded from analysis, as detailed in the Supplementary Results. The final dataset analyzed, therefore, consisted of 102 MS eyes (35 MS +ON, 67 MS –ON) and 73 HC eyes. Additionally, a small amount of data in individual participants/eyes were missing, as described in the Supplementary Results.
The MS and HC cohorts had mean age of 47.1 years (range, 27–81 years) and 50.4 years (range, 24–75 years) and percentage of females of 53.6% and 52.6%, respectively. The median HCVA was −0.14, −0.06, and 0.01 logMAR in the HC, MS −ON, and MS +ON groups, respectively. Corresponding median LCVA values were 0.32, 0.48, and 0.64 logMAR, respectively. In the MS +ON cohort, HCVA and LCVA data were missing in one and six eyes, respectively, because the participants were unable to read any letters on the test charts owing to vision loss after ON. These missing HCVA and LCVA values were not imputed, because they did not meet the imputation criteria of being missing at random (because missing values were associated with poorer visual outcomes). The median difference between HCVA and LCVA was 0.46, 0.50, and 0.62 logMAR in the HC, MS −ON, and MS +ON groups, respectively, corresponding with 23, 25, and 31 letters on the test chart. Age and sex characteristics and visual performance in the subgroups are summarized in Table 1.
Table 1.
Descriptive Statistics Summarizing the Age and Sex Distributions, and Visual Performance, of the Study Cohort and Individual Subgroups at Both the Participant and Eye Levels
| Variable | HC | PwMS | |
|---|---|---|---|
| Participants | 38 | 56 | |
| Age, years mean ± SD | 50.4 ± 12.7 | 47.1 ± 10.6 | |
| Age, years range | 27–81 | 24–75 | |
| Female (%) | 20 (52.6) | 30 (53.6) | |
| HC | MS −ON | MS +ON | |
| Eyes | 73 | 67 | 35 |
| Age, years mean ± SD | 49.67 ± 12.2 | 48.8 ± 9.7 | 44.7 ± 12.0 |
| Female (%) | 38 (52.1) | 34 (50.7) | 21 (60.0) |
| HCVA mean ± SD | −0.13 ± 0.09 | −0.04 ± 0.09 | 0.08 ± 0.23 |
| HCVA median [IQR] | 0.140 [0.14] | −0.06 [0.08] | 0.0100 [0.32] |
| HCVA missing (%) | 0 (0) | 0 (0) | 1 (2.9) |
| LCVA mean ± SD | 0.34 ± 0.12 | 0.50 ± 0.19 | 0.66 ± 0.26 |
| LCVA median [IQR] | 0.32 [0.14] | 0.48 [0.23] | 0.64 [0.32] |
| LCVA missing (%) | 0 (0) | 0 (0) | 6 (17.1) |
| HCVA-LCVA mean ± SD | 0.47 ± 0.09 | 0.53 ± 0.17 | 0.62 ± 0.15 |
| HCVA-LCVA median [IQR] | 0.460 [0.12] | 0.50 [0.17] | 0.620 [0.18] |
| HCVA-LCVA missing (%) | 0 (0) | 0 (0) | 6 (17.1) |
IQR, interquartile range.
HCVA − LCVA is the arithmetic difference between HCVA and LCVA. All HCVA, LCVA, and HCVA − LCVA values are provided as the logMAR. Missing HCVA and LCVA values represent the number of eyes where it was not possible to quantify visual performance; in all cases this occurred in eyes with previous ON.
In pwMS, the EDSS ranged from 3.0 to 8.0 (median, 4.0) and the mean disease duration was equivalent to just over 15 years. Of the 102 MS eyes analyzed, 55 were from individuals with RRMS, 30 SPMS, and 17 PPMS. The majority of pwMS were medically treated, corresponding with 91.2% of analyzed eyes. Although ≥80% of MS eyes were from participants in whom there was no clinical or radiological evidence of disease activity, there was evidence of progression in approximately 40% of these eyes. Disease characteristics of the MS cohort are summarized in Table 2.
Table 2.
Summary of the Disease Characteristics of the Multiple Sclerosis Cohort at the Eye Level, Including Eyes Both With (MS +ON) and Without (MS −ON) Previous ON
| Variable | Levels | Overall MS | MS −ON | MS +ON |
|---|---|---|---|---|
| Eyes | 102 | 67 | 35 | |
| MS type (%) | RRMS | 55 (53.9) | 31 (46.3) | 24 (68.6) |
| SPMS | 30 (29.4) | 20 (29.9) | 10 (28.6) | |
| PPMS | 17 (16.7) | 16 (23.9) | 1 (2.9) | |
| DD, mean ± SD | 183.6 ± 112.6 | 168.6 ± 102.9 | 212.3 ± 125.8 | |
| Treated (%) | No | 9 (8.8) | 6 (9.0) | 3 (8.6) |
| Yes | 93 (91.2) | 61 (91.0) | 32 (91.4) | |
| DMT (%) | None | 9 (8.8) | 6 (9.0) | 3 (8.6) |
| Natulizumab | 15 (14.7) | 9 (13.4) | 6 (17.1) | |
| Ocrelizumab | 31 (30.4) | 22 (32.8) | 9 (25.7) | |
| Rituximab | 31 (30.4) | 21 (31.3) | 10 (28.6) | |
| Fingolimod | 4 (3.9) | 4 (6.0) | 0 (0.0) | |
| GA | 2 (2.0) | 2 (3.0) | 0 (0.0) | |
| DMF | 4 (3.9) | 0 (0.0) | 4 (11.4) | |
| Teriflunomide | 5 (4.9) | 2 (3.0) | 3 (8.6) | |
| Cladribine | 1 (1.0) | 1 (1.5) | 0 (0.0) | |
| Active (clinical) (%) | No | 88 (86.3) | 57 (85.1) | 31 (88.6) |
| Yes | 12 (11.8) | 8 (11.9) | 4 (11.4) | |
| Missing | 2 (2.0) | 2 (3.0) | 0 (0.0) | |
| Active (radiological) (%) | No | 82 (80.4) | 54 (80.6) | 28 (80.0) |
| Yes | 14 (13.7) | 9 (13.4) | 5 (14.3) | |
| Missing | 6 (5.9) | 4 (6.0) | 2 (5.7) | |
| Progression (%) | No | 59 (57.8) | 38 (56.7) | 21 (60.0) |
| Yes | 41 (40.2) | 27 (40.3) | 14 (40.0) | |
| Missing | 2 (2.0) | 2 (3.0) | 0 (0.0) | |
| EDSS, median (range) | 4.0 (3.0–8.0) | 4.25 (3.0–8.0) | 3.75 (3.0–8.0) |
DD, disease duration; DMF, dimethyl fumarate; DMT, disease-modifying therapy; GA, glatarimer acetate; MS, multiple sclerosis; PPMS, primary progressive multiple sclerosis.
DD is provided in months.
ERG
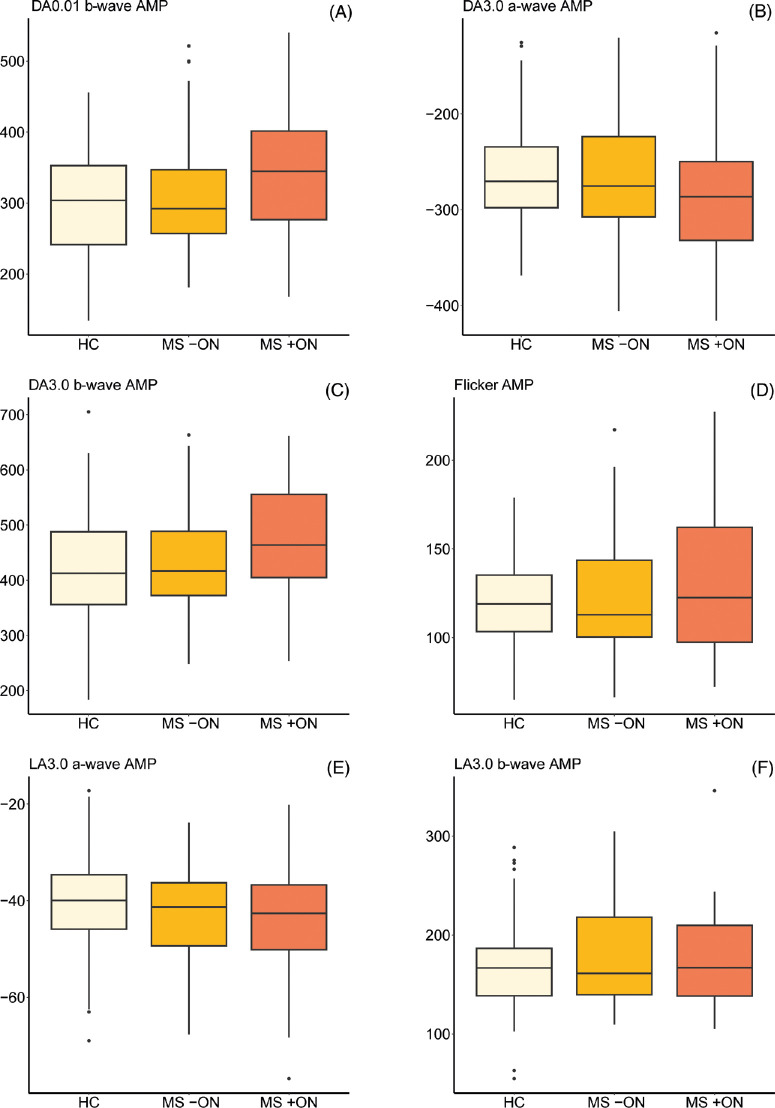
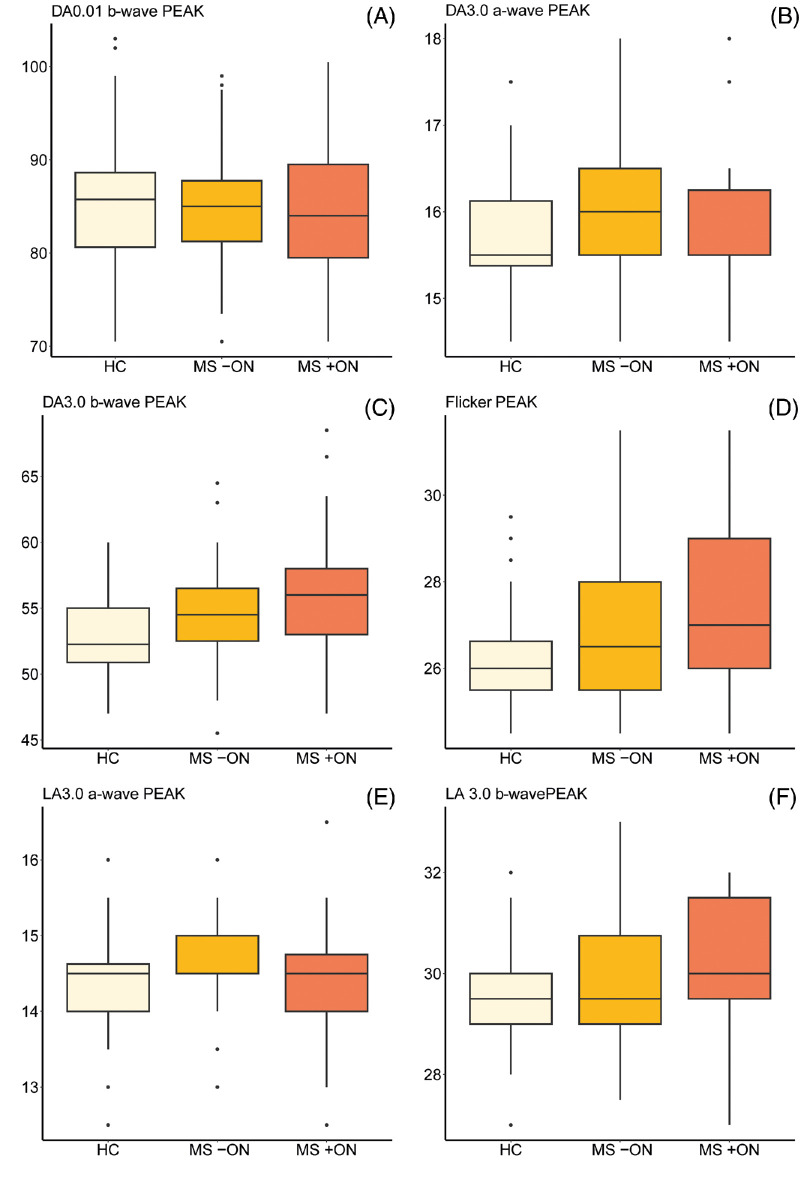
Descriptive statistics for the ERG results are provided in Table 3 (including the amount of missing or imputed data for each condition), with boxplots showing ERG amplitudes and peak times presented in Figures 1 and 2, respectively. Results of the GEE models comparing ERG findings in the subgroups are shown in Table 4. The analyses revealed prolonged DA3.0 b-wave peak times in both MS +ON and MS –ON (P = 0.001 and 0.014, representing strong and moderate evidence, respectively). We also recorded evidence of delayed flicker ERG responses in MS +ON and MS –ON (P = 0.014 and 0.009, representing moderate and strong evidence, respectively). No evidence of any group differences in any other ERG parameters was recorded. Qualitatively, no patients or eyes exhibited a negative ERG configuration.46
Table 3.
Descriptive Statistics of ERG Results in the Study Cohort
| HC | MS −ON | MS +ON | |
|---|---|---|---|
| No. of eyes | 73 | 67 | 35 |
| DA0.01b.AMP | |||
| Mean ± SD | 302.0 ± 76.0 | 313.0 ± 82.3 | 344.0 ± 85.9 |
| Median [IQR] | 304 [111.0] | 292 [89.8] | 345 [125.0] |
| Missing (%) | 1 (1.4) | 0 (0.0) | 0 (0.0) |
| DA0.01b.PEAK | |||
| Mean ± SD | 85.5 ± 7.31 | 84.6 ± 5.87 | 84.7 ± 6.76 |
| Median [IQR] | 85.8 [8.0] | 85.0 [6.5] | 84.0 [10.0] |
| Missing (%) | 1 (1.4) | 0 (0.0) | 0 (0.0) |
| DA3.0a.AMP | |||
| Mean ± SD | −262.0 ± 54.3 | −267.0 ± 57.8 | −283.0 ± 73.0 |
| Median [IQR] | −270.0 [63.6] | −275.0 [83.9] | −286.0 [82.2] |
| Missing (%) | 1 (1.4) | 0 (0.0) | 0 (0.0) |
| DA3.0a.PEAK | |||
| Mean ± SD | 15.8 ± 0.7 | 16.0 ± 0.7 | 15.8 ± 0.7 |
| Median [IQR] | 15.5 [0.8] | 16.0 [1.0] | 15.5 [0.8] |
| Missing (%) | 1 (1.4) | 0 (0.0) | 0 (0.0) |
| DA 3.0b.AMP | |||
| Mean ± SD | 420 ± 107 | 439 ± 94 3 | 473 ± 102 |
| Median [IQR] | 413.0 [132.0] | 417.0 [116.0] | 464.0 [150.0] |
| Missing (%) | 1 (1.4) | 0 (0.0) | 0 (0.0) |
| DA3.0b.PEAK | |||
| Mean ± SD | 52.8 ± 3.1 | 54.9 ± 3.6 | 56.0 ± 4.5 |
| Median [IQR] | 52.3 [4.1] | 54.5 [4.0] | 56.0 [5.0] |
| Missing (%) | 1 (1.4) | 1 (1.5) | 0 (0.0) |
| LA3.0a.AMP | |||
| Mean (SD) | −40.6 ± 10.0 | −42.9 ± 10.5) | −44.2 ± 2.0 |
| Median [IQR] | −40.0 [11.3] | −41.3 [13.1] | −42.6 [13.4] |
| Missing (%) | 1 (1.4) | 0 (0.0) | 2 (5.7) |
| LA3.0a.PEAK | |||
| Mean ± SD | 14.4 ± 0.6 | 14.6 ± 0.5 | 14.4 ± 0.8 |
| Median [IQR] | 14.5 [0.6] | 14.5 [0.5] | 14.5 [0.8] |
| Missing (%) | 1 (1.4) | 0 (0.0) | 0 (0.0) |
| LA3.0b.AMP | |||
| Mean ± SD | 167.0 ± 45.6 | 178.0 ± 51.3 | 177.0 ± 50.1 |
| Median [IQR] | 167.0 [47.9] | 161.0 [78.5] | 167.0 [71.4] |
| Missing (%) | 1 (1.4) | 0 (0.0) | 0 (0.0) |
| LA3.0b.PEAK | |||
| Mean ± SD | 29.7 ± 1.1 | 29.8 ± 1.3 | 30.3 ± 1.4 |
| Median [IQR] | 29.5 [1.0] | 29.5 [1.8] | 30.0 [2.0] |
| Missing (%) | 1 (1.4) | 0 (0.0) | 0 (0.0) |
| Flicker.AMP | |||
| Mean ± SD | 118.0 ± 24.4 | 120.0 ± 32.7 | 129.0 ± 39.3 |
| Median [IQR] | 119.0 [31.8] | 113.0 [43.3] | 123.0 [64.7] |
| Missing (%) | 5 (6.8) | 2 (3.0) | 0 (0.0) |
| Flicker.PEAK | |||
| Mean ± SD | 26.1 ± 1.2 | 27.1 ± 1.8 | 27.4 ± 1.7 |
| Median [IQR] | 260 [1.1] | 26.5 [2.5] | 27.0 [3.0] |
| Missing (%) | 5 (6.8) | 2 (3.0) | 0 (0.0) |
AMP, response amplitude (in microvolts); DA, dark adapted; IQR, interquartile range; LA, light adapted; PEAK, response peak time (in milliseconds).
Figure 1.
(A–F) Boxplots showing ERG response amplitudes in eyes of people with multiple sclerosis both with (MS +ON) and without (MS −ON) previous ON with those of HC participants. Units on all y axes are microvolts. Median values and IQRs are indicated by horizontal lines and boxes, respectively; whiskers show the lowest and highest data points still within 1.5 IQR of the lower and upper quartiles. Individual outlying data points are represented by black dots. AMP, response amplitude; DA, dark adapted; LA, light adapted.
Figure 2.
(A–F) Boxplots showing ERG response peak times in eyes of people with multiple sclerosis both with (MS +ON) and without (MS −ON) previous ON with those of HC participants. Units on all y axes are milliseconds. Median values and IQRs are indicated by horizontal lines and boxes, respectively; whiskers show the lowest and highest data points still within 1.5 IQR of the lower and upper quartiles. Individual outlying data points are represented by black dots. DA, dark adapted; LA, light adapted; PEAK, response peak time.
Table 4.
Results of GEE Models Adjusted for Age and Sex Comparing Electroretinography (ERG) Results in Eyes of People With Multiple Sclerosis Both With (MS +ON) and Without (MS −ON) Previous Optic Neuritis With Those of HC Participants
| Variable | Coefficient | Estimate | SE | 95% CI | P Value |
|---|---|---|---|---|---|
| DA0.01b.AMP | HC (intercept) | 440.04 | 35.24 | 370.96 to 509.12 | |
| MS −ON | 11.54 | 15.22 | −18.3 to 41.37 | 0.77 | |
| MS +ON | 18.97 | 16.49 | −13.34 to 51.28 | 0.55 | |
| DA0.01b.PEAK | HC (intercept) | 74.87 | 2.76 | 69.47 to 80.27 | |
| MS −ON | −0.55 | 1.17 | −2.84 to 1.75 | 0.81 | |
| MS +ON | 0.23 | 1.44 | −2.59 to 3.06 | 0.95 | |
| DA3.0a.AMP | HC (intercept) | −387.52 | 23.62 | −433.81 to −341.22 | |
| MS −ON | −0.43 | 10.31 | −20.63 to 19.78 | 0.97 | |
| MS +ON | −3.63 | 12.12 | −27.39 to 20.13 | 0.88 | |
| DA3.0a.PEAK | HC (intercept) | 14.04 | 0.27 | 13.51 to 14.57 | |
| MS −ON | 0.19 | 0.12 | −0.03 to 0.42 | 0.28 | |
| MS +ON | 0.30 | 0.13 | 0.05 to 0.54 | 0.089 | |
| DA3.0b.AMP | HC (intercept) | 589.10 | 43.26 | 504.32 to 673.89 | |
| MS −ON | 14.33 | 19.29 | −23.47 to 52.13 | 0.77 | |
| MS +ON | 29.22 | 20.48 | −10.91 to 69.36 | 0.37 | |
| DA3.0b.PEAK | HC (intercept) | 48.48 | 1.36 | 45.8 to 51.15 | |
| MS −ON | 2.10 | 0.67 | 0.78 to 3.42 | 0.014 | |
| MS +ON | 3.42 | 0.85 | 1.75 to 5.08 | 0.001 | |
| LA3.0a.AMP | HC (intercept) | −56.69 | 4.28 | −65.08 to −48.29 | |
| MS −ON | −1.32 | 1.94 | −5.13 to 2.48 | 0.77 | |
| MS +ON | −1.18 | 2.31 | −5.7 to 3.34 | 0.81 | |
| LA3.0a.PEAK | HC (intercept) | 13.60 | 0.26 | 13.08 to 14.12 | |
| MS −ON | 0.18 | 0.11 | −0.03 to 0.38 | 0.28 | |
| MS +ON | 0.08 | 0.16 | −0.23 to 0.39 | 0.81 | |
| LA3.0b.AMP | HC (intercept) | 259.44 | 19.42 | 221.39 to 297.5 | |
| MS −ON | 5.67 | 8.69 | −11.36 to 22.7 | 0.77 | |
| MS +ON | 2.67 | 9.24 | −15.44 to 20.78 | 0.88 | |
| LA3.0b.PEAK | HC (intercept) | 27.72 | 0.55 | 26.64 to 28.8 | |
| MS −ON | 0.36 | 0.24 | −0.11 to 0.82 | 0.35 | |
| MS +ON | 0.49 | 0.25 | −0.01 to 0.98 | 0.22 | |
| Flicker.AMP | HC (intercept) | 167.16 | 12.20 | 143.24 to 191.08 | |
| MS −ON | 4.25 | 5.17 | −5.88 to 14.37 | 0.77 | |
| MS +ON | 0.21 | 5.78 | −11.12 to 11.53 | 0.97 | |
| Flicker.PEAK | HC (intercept) | 23.78 | 0.71 | 22.39 to 25.16 | |
| MS −ON | 1.01 | 0.30 | 0.43 to 1.6 | 0.009 | |
| MS +ON | 1.09 | 0.36 | 0.39 to 1.79 | 0.014 |
AMP, response amplitude (in microvolts); CI, confidence interval; PEAK, response peak time (in milliseconds).
Corrected P values representing moderate, strong, or very strong evidence of a difference between the relevant MS subgroup and HC cohort are highlighted in bold (more details available in the Methods section and the Statistical Analysis subsection).
We also observed inner retinal thinning in MS +ON more than MS –ON eyes relative to HC, with slight thickening of the inner nuclear layer in MS +ON eyes. Microcystic macular oedema (MMO) was not observed in any eyes. The OCT findings are described fully in Supplementary Results and presented in Supplementary Table S2, Supplementary Table S3, and Supplementary Figure S1.
Effects of MS on ERG Outcomes
No evidence of any effects of EDSS, disease duration (in years), history of ON, or treatment status (yes/no) on bipolar function (DA3.0 and LA3.0 b-wave peak times) were recorded, as shown in Table 5 and Figure 3. Visualization of the effects of MS subtype and disease status (clinically active, radiologically active, or progression) on DA3.0 and LA3.0 b-wave (Fig. 4) peak times did not suggest any relevant relationships. The corresponding exploratory GEE analyses were also suggestive of no effects of MS subtype and disease status on the ERG outcome measures, as evidenced by the 95% confidence intervals encompassing an effect size of zero (Supplementary Table S3).
Table 5.
Results of GEE Models Analyzing the Influence of Multiple Sclerosis-Related Disability (EDSS), Disease Duration, Previous Optic Neuritis (ON), and Binarized Treatment Status on Selected ERC Peak Time Outcomes*
| Variable | Coefficient | Estimate | SE | 95% CI | P Value |
|---|---|---|---|---|---|
| DA3.0b.PEAK | (Intercept) | 53.41 | 2.36 | 48.75 to 58.04 | |
| EDSS | −0.10 | 0.14 | −0.38 to 0.17 | 0.57 | |
| (Intercept) | 53.14 | 2.14 | 48.94 to 57.33 | ||
| DD (years) | 0.06 | 0.04 | −0.02 to 0.15 | 0.41 | |
| (Intercept) | 51.88 | 2.14 | 47.70 to 56.07 | ||
| ON (yes) | 1.22 | 0.85 | −0.45 to 2.89 | 0.40 | |
| (Intercept) | 51.75 | 2.82 | 46.23 to 57.27 | ||
| Treated (yes) | 0.85 | 1.26 | −1.61 to 3.32 | 0.57 | |
| LA3.0b.PEAK | (Intercept) | 28.11 | 0.84 | 26.47 to 29.75 | |
| EDSS | 0.02 | 0.06 | −0.09 to 0.13 | 0.71 | |
| (Intercept) | 28.31 | 0.76 | 26.82 to 29.79 | ||
| DD (years) | 0.02 | 0.02 | −0.02 to 0.05 | 0.56 | |
| (Intercept) | 28.13 | 0.73 | 26.70 to 29.57 | ||
| ON (yes) | 0.11 | 0.12 | −0.13 to 0.36 | 0.56 | |
| (Intercept) | 27.19 | 1.00 | 25.24 to 29.14 | ||
| Treated (yes) | 0.83 | 0.54 | −0.22 to 1.88 | 0.41 |
CI, confidence interval; DA, dark adapted; DD, disease duration; EDSS, expanded disability status scale; LA, light adapted.
(dark- and light-adapted 3.0 b-wave peak times). For each model, the intercept is the expected value with all predictors as zero, with the estimate quantifying the expected change to the intercept when increasing EDSS by 1.0, DD by 1 year, or changing ON history and treatment status from no to yes, respectively, with all other factors remaining constant.
Figure 3.
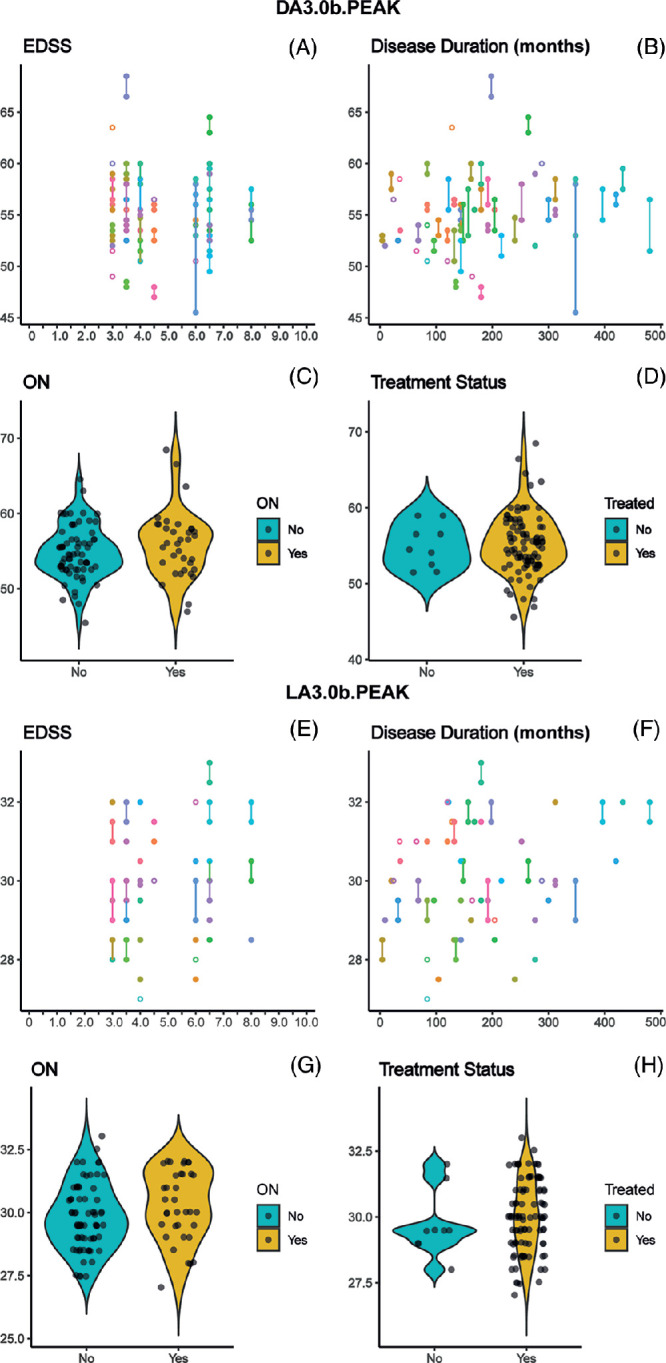
(A–H) ERG peak times plotted against EDSS (A, E), disease duration (B, F), history of ON (C, G), and binarized treatment status (D, h). Peak times selected for display and analysis were the dark (A–d) and light adapted (E–H) 3.0 single flash responses, as discussed in the Statistical Analysis subsection. On the scatterplots (A, B, E, F), data points from both eyes of individual participants are connected by a line; in the violin plots (C, D, G, H), individual data points have been horizontally jittered for clarity. DA, dark adapted; LA, light adapted.
Figure 4.
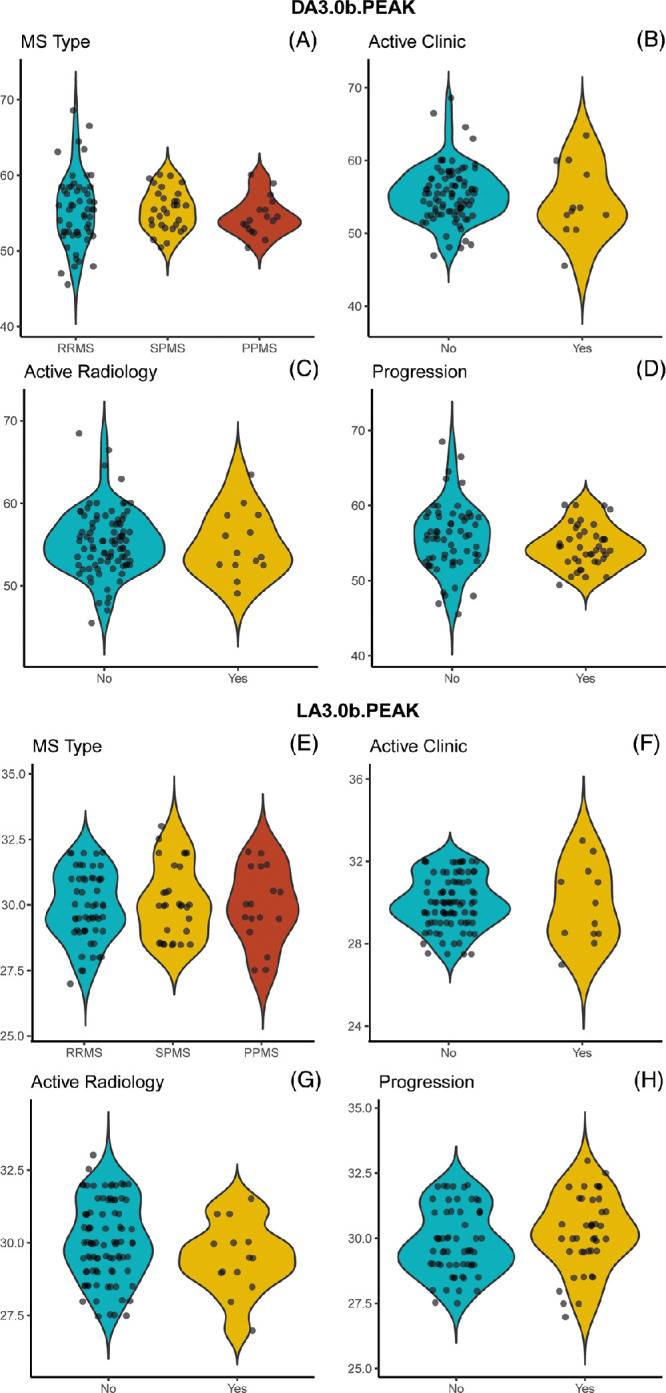
(A–H) ERG peak times plotted against multiple sclerosis subtype (A, e) And Disease activity (clinically active, radiologically active, with evidence of progression, B–D and F–H, respectively). Peak times selected for display and analysis were the dark (A–D) and light adapted (E–H) 3.0 single flash responses, as discussed in the Statistical Analysis subsection. Data points are horizontally jittered for clarity. DA, dark adapted; LA, light adapted.
To further mitigate against possible confounding effects of PPMS (which is distinct phenotypically from RRMS and SPMS) on our analyses, we reanalyzed the effects of EDSS, DD, history of ON, and treatment status on both DA3.0 and LA3.0 b-wave peak times after excluding all PPMS eyes. The reanalyses revealed no evidence of any relationships between these MS parameters and the relevant ERG outcomes in RRMS and SPMS eyes (all corrected P values were 0.89–0.99; data not shown).
The robustness of the data was probed using a sensitivity analysis, as described in the Supplementary Results.
Discussion
We document here, for the first time, evidence of presumed retinal bipolar cell dysfunction as measured with the ISCEV standard full-field ERG37 in individuals with moderate to severe MS, evidenced by delays to DA3.0 b-wave and LA flicker peak times in both MS +ON and MS −ON eyes relative to HC eyes. Qualitatively, these subtle changes seem to be similar to those described in participants with early and/or mild MS,11,13–17 although some previous authors found delays only to cone-isolating single flash stimuli,12 a condition in which we did not observe any evidence of abnormality in the present study. We did not observe any evidence of difference in ERG amplitudes between MS +ON, MS –ON, and HC eyes, a finding also compatible with previous literature studying early/mild MS. These earlier studies described normal amplitudes for most of the different ERG conditions, with occasional either subnormal or supernormal amplitudes for individual conditions,11–14,16,17,47–49 as summarized in Supplementary Table S1. In combination with these previous findings, our study thus confirms that ERG amplitudes are unlikely to be useful in the study of MS. Other authors have documented delayed cone-driven ERGs of normal amplitude in diseases such as AMD50 and early stage dominant RP,51 and flicker ERG is much more likely to be delayed than reduced in participants with birdshot chorioretinopathy.52 This dissociation between full-field ERG peak times and amplitudes recorded in other diseases therefore seems to be also present in pwMS. The insensitivity of peak times to variations in DTL electrode placement53 and typically narrower normative ranges of peak times relative to amplitudes may enable detection of subtle intergroup differences, as in the present study.
Presumed bipolar dysfunction in the eyes of pwMS seems to affect the cone system preferentially, as evidenced in our study by delayed flicker (entirely cone driven) and DA3.0 b-wave (partly cone driven) responses and in previously studied participants with early or mild MS.11–14,16,17 One potentially contributory factor to this cone system preponderance was recently documented by McIlwaine et al.,54 who measured decreased density of cone photoreceptors in both MS +ON and MS −ON eyes using adaptive optics imaging. Analysis of rod and bipolar cell densities would be of great value in further elucidating the physiological substrates of retinal dysfunction in MS; however, we are not aware that current hardware and software platforms permit such imaging in vivo in humans. Additionally, we speculate that the typically narrower, more sharply defined peaks in cone- relative to rod-driven ERGs may enable more precise quantification of ERG parameters, and thus enable the detection of smaller intergroup differences.
We confirmed that previous ON did not exert a measurable influence on the ERG parameters analyzed (Table 5), as shown previously in individuals with mild or early MS.11 Recent findings by Ziccardi et al.55 and previous preliminary observations by our group56 are, however, consistent with functional impairment of the preganglionic retina at the central macular region, as measured with the multifocal ERG, in pwMS who exhibited poor visual recovery after ON. The full-field ERG used in the present study receives minimal contribution from the central macular region57 and so we cannot compare our findings with this previous work directly.55,56 We propose that any preganglionic retrograde effects of ON are likely localized to the macular region and do not affect global retinal function.
Disease severity, as measured by EDSS, also did not exert a measurable influence on the ERG in our cohort. Other authors13 previously recorded a positive correlation between EDSS and LA3.0 b-wave (but not DA3.0 b-wave) peak time; however, their study cohort was made up of individuals with relatively mild disease, as evidenced by their median EDSS of 1.0. Using a different study design and analysis method, we have recorded previously that longitudinal binarized increases in EDSS (i.e., increase/no increase) are associated with prolongation of the DA3.0 b-wave peak time in an early or mild MS cohort with median EDSS 1.0.21 Therefore, although ERG may be associated with clinical disability early in the MS disease course, the lack of correlation in patients with more advanced disease limits potential usefulness in pwMS. Previous authors13 also recorded no statistically significant effects of disease duration on multiple ERG parameters in their early MS cohort, a finding confirmed in the present study despite the apparent positive correlation visible on inspection of Figures 3B, 3F. We hypothesize that disease duration is likely to be correlated positively with age, rendering it more challenging to distinguish effects of disease duration after adjustment of the GEE models for age. Our exploratory analyses were not consistent with any effects of MS phenotype or clinical status on our selected ERG parameters, however the small size of some subgroups (e.g., <12% of analyzed MS eyes were classed as clinically active) likely decreased the potential power of these analyses. Regardless, the lack of measurable influence of EDSS, disease activity, duration, subtype, and treatment on ERG outcome measures suggests that, despite now abundant findings of outer retinal dysfunction,11–17 on current evidence ERG is unlikely to play a useful role in assessing the disease course of MS. This result may be due, at least in part, to the relatively mild nature of the retinal dysfunction described here. Larger studies would be required to uncover potentially subtle relationships with MS clinical markers.
Strengths and Limitations
Strengths of our study include the large number of pwMS with significant disability (up to EDSS 8.0) and our HC cohort of comparable age and sex. Our use of GEE, permitting analysis of both eyes, maximized the sample size and, therefore, the power of our analyses. Inclusion of OCT, HCVA, and LCVA enabled us to categorize MS eyes as MS +ON or MS −ON as accurately as possible, which was vital, given that some participants had a disease duration of decades and that records of older clinical relapses that did not take place at our center were occasionally incomplete.
With regard to potential limitations, before data collection we selected LA3.0 b-wave peak time as a parameter for detailed analysis based on the results of previous work from our group and others, which led us to expect that this would likely be one of any abnormal ERG variables recorded. However, after data analysis was complete, we found no evidence of a difference in this parameter between the MS +ON and MS −ON and HC eyes. It is, therefore, possible that flicker ERG peak time may have been a more useful outcome measure to study in detail; however, we maintained compliance with our preplanned analysis to avoid potential post hoc bias.58 By the time data collection had begun, ocrelizumab had been approved by local regulators59 for use in RRMS and PPMS. As a consequence, only nine eyes were analyzed from untreated participants (whereas previously all individuals with PPMS would have been untreated) and the lack of measurable effect of treatment status on ERG variables should be appraised cautiously owing to the small size of the untreated group. Finally, the clinical relevance of the relatively subtle functional changes documented in our cohort is currently unknown.
In conclusion, we have documented, for the first time, the effects of moderate to severe MS on presumed retinal bipolar cell function, not ascribable to previous ON. However, the findings do not seem to be unambiguously distinct from those previously documented in individuals with earlier or milder MS, and seem to be unaffected by MS phenotype, duration, treatment status, disease status, and severity. It, therefore, appears that bipolar dysfunction, although common (or even ubiquitous) in pwMS, is unlikely to play any useful role as a marker of disease severity and course. ERG b-wave and/or flicker peak times may, however, have potential usefulness as a diagnostic adjunct.
Supplementary Material
Acknowledgments
The authors thank Alice Hakim-Brunner for assistance with participant recruitment.
Supported by the Clinical Research Priority Program Multiple Sclerosis (CRPP MS) of the University of Zurich. JVMH is funded by the Bruppacher Stiftung. All other authors report no external funding related to the study.
This work was previously presented in part at the 59th symposium of the International Society for Clinical Electrophyisology of Vision, Liverpool, 2022.
Disclosure: J.V.M. Hanson, None; S. Single, None; R.B. Eberle, None; V. Kana, Biogen (R), Roche (R), Novartis (R), Merck (R), Behring (R); B.V. Ineichen, Behring (R); C. Gerth-Kahlert, None
References
- 1. Kingwell E, Marriott JJ, Jette N, et al.. Incidence and prevalence of multiple sclerosis in Europe: a systematic review. BMC Neurol. 2013; 13: 128. Accessed Sept 28, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jakimovski D, Bittner S, Zivadinov R, et al.. Multiple sclerosis. Lancet. 2024; 403: 183–202. Accessed Nov 07, 2023. [DOI] [PubMed] [Google Scholar]
- 3. Thompson AJ, Banwell BL, Barkhof F, et al.. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018; 17: 162–173. Accessed 26 Dec, 2017. [DOI] [PubMed] [Google Scholar]
- 4. Lublin FD, Reingold SC, National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Defining the clinical course of multiple sclerosis. Results of an international survey. Neurology. 1996; 46: 907–911. [DOI] [PubMed] [Google Scholar]
- 5. Lublin FD, Reingold SC, Cohen JA, et al.. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014; 83: 278–286. Accessed 30 May, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Toosy AT, Mason DF, Miller DH.. Optic neuritis. Lancet Neurol. 2014; 13: 83–99. [DOI] [PubMed] [Google Scholar]
- 7. Ikuta F, Zimmerman HM.. Distribution of plaques in seventy autopsy cases of multiple sclerosis in the United States. Neurology. 1976; 26: 26–28. Accessed 01 Jun, 1976. [DOI] [PubMed] [Google Scholar]
- 8. Petzold A, Balcer LJ, Calabresi PA, et al.. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017; 16: 797–812. Accessed 19 Sept, 2017. [DOI] [PubMed] [Google Scholar]
- 9. Wicki CA, Manogaran P, Simic T, et al.. Bilateral retinal pathology following a first-ever clinical episode of autoimmune optic neuritis. Neurol Neuroimmunol Neuroinflamm. 2020; 7: e671. Accessed 01 Jan, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hokazono K, Raza AS, Oyamada MK, et al.. Pattern electroretinogram in neuromyelitis optica and multiple sclerosis with or without optic neuritis and its correlation with FD-OCT and perimetry. Doc Ophthalmol. 2013; 127: 201–215. [DOI] [PubMed] [Google Scholar]
- 11. Hanson JVM, Hediger M, Manogaran P, et al.. Outer retinal dysfunction in the absence of structural abnormalities in multiple sclerosis. Invest Ophthalmol Vis Sci. 2018; 59: 549–560. Accessed 01 Jan, 2018. [DOI] [PubMed] [Google Scholar]
- 12. Sriram P, Wang C, Yiannikas C, et al.. Relationship between optical coherence tomography and electrophysiology of the visual pathway in non-optic neuritis eyes of multiple sclerosis patients. PLoS One. 2014; 9: e102546. Accessed 29 Aug, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. You Y, Graham EC, Shen T, et al.. Progressive inner nuclear layer dysfunction in non-optic neuritis eyes in MS. Neurol Neuroimmunol Neuroinflamm. 2018; 5: e427. Accessed 21 Dec, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forooghian F, Sproule M, Westall C, et al.. Electroretinographic abnormalities in multiple sclerosis: possible role for retinal autoantibodies. Doc Ophthalmol. 2006; 113: 123–132. [DOI] [PubMed] [Google Scholar]
- 15. Gundogan FC, Demirkaya S, Sobaci G.. Is optical coherence tomography really a new biomarker candidate in multiple sclerosis? A structural and functional evaluation. Invest Ophthalmol Vis Sci. 2007; 48: 5773–5781. Accessed 07 Dec, 2007. [DOI] [PubMed] [Google Scholar]
- 16. Forooghian F, Adamus G, Sproule M, et al.. Enolase autoantibodies and retinal function in multiple sclerosis patients. Graefes Arch Clin Exp Ophthalmol. 2007; 245: 1077–1084. Accessed 16 Jan, 2007. [DOI] [PubMed] [Google Scholar]
- 17. You Y, Zhu L, Zhang T, et al.. Evidence of muller glial dysfunction in patients with aquaporin-4 immunoglobulin g-positive neuromyelitis optica spectrum disorder. Ophthalmology. 2019; 126: 801–810. Accessed 04 Feb, 2019. [DOI] [PubMed] [Google Scholar]
- 18. Frishman LJ. Electrogenesis of the Electroretinogram. In: Schachat AP, Wilkinson CP, Hinton DR, et al. (eds). Ryan's Retina. 6th ed. New York: Elsevier; 2018: 224–248. [Google Scholar]
- 19. Sieving PA, Murayama K, Naarendorp F.. Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci. 1994; 11: 519–532. [DOI] [PubMed] [Google Scholar]
- 20. Bhatt Y, Hunt DM, Carvalho LS.. The origins of the full-field flash electroretinogram b-wave. Front Mol Neurosci. 2023; 16: 1153934. Accessed 03 Jul, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hanson JVM, Ng M-Y, Hayward-Koennecke HK, et al.. A three-year longitudinal study of retinal function and structure in patients with multiple sclerosis. Doc Ophthalmol. 2022; 144: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boquete L, López-Guillén E, Vilades E, et al.. Diagnostic ability of multifocal electroretinogram in early multiple sclerosis using a new signal analysis method. PLoS One. 2019; 14: e0224500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Santiago L, Ortiz Del Castillo M, Garcia-Martin E, et al.. Empirical mode decomposition-based filter applied to multifocal electroretinograms in multiple sclerosis diagnosis. Sensors (Basel, Switzerland). 2019; 20: 7. Accessed 22 Dec, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. López-Dorado A, Pérez J, Rodrigo MJ, et al.. Diagnosis of multiple sclerosis using multifocal ERG data feature fusion. Inf Fusion. 2021; 76: 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Filgueiras TG, Oyamada MK, Preti RC, et al.. Outer retinal dysfunction on multifocal electroretinography may help differentiating multiple sclerosis from neuromyelitis optica spectrum disorder. Front Neurol. 2019; 10: 928. Original Research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanson JVM, Schippling S, Gerth-Kahlert C.. Commentary: outer retinal dysfunction on multifocal electroretinography may help differentiating multiple sclerosis from neuromyelitis optica spectrum disorder. Front Neurol. 2020; 11: 282. General Commentary. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weleber RG, Watzke RC, Shults WT, et al.. Clinical and electrophysiologic characterization of paraneoplastic and autoimmune retinopathies associated with antienolase antibodies. Am J Ophthalmol. 2005; 139: 780–794. [DOI] [PubMed] [Google Scholar]
- 28. Ohta K, Kikuchi T, Yoshida N.. Slowly progressive non-neoplastic autoimmune-like retinopathy. Graefes Arch Clin Exp Ophthalmol. 2011; 249: 155–158. Accessed 14 Jul, 2010. [DOI] [PubMed] [Google Scholar]
- 29. Zacks DN, Samson CM, Loewenstein J, Foster CS.. Electroretinograms as an indicator of disease activity in birdshot retinochoroidopathy. Graefes Arch Clin Exp Ophthalmol. 2002; 240: 601–607. Accessed 23 Aug, 2002. [DOI] [PubMed] [Google Scholar]
- 30. Uludag G, Onal S, Arf S, et al.. Electroretinographic improvement after rituximab therapy in a patient with autoimmune retinopathy. Am J Ophthalmol Case Rep. 2016; 2: 4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holder GE, Robson AG, Pavesio C, Graham EM.. Electrophysiological characterisation and monitoring in the management of birdshot chorioretinopathy. Br J Ophthalmol. 2005; 89: 709–718. Accessed 01 Jun, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin H, Dao D, Sen HN.. Diagnosis and treatment of autoimmune retinopathy. Ann Eye Sci. 2020; 5: 27. [Google Scholar]
- 33. Cree BAC, Hollenbach JA, Bove R, et al.. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. 2019; 85: 653–666. Accessed 30 Mar, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nolan RC, Galetta SL, Frohman TC, et al.. Optimal intereye difference thresholds in retinal nerve fiber layer thickness for predicting a unilateral optic nerve lesion in multiple sclerosis. J Neuroophthalmol. 2018;38:451–458.. Accessed Feb 01, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fisher JB, Jacobs DA, Markowitz CE, et al.. Relation of visual function to retinal nerve fiber layer thickness in multiple sclerosis. Ophthalmology. 2006; 113: 324–332. [DOI] [PubMed] [Google Scholar]
- 36. Balcer LJ, Raynowska J, Nolan R, et al.. Validity of low-contrast letter acuity as a visual performance outcome measure for multiple sclerosis. Mult Scler. 2017; 23: 734–747. Accessed 17 Feb, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McCulloch DL, Marmor MF, Brigell MG, et al.. ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015; 130: 1–12. [DOI] [PubMed] [Google Scholar]
- 38. Marmor MF, Arden GB, Nilsson SEG, Zrenner E.. Standard for clinical electroretinography: International Standardization Committee. Archives of Ophthalmology. 1989; 107: 816–819. [DOI] [PubMed] [Google Scholar]
- 39. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 40. Stekhoven DJ, Bühlmann P.. MissForest–non-parametric missing value imputation for mixed-type data. Bioinformatics. 2012; 28: 112–118. Accessed 28 Oct, 2011. [DOI] [PubMed] [Google Scholar]
- 41. Liang K-Y, Zeger SL.. Longitudinal data analysis using generalized linear models. Biometrika. 1986; 73: 13–22. [Google Scholar]
- 42. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B Methodol. 1995; 57: 289–300. [Google Scholar]
- 43. Bland M. An Introduction to Medical Statistics. 4th ed. Oxford, UK: Oxford University Press; 2015. [Google Scholar]
- 44. R Core Team. R: A Language and Environment for Statistical Computing. 4.2.2 ed. Vienna, Austria: R Foundation for Statistical Computing; 2022. [Google Scholar]
- 45. Saidha S, Syc SB, Ibrahim MA, et al.. Primary retinal pathology in multiple sclerosis as detected by optical coherence tomography. Brain. 2011; 134: 518–533. [DOI] [PubMed] [Google Scholar]
- 46. Jiang X, Mahroo OA.. Negative electroretinograms: genetic and acquired causes, diagnostic approaches and physiological insights. Eye. 2021; 35: 2419–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feinsod M, Abramsky O, Auerbach E.. Electrophysiological examinations of the visual system in multiple sclerosis. J Neurol Sci. 1973; 20: 161–175. [DOI] [PubMed] [Google Scholar]
- 48. Feinsod M, Rowe H, Auerbach E. Changes in the electroretinogram in patients with optic nerve lesions. Doc Ophthalmol. 1971; 29: 169–200. Accessed 14 May, 1971. [DOI] [PubMed] [Google Scholar]
- 49. Fraser CL, Holder GE.. Electroretinogram findings in unilateral optic neuritis. Doc Ophthalmol. 2011; 123: 173–178. [DOI] [PubMed] [Google Scholar]
- 50. Forshaw TRJ, Kjaer TW, Andréasson S, Sørensen TL. Full-field electroretinography in age-related macular degeneration: an overall retinal response. Acta Ophthalmol. 2021; 99: e253–e259. Accessed 24 Aug, 2020. [DOI] [PubMed] [Google Scholar]
- 51. Berson EL, Gouras P, Hoff M.. Temporal aspects of the electroretinogram. Arch Ophthalmol. 1969; 81: 207–214. [DOI] [PubMed] [Google Scholar]
- 52. Sobrin L, Lam BL, Liu M, et al.. Electroretinographic monitoring in birdshot chorioretinopathy. Am J Ophthalmol. 2005; 140: 52–64. Accessed 26 Jul, 2005. [DOI] [PubMed] [Google Scholar]
- 53. Brouwer AH, de Wit GC, de Boer JH, van Genderen MM.. Effects of DTL electrode position on the amplitude and implicit time of the electroretinogram. Doc Ophthalmol. 2020; 140: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McIlwaine G, Csincsik L, Coey R, et al.. Reduced cone density is associated with multiple sclerosis. Ophthal Sci. 2023; 3: 100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ziccardi L, Barbano L, Boffa L, et al.. Functional assessment of outer and middle macular layers in multiple sclerosis. J Clin Med. 2020; 9: 3766. Accessed 04 Dec, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hanson JVM, Lukas S, Landau K, et al.. Evidence of functional retinal impairment in the absence of sustained structural damage following MS-related optic neuritis. Multiple Sclerosis Journal. 2015; 21: 509–510. [Google Scholar]
- 57. Robson AG, Frishman LJ, Grigg J, et al.. ISCEV Standard for full-field clinical electroretinography (2022 update). Doc Ophthalmol. 2022; 144: 165–177. Accessed 05 May, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Munafò MR, Nosek BA, Bishop DVM, et al.. A manifesto for reproducible science. Nat Human Behav. 2017; 1: 0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Swissmedic. Ocrevus, Konzentrat zur Herstellung einer Infusionslösung (Ocrelizumabum). Available at: https://www.swissmedic.ch/swissmedic/en/home/humanarzneimittel/authorisations/new-medicines/ocrevus_konzentrat_zur_herstellung_einer_infusionsloesung_ocrelizumabum.html. 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.