Abstract
Mitochondrial dynamics orchestrate many essential cellular functions, including metabolism, which is instrumental in promoting cancer growth and metastatic progression. However, how mitochondrial dynamics influences metastatic progression remains poorly understood. Here, we show that breast cancer cells with low metastatic potential exhibit a more fused mitochondrial network compared to highly metastatic cells. To study the impact of mitochondrial dynamics on metastasis, we promoted mitochondrial elongation in metastatic breast cancer cells by individual genetic deletion of three key regulators of mitochondrial fission (Drp1, Fis1, Mff) or by pharmacological intervention with leflunomide. Omics analyses revealed that mitochondrial elongation causes substantial alterations in metabolic pathways and processes related to cell adhesion. In vivo, enhanced mitochondrial elongation by loss of mitochondrial fission mediators or treatment with leflunomide notably reduced metastasis formation. Furthermore, the transcriptomic signature associated with elongated mitochondria correlated with improved clinical outcome in patients with breast cancer. Overall, our findings highlight mitochondrial dynamics as a potential therapeutic target in breast cancer.
Mitochondrial dynamics emerges as a therapeutic target in breast cancer metastasis.
INTRODUCTION
Metastasis is the leading cause of mortality in patients with breast cancer and remains largely incurable. Cancer cells leaving the primary site must surmount a number of obstacles to successfully colonize distant organs. Changes in energy metabolism, such as increased oxidative phosphorylation (OXPHOS), can confer enhanced bioenergetic plasticity to disseminating cancer cells, allowing them to adapt to rapidly changing conditions in their environment, thus contributing to metastatic progression (1–4).
Mitochondria do not function as static isolated organelles. Rather, they are constantly undergoing cycles of fission and fusion, a phenomenon known as mitochondrial dynamics. The balance between fission and fusion events determines mitochondrial network architecture and plays a role in regulating several processes, including bioenergetic adaptation to nutrient availability (5), apoptosis (6), proliferation (7), and autophagy (8). Furthermore, recent findings point toward a link between mitochondrial dynamics and the cellular sensing and transduction of external mechanical forces, such as stiffness of the surrounding extracellular matrix (ECM). ECM stiffness influences cell proliferation, differentiation, migration, and death (9). In cancer, high stiffness of the primary tumor ECM promotes growth and invasiveness (9). However, breast cancer cells metastasize to organs with relatively low stiffness, such as brain, liver, and lungs (10). Recent work showed that low ECM stiffness promoted mitochondrial fission in metastatic breast cancer cells (10).
A growing body of evidence suggests that dysregulation of mitochondrial dynamics can fuel tumor growth. Mitochondrial fission proteins are up-regulated in tumors compared to normal tissues (11–14) and limiting mitochondrial fission by genetic (15, 16) or pharmacological inhibition of dynamin-1-like protein (Drp1) (15–17), or overexpression of mitochondrial fusion mediators (15, 17) resulted in reduced tumor growth in several cancer models. Mitochondrial fission is carried out by the GTPase (guanosine triphosphatase) Drp1, which is recruited by mitochondrial fission 1 protein (Fis1) or mitochondrial fission factor (Mff) to the mitochondrial surface, causing it to oligomerize and form rings around dividing mitochondria.
The role of mitochondrial dynamics in metastatic progression is less understood. Previous studies have investigated the individual contribution of mitochondrial fission proteins to metastatic disease (18, 19). Knockdown of Drp1 in brain-tropic latent breast cancer metastatic cells decreased the number of metastatic lesions in a mouse xenograft breast cancer model (18). Likewise, inhibition of Drp1 reduced metastatic burden in hepatocellular carcinoma (19). Furthermore, high expression of Fis1 in human gastric tumors was correlated with metastatic progression (12). A similar trend was reported in patients with prostate cancer, where Mff protein levels were elevated in primary tumors compared to normal tissue, and further increased in metastatic sites (20). Here, we took an integrative approach to study the role of mitochondrial fission in breast cancer metastasis by individually deleting three key proteins involved in mitochondrial fission and focusing our analyses on shared phenotypes among the three genetic cell models. We found that promoting mitochondrial elongation in breast cancer cells by deletion of Drp1, Fis1, or Mff drastically reduced metastasis in vivo, and this phenotype was linked to changes in citric acid cycle (CAC) and mechanosensing pathways. Treatment of breast cancer cells with the antirheumatic drug leflunomide (Leflu) (21) also promoted mitochondrial elongation and reduced lung metastasis in vivo, highlighting the translational potential of targeting mitochondrial dynamics in cancer.
RESULTS
Breast cancer cells with low metastatic potential display increased mitochondrial length
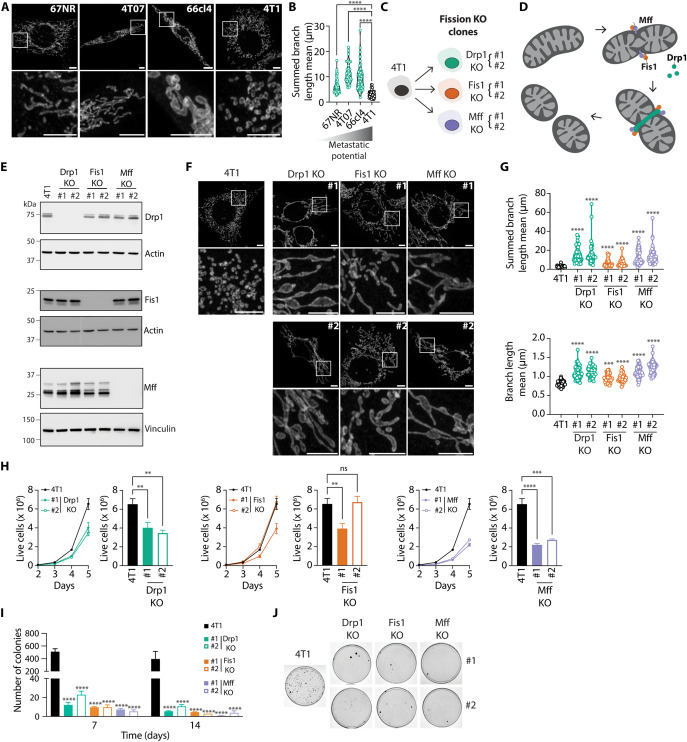
To gain insight into the relationship between mitochondrial dynamics and metastasis, we assessed the mitochondrial length of four cell lines derived from a single spontaneous murine mammary tumor, which display different metastatic potential (Fig. 1, A and B). 67NR and 4T07 cells are tumorigenic but nonmetastatic, whereas 66cl4 cells are weakly metastatic and can only metastasize to the lungs. Conversely, 4T1 cells are highly metastatic, being able to colonize lungs, liver, and bones (22). We observed that cell lines with nonexistent (67NR and 4T07) or low (66cl4) metastatic potential exhibited significantly longer mitochondria than the broadly metastatic cell line 4T1.
Fig. 1. Increased mitochondrial length is associated with low metastatic potential in breast cancer cells.
(A and B) Representative images (A, scale bars, 5 μm) and quantification of mitochondrial length (B) in Tom 20–stained 67NR, 4T07, 66cl4, and 4T1 breast cancer cells. In (B), each data point represents an individual cell (n = 34 to 81 per group). ****P < 0.0001 by Welch’s analysis of variance (ANOVA) and Dunnett’s T3 post-hoc test. (C) Schematic representation of the 4T1 KO cell lines generated for this study. Two independent clones (#1 and #2) were derived from each individual KO cell population (Drp1, Fis1, and Mff). (D) Schematic representation of mitochondrial fission highlighting the mitochondrial fission proteins Drp1, Fis1, and Mff. (E) Validation of KO cell lines by immunoblot. Representative images of three independent experiments. Actin or Vinculin served as loading controls. (F and G) Representative images (F, scale bars, 5 μm) and quantification of mitochondrial length (G) in Tom 20–stained parental, Drp1 KO, Fis1 KO, and Mff KO 4T1 breast cancer cells. In (G), each data point represents an individual cell (n = 33 to 42 per group). ****P < 0.0001 by Welch’s ANOVA and Dunnett’s T3 post-hoc test. (H) Live cell counts of parental versus Drp1 KO, Fis1 KO, and Mff KO 4T1 breast cancer cells over the course of 5 days. Bar graphs depict live cell counts on day 5. Results represented as mean ± SEM of three independent experiments. ****P < 0.0001, ***P < 0.001, **P < 0.01 by one-way ANOVA and Dunnett’s post-hoc test. (I and J) Number of colonies (I) and representative images of colonies (J) observed in 4T1 parental versus Drp1 KO, Fis1 KO, and Mff KO clones after culture in ultralow attachment plates for 7 (I) and 14 (I and J) days. In (I), results are represented as mean ± SEM of five (7 days) or three (14 days) independent experiments. ****P < 0.0001 by two-way ANOVA and Dunnett’s post-hoc test. ns, nonsignificant.
The link between mitochondrial length and metastatic potential (Fig. 1, A and B) led us to hypothesize that increased mitochondrial elongation may limit the metastatic capacity of breast cancer cells. To test this idea, we generated two independent CRISPR-Cas9 knockout (KO) clones using the highly metastatic triple-negative breast cancer (TNBC) cell line 4T1 for each of the three key proteins involved in mitochondrial fission: Drp1, Fis1, and Mff (fission KO, Fig. 1C). Mitochondrial fission is predominantly orchestrated by Drp1, which is recruited from the cytosol to the outer mitochondrial membrane by different adaptor proteins, including Fis1 and Mff (23) (Fig. 1D). Each adaptor protein can mediate the recruitment of Drp1 in an independent fashion (23). Once at the mitochondrial surface, Drp1 oligomerizes and forms a ring that wraps around and constricts mitochondria (24). Immunoblot analyses confirmed the individual deletion of Drp1, Fis1, and Mff in all of the independent KO clones generated for each target (Fig. 1E). Imaging of mitochondrial morphology revealed that all fission KO cell lines exhibited drastically enhanced mitochondrial elongation as evidenced by increased mitochondrial branch length (Fig. 1, F and G).
All fission KO cells, except for Fis1 KO clone #2, displayed reduced cell counts in vitro when compared to parental 4T1 cells (Fig. 1H). However, cell viability was not affected in any of the KO clones (fig. S1A), suggesting that the decrease in cell counts observed in the fission KO cells is due to lower proliferation rates. To effectively colonize distant organs, cancer cells must be able to survive in circulation and resist death by anoikis. Therefore, we evaluated the ability of fission KO cells to survive in culture in ultralow attachment plates. Most KO cell lines showed significantly more cell death than parental 4T1 cells after 7 days of culture in ultralow attachment plates (fig. S1B). To study their ability to reattach to substrates after culture in anoikis-promoting conditions, we replated cells grown on ultralow attachment plates on regular cell culture dishes. The number of colonies formed by all fission KO cells was significantly lower than parental 4T1 cells after 7 and 14 days of culture under anoikis-promoting conditions (Fig. 1, I and J, and fig. S1C). Overall, these findings indicate that limiting mitochondrial fission renders breast cancer cells more sensitive to anoikis in vitro.
Transcriptomics and metabolomics analyses uncover changes in cell adhesion and CAC in mitochondrial fission KO cells
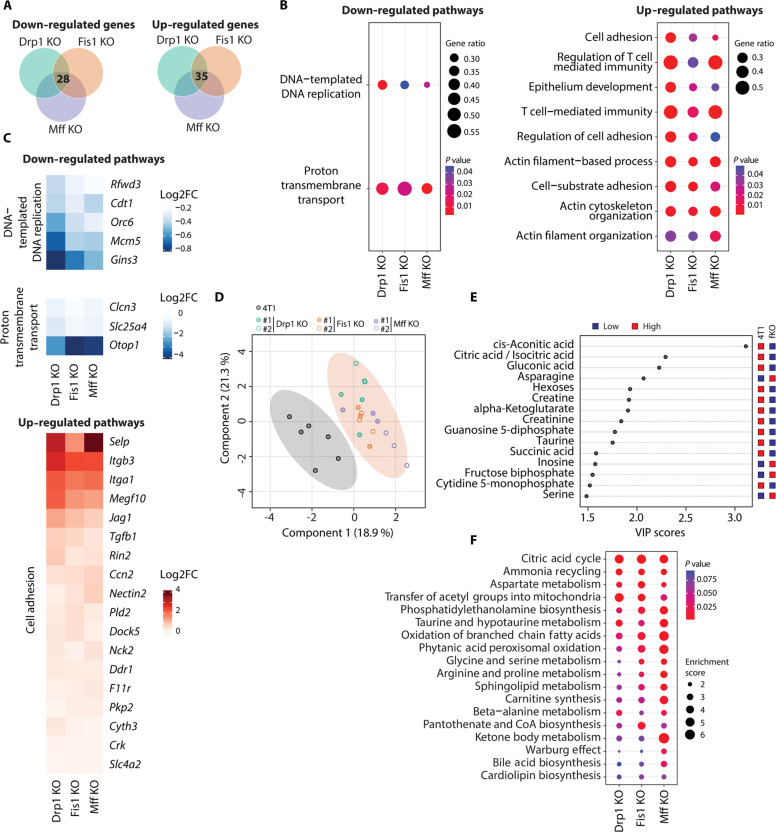
We then sought to identify shared mechanisms across all fission KO cell lines that might account for their common phenotypes. First, we performed global transcriptomics analyses of all fission KO cell lines (Drp1 KO, Fis1 KO, and Mff KO) and focused on identifying transcripts and associated pathways displaying common changes when compared with parental 4T1 cells. Differential expression analysis revealed 28 down-regulated and 35 up-regulated genes, which were common to all fission KO cell lines with a fold change >1.5 relative to parental 4T1 cells (Fig. 2A and fig. S2). Next, we conducted gene set enrichment analyses (GSEAs) for each KO versus parental and compared results to discover altered pathways present in all conditions. In agreement with the decrease in proliferation we observed in the KO cell lines, our analyses uncovered “DNA-templated DNA replication” as one of the down-regulated pathways common to all fission KO cells (Fig. 2B, left). “Proton transmembrane transport” was also down-regulated in all the fission KO cell lines (Fig. 2B, left). Genes driving this signature included Clcn3 and Slc25a4 (Fig. 2C, middle), both of which have been implicated in promoting metastasis. Clcn3 encodes a voltage-gated chloride channel reported to promote cancer metastasis by inducing membrane ruffle formation (25). Slc25a4, a mitochondrial ADP/ATP transporter, was found to be up-regulated in distant metastasis from patients with TNBC (26). Down-regulation of Clcn3 and Slc25a4 in fission KO cells was confirmed by quantitative polymerase chain reaction (qPCR) analysis (fig. S3A, top and middle). In contrast, up-regulated pathways were predominantly linked to cell adhesion and cytoskeleton organization (Fig. 2B, right). Discoidin domain receptor 1 (Ddr1), a gene recently implicated in facilitating cell adhesion and limiting cancer cell invasiveness (27), was up-regulated in all fission KO cells (Fig. 2C, bottom). We confirmed that Ddr1 expression was induced in fission KO cells at mRNA (fig. S3A, bottom) and protein (fig. S3, B and C) levels. To evaluate changes in cell adhesion and cytoskeletal organization, cells were seeded on ECM-coated coverslips and evaluated through immunofluorescence of adhesion marker paxillin and cytoskeletal marker phalloidin (fig. S4). Consistent with Ddr1 promoting adhesion to collagen, we observed a mild increase in the number of cell adhesions in most KO cells when they were grown on collagen coated coverslips (fig. S4E) compared to a more muted increase when cells were grown on fibronectin (fig. S4A). Adhesion area and cell aspect ratio were unchanged (fig. S4, B, C, F and G).
Fig. 2. Transcriptomic and metabolomic signatures of mitochondrial elongation in breast cancer cells.
(A) Venn diagrams depicting common down-regulated and up-regulated transcripts in all KO cell lines versus parental 4T1 breast cancer cells that were identified by DESeq analysis of RNA-seq data (n = 3), |FC| > 1.5 and P adjusted value <0.05. (B) Dot plots of common down-regulated and up-regulated pathways in all KO cell lines versus parental 4T1 breast cancer cells, according to GSEA. GeneRatio is calculated as follows: count of core enrichment genes/count of genes present in the indicated pathway. (C) Heatmaps showing common affected genes in all KO cell lines versus parental 4T1 breast cancer cells from selected pathways in (B). (D) PLS-DA of metabolite profile data between parental and fission KO 4T1 cell lines. All KO cell lines were grouped into one category (fKO), contained in the light shaded orange circle. Each data point represents one sample (n = 6 for parental 4T1, n = 3 for each KO clone). (E) VIP scores of top 15 metabolites. All fission KO cell lines were grouped into one category (fKO) and compared to parental 4T1 breast cancer cells. (F) Dot plots of common altered metabolic pathways in all KO cell lines versus parental 4T1 breast cancer cells, according to MSEA.
Mitochondrial dynamics can influence mitochondrial metabolism; thus, we conducted metabolomic analyses to probe for the consequences of abrogated mitochondrial fission in 4T1 breast cancer cells. To focus on the similarities between the three fission KO models (Drp1 KO, Fis1 KO, and Mff KO), we performed a partial least squares discriminant analysis (PLS-DA) of the three KO groups compared with parental 4T1 cells (Fig. 2D). We detected substantial differences between the cell models with elongated mitochondria and the parental control cells. Variable importance in projection (VIP) scores demonstrated that many of the key metabolites responsible for the separation between 4T1 cells with elongated mitochondria and the control 4T1 cells were involved in the CAC (Fig. 2E). In agreement with these findings, metabolite set enrichment analysis (MSEA) yielded CAC as the top altered metabolic pathway in cells with impaired mitochondrial fission (Fig. 2F). Furthermore, many of the affected pathways in fission KO cells identified by MSEA were related to fatty acid oxidation (FAO, oxidation of branched chain fatty acids, phytanic and peroxisomal oxidation, and carnitine synthesis; Fig. 2F).
4T1 mitochondrial fission KO cells display reduced bioenergetic scope
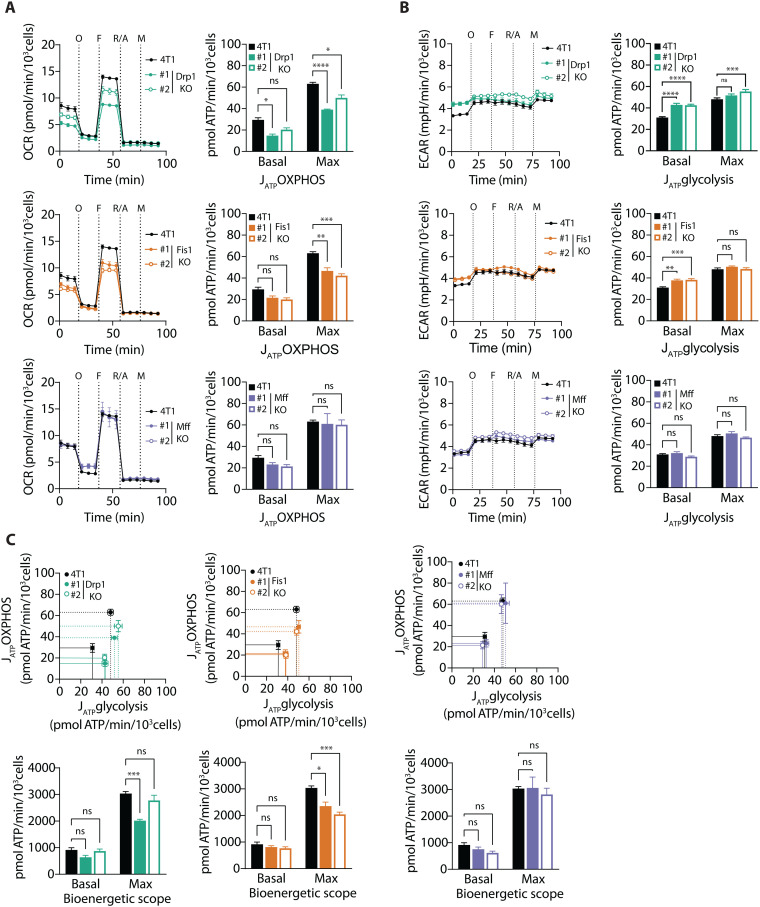
Because metabolomic analyses uncovered several alterations in the metabolic pathways of fission KO cells, with CAC being the most significant one, we sought to evaluate the bioenergetic profile of the fission KO cells by real-time extracellular flux analysis. In general, fission KO cells displayed lower OXPHOS-driven ATP production than parental 4T1 cells, although this was more pronounced for the Drp1 and Fis1 KO cells (Fig. 3A, top and middle panels). We also detected an overall decrease in mitochondrial membrane potential in all fission KO cells, which was less prominent in Fis1 KO clone #2 (fig. S5A), as well as a slight decrease in mitochondrial–to–nuclear DNA ratio, suggesting a reduction in mitochondrial content (fig. S5B). The altered respiration phenotype was accompanied by an increase in glycolytic ATP production (Fig. 3B, top and middle panels). However, this was not sufficient to fully compensate for the decrease in OXPHOS-linked ATP production, resulting in a reduction in the maximal bioenergetic scope for Drp1 KO and Fis1 KO cells (Fig. 3C, top and middle panels). Mff KO cells displayed lower oligomycin-sensitive oxygen consumption rates (OCRs), compared with parental 4T1 cells (Fig. 3A, bottom left panel). Nevertheless, neither their glycolytic ATP production nor their maximal bioenergetic capacity showed significant differences compared with 4T1 controls (Fig. 3, B and C, bottom panels).
Fig. 3. Loss of mitochondrial fission mediators decreases bioenergetic scope of breast cancer cells.
(A) Left: Oxygen consumption rate (OCR) after injection of oligomycin (O), FCCP (F), rotenone/antimycin A (R/A) and monensin (M). Right: Rate of ATP production by oxidative phosphorylation (JATP OXPHOS) in Drp1 KO, Fis1 KO, and Mff KO clones versus parental 4T1 breast cancer cells. ****P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05 by one-way ANOVA and Dunnett’s post-hoc test. (B) Left: Extracellular acidification rate (ECAR) after injection of oligomycin (O), FCCP (F), rotenone/antimycin A (R/A), and monensin (M). Right: rate of ATP production by glycolysis (JATP glycolysis) in Drp1 KO, Fis1 KO, and Mff KO clones versus parental 4T1 breast cancer cells. ****P < 0.0001, ***P < 0.001, **P < 0.01, by one-way ANOVA and Dunnett’s post-hoc test. (C) Left: Bioenergetic space plots of Drp1 KO, Fis1 KO, and Mff KO clones versus parental 4T1 breast cancer cells. Data points with dashed lines depict maximal ATP production rates, whereas data points with solid lines depict ATP production rates under basal conditions. The areas delimited by the dashed and solid lines represent the maximal and basal bioenergetic scope, respectively. Right: Calculated bioenergetic scope values under basal and maximal conditions. ***P < 0.001, *P < 0.05 by two-way ANOVA and Dunnett’s post-hoc test. All results shown in this figure are depicted as mean ± SEM of four independent experiments. ns, nonsignificant.
Loss of mitochondrial fission mediators in breast cancer cells impairs metastasis formation in vivo
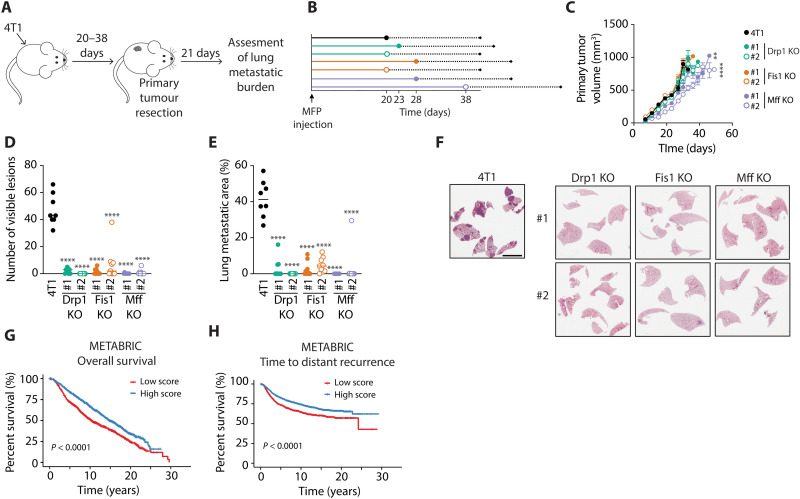
To determine whether impaired mitochondrial fission interferes with the metastatic ability of breast cancer cells in vivo, we first injected either 4T1 Drp1, Fis1, or Mff KO cells into the mammary fat pads of mice and tumors were allowed to reach a predefined volume before resection (Fig. 4, A and B). Lung metastatic burden was evaluated at end point. We did not detect any statistically significant differences in the growth rates of the fission KO mammary tumors when compared with parental 4T1 controls, except for the Mff KO clones, which exhibited a marginal reduction in tumor growth (Fig. 4C). Only Mff KO clone #2 took slightly longer to reach the resectable volume (Fig. 4B). Notably, the number of visible metastatic lesions and the total metastatic area in the lungs of mice bearing 4T1 fission KO tumors were markedly reduced compared with mice that developed parental 4T1 tumors (Fig. 4, D to F). Several mice bearing fission KO tumors did not develop any detectable metastatic lesions. Similar results were obtained by reducing Drp1 or Fis1 expression in 4T1 cells by stable shRNA-mediated knockdown (fig. S6, A to G). These results reinforce the conclusion that the observed effects on spontaneous lung metastasis are driven by the loss of Drp1 or Fis1, and not due to potential off-target effects of the CRISPR-Cas9 deletion. As an alternative approach, we performed experimental metastasis assays in which 4T1 fission KO cells were injected into the lateral tail veins of mice (fig. S6H). Administration of 4T1 fission KO cells resulted in decreased lung metastatic burden when compared to mice injected with parental 4T1 breast cancer cells (fig. S6, I and J). However, the effect observed in the experimental metastasis assay was less pronounced when compared to the spontaneous metastasis assay (Fig. 4, D to F).
Fig. 4. Loss of mitochondrial fission mediators in triple-negative breast cancer cells limits lung metastasis in an immunocompetent mouse model.
(A) Schematic depicting the experimental design of the spontaneous metastasis assays conducted to assess metastatic potential of parental versus Drp1 KO, Fis1 KO, and Mff KO 4T1 breast cancer cells in vivo. Cells were injected into the mammary fat pads (MFPs) of mice and tumor growth was assessed for 20 to 38 days. Tumors were resected when they reached 500 mm3. Twenty-one days after resection, lung metastatic burden was evaluated. (B) Diagram showing the median time required for each cell line to reach resection size. (C) Primary tumor growth of parental versus Drp1 KO, Fis1 KO, and Mff KO 4T1 breast cancer cells. Graph shows mean tumor volume over time ± SEM (n = 9 per group). ****P < 0.0001, **P < 0.01 by mixed-effects model and Dunnett’s posttest. (D and E) Number of visible lesions (D) and total metastatic lesion area (E) quantified in the lungs of mice bearing parental versus Drp1 KO, Fis1 KO, and Mff KO 4T1 tumors. Results are shown as individual data points, where each data point represents an individual mouse, and mean is depicted as a line (n = 8 for parental 4T1, n = 9 for all KOs). ****P < 0.0001, by one-way ANOVA and Dunnett’s post-hoc test. (F) Representative images of H&E-stained lungs from mice injected with parental versus Drp1 KO, Fis1 KO, and Mff KO 4T1 breast cancer cells. Scale bar, 4 mm. (G and H) Kaplan-Meier overall survival (G) and time to distant recurrence (H) analysis of patients from the METABRIC study. A gene expression signature associated with impaired mitochondrial fission was constructed using the genes identified in Fig. 2A. Patients were divided in two groups according to their gene signature score.
To assess the clinical relevance of these findings, we derived a fission KO gene expression signature using the differentially expressed genes common to all 4T1 fission KO models identified in the transcriptomic data (Fig. 2A and fig. S2). We then computed fission KO signature scores for all patients comprised in the METABRIC dataset (28–30), and the cohort was divided into two groups according to their scores. The high-scoring group exhibited significantly improved survival (Fig. 4G), in agreement with our data demonstrating that disrupting mitochondrial fission limits breast cancer metastasis and progression. We performed survival analyses by breast cancer subtype and discovered that high fission KO signature scores correlated with significantly improved outcome in most subtypes, including the basal-like and HER2-positive breast cancer subtypes (fig. S6K), which are commonly associated with poor prognosis. In addition, we analyzed the time to distant recurrence as a measure of metastatic progression. Patients within the high-scoring group displayed a significant delay in distant recurrence (Fig. 4H). Collectively, these results suggest that limited mitochondrial fission is associated with improved outcome in patients with breast cancer.
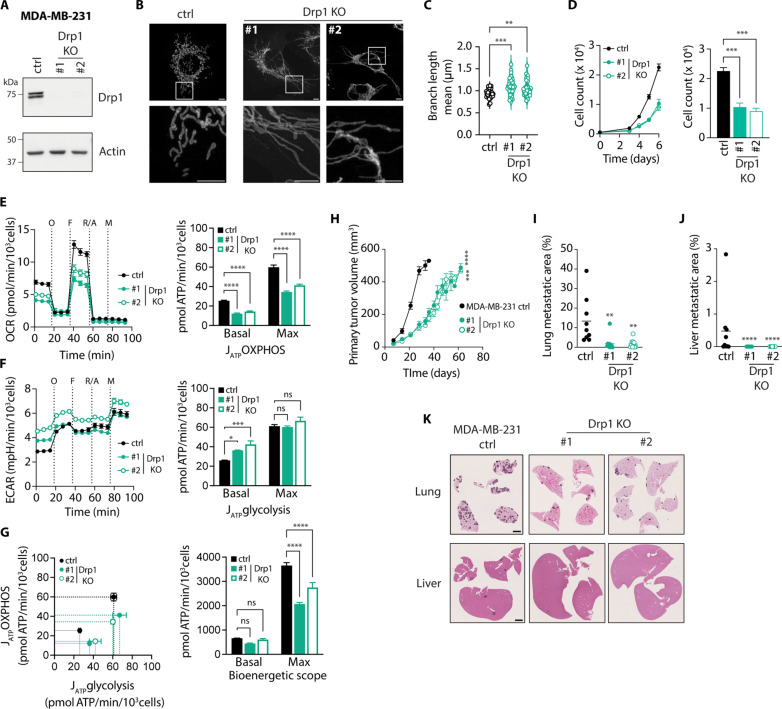
To further confirm that our findings can be transferred to other models of breast cancer metastasis, we generated Drp1 KO clones from the MDA-MB-231 human TNBC cell line (Fig. 5A). Consistent with our previous observations, MDA-MB-231 cells displayed increased mitochondrial length when Drp1 expression was eliminated (Fig. 5, B and C). Of note, the differences in mitochondrial branch length between Drp1 KO and control MDA-MB-231 cells were not as notable as in the 4T1 model, perhaps due to MDA-MB-231 breast cancer cells having a more fused mitochondrial network at baseline. Drp1 KO MDA-MB-231 cells showed considerably reduced proliferation rates in vitro compared with control cells (Fig. 5D). Deletion of Drp1 in MDA-MB-231 cells also affected respiration, resulting in lower ATP production from OXPHOS (Fig. 5E). In addition, Drp1 KO MDA-MB-231 cells displayed elevated basal glycolytic ATP production (Fig. 5F). However, maximal ATP production rates from glycolysis remained unchanged upon Drp1 deletion. Therefore, maximal bioenergetic scope in Drp1 KO was significantly reduced compared to control MDA-MB-231 cells (Fig. 5G), recapitulating the effects of Drp1 KO in 4T1 cells.
Fig. 5. Loss of mitochondrial fission mediators in human breast cancer cells impairs their proliferation, bioenergetic scope, and metastatic capacity.
(A) Representative immunoblot of MDA-MB-231 Drp1 KO cell lines showing Drp1 loss. Actin was used as loading control. (B and C) Representative images (B, scale bars, 5 μm) and quantification of mitochondrial length (C) in Tom 20–stained MDA-MB-231 cells. In (C), n = 24 to 45 per group. ***P < 0.001, **P < 0.01, by one-way ANOVA and Dunnett’s post-hoc test. (D) Left: Proliferation curve of MDA-MB-231 cells. Right: Day 6 cell counts. ***P < 0.001 by one-way ANOVA and Dunnett’s post-hoc test. (E) Oxygen consumption rate (OCR, left) and ATP production rate by OXPHOS (JATP OXPHOS, right) in MDA-MB-23 cells. (F) ECAR (left) and ATP production rate by glycolysis (JATP glycolysis, right) in MDA-MB-231 cells. ****P < 0.0001, ***P < 0.001, *P < 0.05 by one-way ANOVA and Dunnett’s post-hoc test (E and F). (G) Left: Bioenergetic space plots of MDA-MB-231 cells. Solid lines depict basal and dashed lines depict maximal ATP production rates. Areas delimited by solid and dashed lines represent basal and maximal bioenergetic scope, respectively. Right: Basal and maximal bioenergetic scope. ****P < 0.0001 by two-way ANOVA and Dunnett’s post-hoc test. (H) Growth curve of MDA-MB-231 tumors (n = 9 for ctrl and n = 10 for Drp1 KO). ****P < 0.0001, ***P < 0.001 by mixed-effects model and Dunnett’s post-hoc test. (I and J) Total metastatic lesion area in lungs (I) and liver (J) of mice bearing MDA-MB-231 tumors. Each data point represents an individual mouse, and mean is depicted as a line (n = 9 for ctrl, n = 10 for Drp1 KO). **P < 0.01 by one-way ANOVA and Dunnett’s post-hoc test (I), ****P < 0.0001 by Kruskal-Wallis and Dunn’s post-hoc test (J). (K) Representative images of H&E-stained lungs and livers from mice bearing MDA-MB-231 tumors. Scale bar, 2 mm. Unless indicated otherwise, all data are presented as mean ± SEM (N = 3). O, oligomycin; F, FCCP; R/A, rotenone/antimycin A; M, monensin; ns, nonsignificant.
Given that the rate of metastasis formation in the 4T1 spontaneous model is low in sites other than the lung, we decided to take advantage of the MDA-MB-231 Drp1 KO cells to evaluate how inhibition of mitochondrial fission impacts breast cancer metastatic dissemination to other organs. Previous studies reported that orthotopic injection of human MDA-MB-231 breast cancer cells into the mammary fat pad of NOD scid gamma mice gave rise to metastases in lung, liver, brain, and bone in the majority of cases (31). In contrast to the 4T1 model, Drp1 KO MDA-MB-231 tumors grew at a considerably slower rate and reached the target volume for resection (500 mm3) approximately 21 days later than control tumors (Fig. 5H). Despite this delay in resection, mice bearing Drp1 KO MDA-MB-231 tumors exhibited a significant reduction in metastatic burden in lungs and liver (Fig. 5, I to K). Together, these results indicate that limiting mitochondrial fission in breast cancer cells markedly hinders their ability to metastasize to distant organs.
Leflu promotes mitochondrial elongation and restricts metastasis formation in vivo
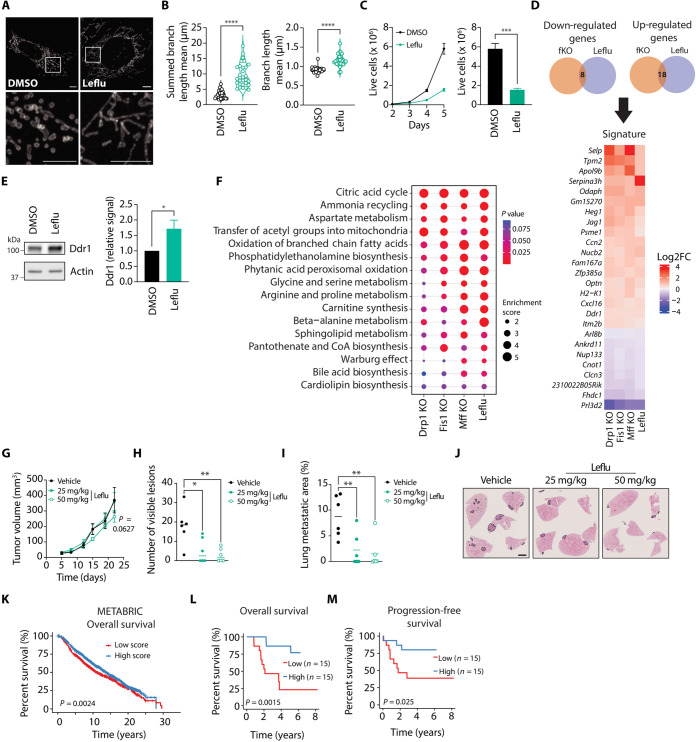
Our findings suggest that elongated mitochondria as a result of mitochondrial fission KO in breast cancer cells negatively affect their metastatic ability. As an alternative approach to promote mitochondrial elongation, we treated 4T1 cells with Leflu. Leflu is an inhibitor of dihydroorotate dehydrogenase (Dhodh), the enzyme responsible for catalyzing the rate-limiting step of the de novo pyrimidine synthesis pathway. Leflu has been reported to induce mitochondrial fusion by promoting Mfn2 transcription (21). In agreement with previous studies in other cell types (15, 21), Leflu treatment increased mitochondrial length in 4T1 cells (Fig. 6, A and B). Analysis of mitochondrial dynamics machinery showed that Leflu promoted Mfn2 expression in 4T1 cells (fig. S7A), consistent with previous reports (21). Drp1 protein levels were also reduced after treatment with Leflu (fig. S7A). Dhodh inhibition by Leflu depletes cells of their pyrimidine pool and restricts proliferation (32). Pyrimidine levels can be restored by uptake of extracellular uridine through the salvage pathway. In agreement with prior studies (21), uridine supplementation blocked the capacity of Leflu to promote mitochondrial elongation (fig. S7, B and C). Consistent with previous observations in other cancer cells (33–35), Leflu treatment limited the proliferative capacity of 4T1 cells in vitro (Fig. 6C).
Fig. 6. Leflu treatment promotes mitochondrial elongation in breast cancer cells and hinders their metastatic capacity in vivo.
(A and B) Representative images (A, scale bars, 5 μm) and quantification of mitochondrial length (B) in Tom 20–stained 4T1 cells treated with Leflu or DMSO. In (B), each data point represents an individual cell (n = 29 per group). ****P < 0.0001 by Welch’s t test. (C) Left: Proliferation curve of Leflu- and DMSO-treated 4T1 cells. Right: Live cell counts on day 5 (N = 4). ***P < 0.001 by unpaired t test. (D) Venn diagrams (top) and heatmap (bottom) depicting common altered genes in fission KO (fKO) and Leflu-treated 4T1 cells. (E) Common altered metabolic pathways in Leflu-treated and fission KO 4T1 cells. (F) Representative immunoblot and densitometric analysis of Ddr1 signal relative to actin signal. *P < 0.05 by unpaired t test (N = 4). (G) Tumor growth curve of Leflu- versus DMSO-treated mice (n = 6 per group). P = 0.0627 by mixed-effects model and Dunnett’s post-hoc test. (H and I) Number of visible lesions (H) and total metastatic area (I) in lungs of Leflu- versus DMSO-treated mice. Each data point represents an individual mouse, and mean is depicted as a line (n = 6 per group). **P < 0.01, *P < 0.05, by one-way ANOVA and Dunnett’s post-hoc test. (J) Representative images of H&E-stained lungs from Leflu- versus DMSO-treated mice. Scale bar, 2 mm. (K) Kaplan-Meier overall survival analysis of patients from the METABRIC study. A gene expression signature associated with enhanced mitochondrial elongation was constructed using the genes identified in (D). Patients were divided into two groups according to their signature score. (L and M) Overall (L) and progression-free (M) survival of patients belonging to an aggressive breast cancer cohort. Patients were categorized according to their signature scores and separated by the median score into two groups (low and high) to conduct survival analyses. Unless indicated otherwise, all data are presented as mean ± SEM.
To further narrow down the transcriptomic signature of mitochondrial elongation, we conducted RNA sequencing (RNA-seq) analysis and GSEA of Leflu-treated 4T1 cells and integrated these results with the transcriptomics results obtained from the three fission KO cell models. There was overlap between the differentially expressed genes detected in Leflu-treated cells and all fission KO cells (Fig. 6D, top). We identified a list containing 26 genes that were either significantly down-regulated or up-regulated in all KO cells and following Leflu treatment (Fig. 6D, bottom). In addition, we observed that some of the genes driving the pathways uncovered by GSEA in fission KOs also appeared in Leflu-treated cells. Clcn3 expression was decreased while Ddr1 was up-regulated after Leflu treatment, similar to the pattern observed in the fission KO cells (Fig. 6D, bottom). Induction of Ddr1 was confirmed at the protein level by immunoblot (Fig. 6E). Supplementation with extracellular uridine abrogated the ability of Leflu to induce Ddr1 expression (fig. S7D).
Analysis of the metabolomic profile in Leflu-treated cells revealed commonalities with the three fission KO cell lines (Fig. 6F). Notably, CAC was the top altered pathway in Leflu-treated cells compared with controls (Fig. 6F). Furthermore, many other altered pathways identified by MSEA were related to FAO (Fig. 6F). Bioenergetic analysis of Leflu-treated cells revealed reduced maximal OXPHOS-linked ATP production compared with control (fig. S7E). Maximal glycolytic ATP production was also reduced by Leflu treatment (fig. S7F). Together, these metabolic changes culminated in a significantly lower maximal bioenergetic capacity for Leflu-treated 4T1 breast cancer cells compared with vehicle-treated controls (fig. S7G).
To evaluate the effect of Leflu on breast cancer metastasis formation in vivo, we injected 4T1 cells into the mammary fat pad of mice and treated them with Leflu for 5 days on, 2 days off throughout the duration of the experiment. Administration of Leflu at two different doses had no major effect on primary tumor growth (Fig. 6G). However, metastasis formation was notably affected by Leflu treatment, as evidenced by a reduction in the number of visible lesions and total metastatic area quantified in the lungs of treated mice (Fig. 6, H to J). Because these results suggest that Leflu treatment might be clinically beneficial, we derived a mitochondrial elongation signature from the common affected genes in all fission KOs and Leflu-treated cells (Fig. 6D). We then performed survival analysis using patient data from the METABRIC study, separating the patients in two groups according to their mitochondrial elongation signature scores. We observed that the group with high scores exhibited better outcome relative to patients with breast tumors possessing low scores (Fig. 6K). Given our interest in identifying actionable targets of metastatic progression, we also computed mitochondrial elongation signature scores in a dataset composed of 30 patients presenting with poor prognosis, aggressive breast cancers (36). To conduct survival analysis, samples were grouped according to the median score to define high and low mitochondrial elongation signature scores. The high-scoring group displayed significantly improved outcome (Fig. 6, L and M), thus supporting our hypothesis that promoting mitochondrial elongation may limit disease progression.
DISCUSSION
Collectively, our data show that increased mitochondrial elongation is linked to lower metastatic potential in breast cancer and that inhibiting mitochondrial fission in breast cancer cells hinders metastasis formation. Previous studies have reported a role for individual proteins involved in mitochondrial fission/fusion in metastatic progression. For instance, inhibition of Drp1 has been shown to reduce metastases in the brain (18). However, proteins involved in mitochondrial dynamics often have additional roles that may obscure the importance of this particular pathway in metastatic progression (20, 37, 38). Here, we opted to take a unifying approach by targeting three different mitochondrial fission proteins and identifying their common transcriptomic and metabolomic signatures. By doing so, we were able to shed light on key pathways associated with their role in mitochondrial fission. Across all the fission KO cells, we observed an up-regulation of genes associated with cell adhesion and cytoskeleton organization, including Ddr1. The role of Ddr1 in cancer is controversial. While some studies suggest that it contributes to disease progression, others report that it limits cancer aggressiveness. In a transgenic mouse breast cancer model, deletion of Ddr1 promoted tumor growth and formation of lung metastases (39). In addition, breast cancer subtypes associated with better prognosis display higher expression of Ddr1 than more aggressive subtypes (39). Recently, a link between mitochondrial dynamics and cell migration involving Ddr1 was described (27). Highly invasive cancer cells rely on weak adhesions to migrate and display lower levels of Ddr1, which binds to fibrillar collagen (27). In contrast, nonmetastatic cells apply strong adhesive traction stress, which is dependent on Ddr1 expression (27). Knockdown of Ddr1 promoted mitochondrial fission and 3D invasion (27). In agreement with this study, we observed increased Ddr1 expression in all our fission KO clones, suggesting an important role for mechanosensing in linking mitochondrial dynamics and metastasis formation. An association between ECM stiffness and mitochondrial dynamics has been described in recent work, where low ECM stiffness found in breast cancer metastasis target organs promoted mitochondrial fission (10). We observed a small yet variable increase in the number of cell adhesions in many of the fission KO clones when cells were plated on collagen and fibronectin. This increase in cell adhesion may limit their migration capacity. Conversely, adhesion area, which may serve as a marker of adhesion maturation and strength, was unchanged. Ddr1 also participates in cell-cell junctions, and its depletion has been reported to disrupt cohesion and boost single-cell motility, which may contribute to blood-borne metastatic dissemination (40).
Metabolomic analyses showed a reduction in CAC metabolites in all fission KO cells, which was concomitant with a reduction in maximal OXPHOS-linked ATP generation. In a pancreatic cancer model, disruption of mitochondrial dynamics resulted in alteration of CAC metabolites (41, 42) as well as in glutamate, alanine, and aspartate metabolism (42). Drp1 KO pancreatic cancer cells also displayed reduced spare respiratory capacity (15, 41). In this model, Drp1 was found to be crucial for supporting homeostatic mitochondrial fusion and fission cycles, thus permitting the removal of damaged organelles (41). Successive rounds of proliferation without mitochondrial fission could lead to accumulation of damaged mitochondrial components, resulting in changes in respiration (43, 44). Studies from our group (1) and others (2, 3, 45) have highlighted the importance of mitochondrial metabolism in breast cancer metastasis. Reduced mitochondrial respiration might render fission KO cells less tolerant to changes in their environment through reduced bioenergetic flexibility.
Furthermore, recent work suggests that mitochondrial length influences mitochondrial respiration through regulating the rate of FAO (46). Mitochondrial fragmentation promotes oxidation of long-chain fatty acids, while mitochondrial fusion limits this process (46, 47). In agreement with these findings, our metabolomic analysis showed that several pathways related to FAO were altered in 4T1 fission KO cells. Similarly, a recent study demonstrated that inhibition of Drp1 results in impaired lipid oxidation and reduced brain metastasis formation (18). Considering that mitochondrial fission proteins also participate in peroxisomal fission and that both organelles regulate lipid metabolism, these results further strengthen the impact of organelle dynamics on their associated functions.
When assessing the impact of inhibiting mitochondrial fission in metastasis formation in vivo, we found that deletion of mitochondrial fission genes in 4T1 cells dramatically hampers their ability to metastasize to the lungs. We observed differences between the two metastatic models we used. The decrease in metastatic burden observed for the fission KO 4T1 cells was much more pronounced in the spontaneous model (~90%) than in the experimental model (~30%). This difference suggests that fission KO cells struggle with the intravasation step of the metastatic cascade. In addition, we found that fission KO cells are less resistant to anoikis, which could hinder their survival in circulation.
In the human TNBC model MDA-MB-231, deletion of Drp1 led to a substantial delay in tumor growth. Unlike 4T1 cells, MDA-MB-231 cells harbor an activating KRAS mutation (48). In other KRAS-mutant cancers, such as pancreatic adenocarcinoma, Drp1 has been deemed crucial for oncogenic transformation and tumor growth (41). Ectopic expression of oncogenic RAS induced mitochondrial fragmentation in a Drp1-dependent manner (49). Oncogenic MAPK signaling caused by mutant RAS results in Drp1 activation through its phosphorylation by ERK1/2 (49, 50). Overall, these findings suggest that KRAS-mutant cancers may be more sensitive to loss of Drp1. The effect of Drp1 deletion on total ATP production was more pronounced in MDA-MB-231 relative to 4T1 cells. In agreement with our observations in the 4T1 model, Drp1 KO MDA-MB-231 cells were less capable of metastasizing to the lungs and liver. The impact of limiting mitochondrial fission on metastasis was conserved between an immunocompetent (Balb/c) and an immunocompromised mouse model (NOD scid gamma), suggesting that enhanced immune recognition or clearance is unlikely a key underlying mechanism.
In addition to targeting mitochondrial fission proteins by genetic manipulation, we pharmacologically induced mitochondrial elongation by Leflu administration. Leflu is an antirheumatic drug that was identified to promote mitochondrial fusion through modulating Mitofusins (21). Leflu-treated cells showed an overlap in their transcriptomic signature with fission KO cells, which included up-regulation of Ddr1. Moreover, Leflu-treated cells displayed alterations in their CAC in a similar fashion to fission KO cells. An untargeted metabolomics study of pancreatic cancer cells where mitochondrial fusion was induced by different approaches uncovered that the CAC cycle was also one of the top altered pathways after Drp1 deletion or Leflu treatment (42).
The target of Leflu, Dhodh, is located in the mitochondrial inner membrane and uses ubiquinone as an electron acceptor, which then delivers the electrons to complex III of the electron transport chain. Dhodh inhibition by Leflu caused a slight decrease in ATP production from OXPHOS in 4T1 cells. This is in agreement with previous reports showing that 5 to 10% of coupled oxygen consumption is derived from Dhodh in 4T1 cells (51). However, the effect of Leflu on cellular bioenergetics may be cell type and dose dependent (21). In patient-derived pancreatic cancer cells, Leflu significantly decreased basal and maximal ATP-linked respiration, but only in those harboring KRAS mutations (15).
We observed that administration of Leflu in vivo reduced metastasis formation in the 4T1 TNBC model. In previous reports, Leflu reduced tumor growth in mouse xenograft models of breast (52), pancreatic (15), prostate (53), and lung (54) cancer. In a mouse model of colorectal cancer, Leflu drastically limited liver metastasis formation despite only having a moderate effect on primary tumor growth (55). In addition, Leflu treatment significantly reduced the formation of lung micrometastases after subcutaneous injection of cells derived from lung adenocarcinoma (54). However, because there was a considerable difference in primary tumor size between the treated and control groups, it cannot be ruled out that the reduced incidence of metastasis reported was due to reduced growth of the tumor at the primary site.
Despite considerable advances made in treating breast cancer, TNBC is still associated with poor prognosis, caused by its aggressive biology, high rate of metastasis, and lack of targeted therapies. Accordingly, there is still a pressing need to identify actionable targets in this breast cancer subtype. Our work suggests that mitochondrial fission could be targeted in TNBC to limit metastatic dissemination. Among other compounds known to promote mitochondrial fusion, Leflu offers the advantage of already being approved by the Food and Drug Administration for use in rheumatoid arthritis. This would allow for its prompt use in clinical settings. There is an ongoing phase 1/2 clinical trial for its use in patients with previously treated HER2-negative metastatic breast cancer (56). Our results showed that in a cohort of patients presenting with difficult-to-treat, aggressive forms of breast cancer, those possessing high mitochondrial elongation signature scores exhibit better outcomes, which is encouraging. Together, our data suggest that promoting mitochondrial elongation in cancer cells limits their ability to metastasize, thus uncovering a potential therapeutic opportunity for preventing disease progression.
MATERIALS AND METHODS
Cell culture
4T1, MDA-MB-231, and CRISPR-KO breast cancer cells were grown at 37°C and 5% CO2 in high-glucose Dulbecco’s modified Eagle’s medium (Wisent no. 319-005 CL), 10% fetal bovine serum (FBS), 20 mM Hepes, and 1% penicillin/streptomycin.
Generation of CRISPR-KO clones
Cell lines with genetic disruptions were generated at the Genomic Engineering and Molecular Biology (GEMb) core facility in the Faculty of Medicine at the University of Ottawa (RRID:SCR_022954). The GEMb facility is supported by CFI-36490, CFI-37607, and CFI-36940. pSpCas9(BB)-2A-Puro (PX459) V2.0 was a gift from F. Zhang (Addgene plasmid no. 62988; http://n2t.net/addgene:62988; RRID:Addgene_62988). plenti-Cas9-Blast was a gift from F. Zhang (Addgene plasmid no. 52962; http://n2t.net/addgene:52962; RRID:Addgene_52962).
For generating 4T1 fission KOs, guides targeting each gene (Dnm1l, Fis1, and Mff) were cloned into PX459v2.0 (Addgene: 62988). Constructs were sequenced and transfected into 4T1 cells. Selection was carried out with Puromycin (Wisent, 5 μg/ml).
MDA-MB-231 cells disrupted in DNM1L were generated using guide pairs from the Transomic sgRNA library cloned into pCLIP-dual-SFFV-ZsGreen-Puromycin-V163 (Transomic Technologies). Briefly, Lipofectamine 3000 (Thermo Fisher Scientific) was used to transfect equal amounts of each guide vector (separately) along with plenti-Cas9-Blast (Addgene: 52962). Following transfection, cells were allowed to recover for 3 days, following which they were passaged and re-transfected 24 hours later with the same vectors, for a total of three rounds of transfection.
For clonal outgrowth, single cells were deposited in 96-well plates containing regular growth media supplemented with 25% conditioned media (centrifuged at 2500g for 10 min and 0.45 μm filtered) and an additional 10% FBS using an SH800 cell sorter (Sony Biosciences).
Immunoblots
Cells were washed with ice-cold phosphate-buffered saline (PBS), lifted off the plate with a cell scraper, and collected in microtubes. After centrifugation at 4°C, cell pellets were lysed with RIPA buffer containing protease and phosphatase inhibitors (cOmplete mini EDTA-free Protease inhibitor cocktail and PhosSTOP, Roche). Protein concentrations were quantified by the BCA assay (Thermo Fisher Scientific). Equivalent amounts of protein were loaded in each lane of SDS–polyacrylamide gel electrophoresis gels. Proteins were transferred to a polyvinylidene difluoride membrane with a Trans-Blot Turbo Transfer System (Bio-Rad). Membranes were blocked with 5% milk in Tween-TBS for 1 hour at room temperature and incubated with the appropriate primary antibody overnight at 4°C. Then, membranes were washed with Tween-TBS and incubated with a secondary antibody against mouse or rabbit conjugated to horseradish peroxidase (Bio-Rad). Blots were imaged in an ImageQuant LAS 4000 (GE) using the Clarity ECL substrate (Bio-Rad).
Immunofluorescence and confocal microscopy
Cells were seeded onto fibronectin-coated coverslips overnight and fixed with 4% paraformaldehyde (PFA) for 15 min at 37°C. Permeabilization and blocking were performed by incubating in PBS containing 0.5% Triton X-100 and 1% BSA for 20 min at room temperature. Staining with primary antibody was performed at room temperature for 1 hour. Coverslips were then washed with PBS and stained with the appropriate secondary antibody for 1 hour at room temperature. Nuclei staining was carried out by adding a DAPI (4′,6-diamidino-2-phenylindole) solution for 5 min. Coverslips were mounted onto slides using ProLong Glass Antifade Mountant (Thermo Fisher Scientific) and stored in the dark at 4°C. Images were taken on an LSM 880 AxioObserver Z1 confocal microscope (Zeiss) with an Airyscan detector, using a 63× Plan-Apochromat oil objective.
For adhesion number and size experiments, 5000 4T1 parental or KO clones were seeded on glass coverslips coated with either 5 μg/cm2 of Human Plasma Fibronectin (EMD Millipore, catalog no. FC010) or type 1 rat-tail collagen (EMD Millipore, catalog no. 08-115). After 24 hours, cells were fixed with 3% paraformaldehyde and stained with anti-Paxillin antibody (Abcam, catalog no. AB23510) and phalloidin Alexa Fluor 488 (Invitrogen, catalog no. A12379). Using a Stellaris-5 confocal microscope (Leica, Wetzlar, Germany), 10 fields of view per cell population were imaged. Raw images were analyzed using the Imaris software (BitPlane, Oxford Instruments, Zurich, Switzerland, Ver. 10.1.1). Briefly, adhesions were first segmented using the “surfaces” function, then smoothed using a surfaces detail of 0.200 μm and a local background subtraction of 0.200 μm. Surfaces were then split by seeding points with a diameter of 0.300 μm. Adhesions smaller than 8 voxels were excluded. Adhesion density was calculated by dividing adhesion number by area (area per cell or cell cluster). Cell aspect ratio was calculated by dividing its long ellipse axis by the short ellipse axis. Images containing cell clusters were identified during segmentation and excluded from cell aspect ratio analysis.
Analysis of mitochondrial morphology
For evaluating mitochondrial length, z-stacks were transformed to 2D images by performing maximum intensity projections and binarized in Fiji (57). Binary images were analyzed with the Fiji plugin MiNA (Mitochondrial Network Analysis) (58).
Cell proliferation
For assessing cell proliferation, 7000 4T1 breast cancer cells were seeded per well in a six-well plate. Cells were harvested from plates by trypsinization and counted using a Countess automated cell counter (Thermo Fisher Scientific) on days 2, 3, 4, and 5 after seeding. Viability was determined using Trypan Blue exclusion.
For MDA-MB-231 cells, 500 cells were seeded per well in 96-well black wall clear bottom plates. On days 3, 4, 5, and 6 after seeding, cells were fixed with 4% PFA and nuclei were stained with DAPI. Images were taken on an EVOS FL Auto wide-field microscope (Thermo Fisher Scientific) using a 4× objective. Nuclei present in images were counted using Fiji. Counts were adjusted to represent cell numbers present in the whole well.
Anoikis assay
A total of 500 cells were seeded into 1 ml of media in 24-well ultralow attachment plates (Corning) and allowed to grow for 7 or 14 days. After the indicated time, 0.1 ml of media was removed and transferred to a standard tissue culture 100-mm plate. Following 7 days of growth, the plates were fixed in formalin, stained with crystal violet (Sigma), and digitally imaged (Chemi-doc Bio-Rad), and the number of colonies was scored manually. The second growth phase on tissue culture dishes allows for the assessment of the number of cells that survive the anchorage-independent growth and are able to reattach.
For assessing cell viability after culture in anoikis-inducing conditions, 500 cells were cultured in 24-well ultralow attachment plates (Corning). After 7 days, cells were harvested and incubated with Accutase (Thermo Fisher Scientific) for 10 min at 37°C. Cell clumps were dislodged by gently pipetting. Cells were washed with annexin V binding buffer (10 mM Hepes, 140 mM NaCl, and 2.5 mM CaCl2, pH 7.4) and incubated with annexin V–fluorescein isothiocyanate at room temperature for 15 min in the dark. Then, propidium iodide was added at 1 μg/ml and samples were immediately analyzed using a LSRFortessa flow cytometer (BD). Dead cells were defined as annexin V+ (PI− and PI+).
Transcriptomics
4T1 Breast cancer cells were harvested in TRIzol (Thermo Fisher Scientific) and stored at −80°C until extraction. RNA was extracted using the TRIzol Plus RNA purification kit (Thermo Fisher Scientific). DNA was removed using an on-column DNase protocol. Library preparation and RNA-seq were performed by the genomics platform of the Institute for Research in Immunology and Cancer of the Université de Montréal (IRIC). RNA was quantified using Qubit (Thermo Fisher Scientific) and quality was assessed with the 2100 Bioanalyzer (Agilent Technologies). Transcriptome libraries were generated using the KAPA RNA HyperPrep (Roche) with a poly-A selection (Thermo Fisher Scientific). Next-generation sequencing was performed on the Illumina NextSeq500, obtaining around 30 million single-end 75-bp reads per sample. Sequences were trimmed for sequencing adapters and low-quality 3′ bases using Trimmomatic version 0.35 (59) and aligned to the reference mouse genome version GRCm38 (gene annotation from Gencode version M25, based on Ensembl 100) using STAR version 2.7.1a (60). As part of quality control, the sequences were aligned to several different genomes to verify that there was no sample contamination. Gene expressions were computed using RSEM (61) to obtain normalized gene and transcript level expression, in FPKM and TPM values, for these stranded RNA libraries. Further analysis was conducted in R (62) (version 4.3.0). The package DESeq2 version 1.30.1 (63) was used to normalize gene read counts and for conducting differential gene expression analysis. Significant gene sets were selected according to a false discovery rate (FDR) cutoff of 5% (Padj < 0.05). GSEA was performed using the clusterProfiler (64) package. Enriched Gene Ontology biological processes were identified using the gseGO function, with a cutoff of Padj < 0.05. The fgsea algorithm (65) was selected for analysis. The compareCluster function was used to compare enriched functional profiles and combine the results into a single object.
Metabolomics
Cells were seeded in six-well plates and harvested 48 hours later for metabolite extraction. In brief, cells were washed with 150 mM ammonium formate and harvested with 50% (v/v) methanol. Following addition of acetonitrile, cell lysates were further homogenized in a MagNALyser (Roche) using ceramic beads. After adding dichloromethane and H2O, cell extracts were partitioned into aqueous and organic layers by centrifugation. The aqueous layer was collected and dried in a refrigerated speed-vac (Labconco). Samples were resuspended in H2O and run on a 6470A tandem quadruple mass spectrometer equipped with a 1290 Infinity II ultrahigh-performance LC (Agilent Technologies) using the Metabolomics Dynamic MRM (multiple reaction monitoring) Database and Method, which uses ion-pairing reverse-phase chromatography. This method was further optimized for phosphate-containing metabolites with the addition of 5 μM InfinityLab deactivator (Agilent Technologies) to mobile phases A and B, which requires decreasing the backflush acetonitrile to 90%. MRM transitions were optimized using authentic standards and quality control samples. Metabolites were quantified by integrating the area under the curve of each compound using external standard calibration curves with Mass Hunter Quant (Agilent Technologies). No corrections for ion suppression or enhancement were performed. Metabolite concentrations were normalized to cell counts. Further data processing analysis was conducted in Metaboanalyst 5.0 (66). MSEA was performed using the Quantitative Enrichment Analysis algorithm, with the Small Molecule Pathway Database as library. A reference metabolome listing all the metabolites that can be detected in our analytical platform was uploaded to the analysis as well. A cutoff of P < 0.1 was set to identify statistically significant altered pathways.
Seahorse extracellular flux analysis
OCR and extracellular acidification rate (ECAR) were measured in an XF96e extracellular flux analyzer (Agilent Technologies) following a modified Mito Stress Test protocol. The day before the assay, 10,000 (4T1) or 15,000 (MDA-MB-231) breast cancer cells were seeded per well in a Seahorse 96-well plate and cultured overnight in their standard growing media and under the same conditions. On the day of the assay, the plate was washed with XF media and incubated at 37°C in a non-CO2 incubator for 1 hour before loading into the Seahorse analyzer. OCR and ECAR were measured three times at basal conditions and after injections of oligomycin (1 μM for 4T1 and 2 μM for MDA-MB-231), FCCP (1 μM for 4T1 and 1.5 μM for MDA-MB-231), rotenone/antimycin A (0.5 μM each), and monensin (20 μM). Hoechst was added to the last injection for nuclei staining. After the Seahorse assay was completed, the plate was imaged using an EVOS FL Auto (Thermo Fisher Scientific) and cell numbers in each well were calculated by nuclei counting. OCR and ECAR were then normalized to cell counts. ATP production and bioenergetic capacity were calculated as previously described by Mookerjee et al. (67).
Mitochondrial membrane potential analysis
Mitochondrial membrane potential was evaluated by TMRM staining. A 100 nM TMRM solution in cell culture media was added to cells plated the day before and incubated at 37°C for 30 min. Cells were then washed with PBS, trypsinized, and resuspended in PBS containing 2% FBS and DAPI (0.2 μg/ml). Samples were immediately analyzed in an LSRFortessa (BD) flow cytometer. Dead DAPI+ cells were excluded from the analysis.
Mitochondrial DNA analysis
DNA was extracted from cells using the PureLink Genomic DNA Mini Kit, following the manufacturer’s instructions. Levels of mitochondrial DNA relative to nuclear DNA were assessed by qPCR using Taqman probes for Rps18 (nuclear) and mt-Nd1 (mitochondrial). Probes binding to one single exon were selected to allow detection of nuclear DNA.
In vivo metastasis assays
All animal studies were approved by the Animal Resource Centre at McGill University under protocol AUP no. 4830 and comply with guidelines set by the Canadian Council of Animal Care.
Tumor cells were injected either into the mammary fat pad or the tail vein of mice. For spontaneous metastasis assays, 10,000 cells (4T1) or 250,000 cells (MDA-MB-231), were injected into the mammary fad pads of female Balb/c mice (Charles River, 4T1 model) or NOD-scid IL2Rgammanull mice (The Jackson Laboratory, MDA-MB-231 model) in a 1:1 mixture of PBS:Matrigel Growth Factor Reduced (Corning). Tumors were measured using caliper measurements at the indicated time points and tumor volumes were calculated using the formula . Tumors were resected when the volume reached ~500 mm3. Mice were euthanized 18 to 22 days after tumor resection and lung and liver (only for MDA-MB-231 model) tissue was collected to analyze metastatic burden. For tail vein injections, 200,000 4T1 cells were injected into the lateral tail vein of female Balb/c mice. Twenty-four days after tumor cell injection, mice were euthanized, and lung tissue was collected to analyze metastatic burden.
For Leflu experiments, mice were treated 5 days after tumor cell injection and daily for 5 days on, 2 days off, by oral gavage with either vehicle or Leflu (25 or 50 mg/kg) until they were euthanized. H&E-stained sections were digitally scanned, and metastatic burden was assessed using ImageScope (Leica Biosystems).
Mitochondrial fission KO and mitochondrial elongation signature
For the analyses conducted using the METABRIC dataset (28–30), expression and clinical data were downloaded from cBioPortal (68, 69) (www.cbioportal.org). Analyses were carried out using R (62) (v.4.1.0), using the singscore package (v.3.17) (70, 71). Briefly, human homologs of genes that were significantly altered in all fission KO 4T1 cell lines (mitochondrial fission KO) or in all KOs and Leflu-treated 4T1 (mitochondrial elongation) were used to compute a score for each patient based on the expression of these genes. Using the maxstat package (v.0.7-25) (72), the best cutoff was calculated to separate individuals with low (Fragmented) versus high (Fused) score. Kaplan-Meier curves were generated with the survival (73) (v.3.2-11) and survminer (74) (v.0.4.9) packages.
In addition, publicly available RNA-seq and clinical data were obtained from Savage et al. (36). Mitochondrial elongation gene set signature scores were computed using the R package GSVA (75). To predict survival outcomes, patients were categorized according to their signature scores and separated by the median score into two groups (low and high). Differences in survival were assessed using Kaplan–Meier analysis and log-rank test statistics using the survival and survminer R packages.
Statistical analyses
Statistical analyses were carried out using GraphPad Prism or R on independent biological replicates. Details on statistical tests and number of replicates are provided in figure legends.
Leflu treatment in vitro
For transcriptomics, metabolomics, qPCR, and immunoblot analyses, cells were cultured in the presence of 10 μM Leflu (Sigma) or dimethyl sulfoxide (DMSO) as vehicle control for 3 days. For Seahorse assays, cells were trypsinized and transferred to Seahorse plates on day 2 of treatment. Treatment was continued overnight in the seahorse plate. For proliferation assays, Leflu was present for the whole duration of the experiment (5 days). For uridine supplementation experiments, uridine (Sigma) was added in the presence or absence of Leflu at the reported concentrations.
Acknowledgments
We would like to acknowledge the University of Ottawa Flow Cytometry and Virometry Core (RRID:SCR_023306), as well as the Cell Biology and Image Acquisition Core (RRID:SCR_021845) funded by the University of Ottawa, Natural Sciences and Engineering Research Council of Canada, and the Canada Foundation for Innovation. Cell lines with genetic disruptions were generated at the Genomic Engineering and Molecular Biology (GEMb) core facility in the Faculty of Medicine at the University of Ottawa (RRID:SCR_022954). Metabolomics analyses were performed at the University of Ottawa Metabolomics Core Facility. RNA-seq was conducted at the Institute for Research in Immunology and Cancer of the Université de Montréal (IRIC) Genomics Core Facility. We would like to thank the Flow Cytometry, Histology cores at the Goodman Cancer Institute and the Advanced Bioimaging Facility (ABIF) at McGill University.
Funding: This work was supported by Fonds de Recherche du Québec Santé—FRQS (Y.A.D.), Next Generation of Scientists Award—Cancer Research Society (Y.A.D.), the Canadian Institutes of Health Research (J.S.P. and P.M.S.), McGill University William Dawson Scholar (P.M.S.), and Canada Research Chair (Tier 1) in Cancer Metabolism (J.S.P.).
Author contributions: Conceptualization: L.M., M.G.A., M.P., P.M.S., and J.S.P. Data curation: L.M. Formal analysis: L.M., M.G.A., Y.A.D., H.K., A.P., and A.N. Funding acquisition: P.M.S. and J.S.P. Investigation: L.M., M.G.A., Y.A.-D., C.S.L., A.N., and M.B. Methodology: L.M., M.G.A., Y.A.D., P.M.S., and J.S.P. Project administration: L.M., M.G.A., H.K., M.P., P.M.S., and J.S.P. Resources: M.P. and P.M.S. Software: L.M., Y.A.D., H.K., and A.P. Supervision: L.M., M.G.A., M.P., P.M.S., and J.S.P. Validation: L.M., M.G.A., P.M.S., and J.S.P. Visualization: L.M. and J.S.P. Writing—original draft: L.M. and J.S.P. Writing—review and editing: L.M., M.G.A., Y.A.-D., H.K., A.P., C.S.L., A.N., M.K., M.P., P.M.S., and J.S.P.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: Sequencing data are available on NCBI GEO under accession number GSE245216. All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
Supplementary Materials
This PDF file includes:
Figs. S1 to S7
REFERENCES AND NOTES
- 1.Andrzejewski S., Klimcakova E., Johnson R. M., Tabariès S., Annis M. G., McGuirk S., Northey J. J., Chénard V., Sriram U., Papadopoli D. J., Siegel P. M., St-Pierre J., PGC-1α promotes breast cancer metastasis and confers bioenergetic flexibility against metabolic drugs. Cell Metab. 26, 778–787.e5 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Lebleu V. S., O’Connell J. T., Gonzalez Herrera K. N., Wikman H., Pantel K., Haigis M. C., De Carvalho F. M., Damascena A., Domingos Chinen L. T., Rocha R. M., Asara J. M., Kalluri R., PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 16, 992–1003 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis R. T., Blake K., Ma D., Gabra M. B. I., Hernandez G. A., Phung A. T., Yang Y., Maurer D., Lefebvre A. E. Y. T., Alshetaiwi H., Xiao Z., Liu J., Locasale J. W., Digman M. A., Mjolsness E., Kong M., Werb Z., Lawson D. A., Transcriptional diversity and bioenergetic shift in human breast cancer metastasis revealed by single-cell RNA sequencing. Nat. Cell Biol. 22, 310–320 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Delaunay S., Pascual G., Feng B., Klann K., Behm M., Hotz-Wagenblatt A., Richter K., Zaoui K., Herpel E., Münch C., Dietmann S., Hess J., Benitah S. A., Frye M., Mitochondrial RNA modifications shape metabolic plasticity in metastasis. Nature 607, 593–603 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liesa M., Shirihai O. S., Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 17, 491–506 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suen D.-F., Norris K. L., Youle R. J., Mitochondrial dynamics and apoptosis. Genes Dev. 22, 1577–1590 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitra K., Mitochondrial fission-fusion as an emerging key regulator of cell proliferation and differentiation. Bioessays 35, 955–964 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Gomes L. C., Di Benedetto G., Scorrano L., During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol. 13, 589–598 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammadi H., Sahai E., Mechanisms and impact of altered tumour mechanics. Nat. Cell Biol. 20, 766–774 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Romani P., Nirchio N., Arboit M., Barbieri V., Tosi A., Michielin F., Shibuya S., Benoist T., Wu D., Hindmarch C. C. T., Giomo M., Urciuolo A., Giamogante F., Roveri A., Chakravarty P., Montagner M., Calì T., Elvassore N., Archer S. L., De Coppi P., Rosato A., Martello G., Dupont S., Mitochondrial fission links ECM mechanotransduction to metabolic redox homeostasis and metastatic chemotherapy resistance. Nat. Cell Biol. 24, 168–180 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao S., Zhang X., Shi Y., Cheng L., Song T., Wu B., Li J., Yang H., MIEF2 over-expression promotes tumor growth and metastasis through reprogramming of glucose metabolism in ovarian cancer. J. Exp. Clin. Cancer Res. 39, 286 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karimi D., Pedram N., Kakaei F., Asadi M., Poursaei E., Kermani T. A., FIS1 overexpression is correlated with tumor metastasis in gastric adenocarcinoma. J. Gastrointest. Cancer 53, 466–471 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Seo J. H., Chae Y. C., Kossenkov A. V., Lee Y. G., Tang H.-Y., Agarwal E., Gabrilovich D. I., Languino L. R., Speicher D. W., Shastrula P. K., Storaci A. M., Ferrero S., Gaudioso G., Caroli M., Tosi D., Giroda M., Vaira V., Rebecca V. W., Herlyn M., Xiao M., Fingerman D., Martorella A., Skordalakes E., Altieri D. C., MFF regulation of mitochondrial cell death is a therapeutic target in cancer. Cancer Res. 79, 6215–6226 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao J., Zhang J., Yu M., Xie Y., Huang Y., Wolff D. W., Abel P. W., Tu Y., Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene 32, 4814–4824 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu M., Nguyen N. D., Huang Y., Lin D., Fujimoto T. N., Molkentine J. M., Deorukhkar A., Kang Y., San Lucas F. A., Fernandes C. J., Koay E. J., Gupta S., Ying H., Koong A. C., Herman J. M., Fleming J. B., Maitra A., Taniguchi C. M., Mitochondrial fusion exploits a therapeutic vulnerability of pancreatic cancer. JCI Insight 4, e126915 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie Q., Wu Q., Horbinski C. M., Flavahan W. A., Yang K., Zhou W., Dombrowski S. M., Huang Z., Fang X., Shi Y., Ferguson A. N., Kashatus D. F., Bao S., Rich J. N., Mitochondrial control by DRP1 in brain tumor initiating cells. Nat. Neurosci. 18, 501–510 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rehman J., Zhang H. J., Toth P. T., Zhang Y., Marsboom G., Hong Z., Salgia R., Husain A. N., Wietholt C., Archer S. L., Inhibition of mitochondrial fission prevents cell cycle progression in lung cancer. FASEB J. 26, 2175–2186 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parida P. K., Marquez-Palencia M., Ghosh S., Khandelwal N., Kim K., Nair V., Liu X.-Z., Vu H. S., Zacharias L. G., Gonzalez-Ericsson P. I., Sanders M. E., Mobley B. C., McDonald J. G., Lemoff A., Peng Y., Lewis C., Vale G., Halberg N., Arteaga C. L., Hanker A. B., DeBerardinis R. J., Malladi S., Limiting mitochondrial plasticity by targeting DRP1 induces metabolic reprogramming and reduces breast cancer brain metastases. Nat. Cancer 4, 893–907 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun X., Cao H., Zhan L., Yin C., Wang G., Liang P., Li J., Wang Z., Liu B., Huang Q., Xing J., Mitochondrial fission promotes cell migration by Ca2+/CaMKII/ERK/FAK pathway in hepatocellular carcinoma. Liver Int. 38, 1263–1272 (2018). [DOI] [PubMed] [Google Scholar]
- 20.Seo J. H., Agarwal E., Chae Y. C., Lee Y. G., Garlick D. S., Storaci A. M., Ferrero S., Gaudioso G., Gianelli U., Vaira V., Altieri D. C., Mitochondrial fission factor is a novel Myc-dependent regulator of mitochondrial permeability in cancer. EBioMedicine 48, 353–363 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miret-Casals L., Sebastián D., Brea J., Rico-Leo E. M., Palacín M., Fernández-Salguero P. M., Loza M. I., Albericio F., Zorzano A., Identification of new activators of mitochondrial fusion reveals a link between mitochondrial morphology and pyrimidine metabolism. Cell Chem. Biol. 25, 268–278 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Aslakson C. J., Miller F. R., Selective events in the metastatic process defined by analysis of the sequential dissemination of subpopulations of a mouse mammary tumor. Cancer Res. 52, 1399–1405 (1992). [PubMed] [Google Scholar]
- 23.Losón O. C., Song Z., Chen H., Chan D. C., Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol. Biol. Cell 24, 659–667 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giacomello M., Pyakurel A., Glytsou C., Scorrano L., The cell biology of mitochondrial membrane dynamics. Nat. Rev. Mol. Cell Biol. 21, 204–224 (2020). [DOI] [PubMed] [Google Scholar]
- 25.Xu B., Jin X., Min L., Li Q., Deng L., Wu H., Lin G., Chen L., Zhang H., Li C., Wang L., Zhu J., Wang W., Chu F., Shen J., Li H., Mao J., Chloride channel-3 promotes tumor metastasis by regulating membrane ruffling and is associated with poor survival. Oncotarget 6, 2434–2450 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roshanzamir F., Robinson J. L., Cook D., Karimi-Jafari M. H., Nielsen J., Metastatic triple negative breast cancer adapts its metabolism to destination tissues while retaining key metabolic signatures. Proc. Natl. Acad. Sci. U.S.A. 119, e2205456119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crosas-Molist E., Graziani V., Maiques O., Pandya P., Monger J., Samain R., George S. L., Malik S., Salise J., Morales V., Le Guennec A., Atkinson R. A., Marti R. M., Matias-Guiu X., Charras G., Conte M. R., Elosegui-Artola A., Holt M., Sanz-Moreno V., AMPK is a mechano-metabolic sensor linking cell adhesion and mitochondrial dynamics to Myosin-dependent cell migration. Nat. Commun. 14, 2740 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rueda O. M., Sammut S.-J., Seoane J. A., Chin S.-F., Caswell-Jin J. L., Callari M., Batra R., Pereira B., Bruna A., Ali H. R., Provenzano E., Liu B., Parisien M., Gillett C., McKinney S., Green A. R., Murphy L., Purushotham A., Ellis I. O., Pharoah P. D., Rueda C., Aparicio S., Caldas C., Curtis C., Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature 567, 399–404 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pereira B., Chin S.-F., Rueda O. M., Vollan H.-K. M., Provenzano E., Bardwell H. A., Pugh M., Jones L., Russell R., Sammut S.-J., Tsui D. W. Y., Liu B., Dawson S.-J., Abraham J., Northen H., Peden J. F., Mukherjee A., Turashvili G., Green A. R., McKinney S., Oloumi A., Shah S., Rosenfeld N., Murphy L., Bentley D. R., Ellis I. O., Purushotham A., Pinder S. E., Børresen-Dale A.-L., Earl H. M., Pharoah P. D., Ross M. T., Aparicio S., Caldas C., The somatic mutation profiles of 2,433 breast cancers refine their genomic and transcriptomic landscapes. Nat. Commun. 7, 11479 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curtis C., Shah S. P., Chin S.-F., Turashvili G., Rueda O. M., Dunning M. J., Speed D., Lynch A. G., Samarajiwa S., Yuan Y., Gräf S., Ha G., Haffari G., Bashashati A., Russell R., Kinney S. M.; METABRIC Group, Langerød A., Green A., Provenzano E., Wishart G., Pinder S., Watson P., Markowetz F., Murphy L., Ellis I., Purushotham A., Børresen-Dale A.-L., Brenton J. D., Tavaré S., Caldas C., Aparicio S., The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486, 346–352 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iorns E., Drews-Elger K., Ward T. M., Dean S., Clarke J., Berry D., El Ashry D., Lippman M., A new mouse model for the study of human breast cancer metastasis. PLOS ONE 7, e47995 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cherwinski H. M., Cohn R. G., Cheung P., Webster D. J., Xu Y. Z., Caulfield J. P., Young J. M., Nakano G., Ransom J. T., The immunosuppressant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesis. J. Pharmacol. Exp. Ther. 275, 1043–1049 (1995). [PubMed] [Google Scholar]
- 33.Liu L., Dong Z., Lei Q., Yang J., Hu H., Li Q., Ji Y., Guo L., Zhang Y., Liu Y., Cui H., Inactivation/deficiency of DHODH induces cell cycle arrest and programed cell death in melanoma. Oncotarget 8, 112354–112370 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hubackova S., Davidova E., Boukalova S., Kovarova J., Bajzikova M., Coelho A., Terp M. G., Ditzel H. J., Rohlena J., Neuzil J., Replication and ribosomal stress induced by targeting pyrimidine synthesis and cellular checkpoints suppress p53-deficient tumors. Cell Death Dis. 11, 110 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu X., Shen J., Mall J. W., Myers J. A., Huang W., Blinder L., Saclarides T. J., Williams J. W., Chong A. S. F., In vitro and in vivo antitumor activity of a novel immunomodulatory drug, leflunomide: Mechanisms of action. Biochem. Pharmacol. 58, 1405–1413 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Savage P., Pacis A., Kuasne H., Liu L., Lai D., Wan A., Dankner M., Martinez C., Muñoz-Ramos V., Pilon V., Monast A., Zhao H., Souleimanova M., Annis M. G., Aguilar-Mahecha A., Lafleur J., Bertos N. R., Asselah J., Bouganim N., Petrecca K., Siegel P. M., Omeroglu A., Shah S. P., Aparicio S., Basik M., Meterissian S., Park M., Chemogenomic profiling of breast cancer patient-derived xenografts reveals targetable vulnerabilities for difficult-to-treat tumors. Commun. Biol. 3, 310 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alirol E., James D., Huber D., Marchetto A., Vergani L., Martinou J. C., Scorrano L., The mitochondrial fission protein hFis1 requires the endoplasmic reticulum gateway to induce apoptosis. Mol. Biol. Cell 17, 4593–4605 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Germain M., Mathai J. P., McBride H. M., Shore G. C., Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. EMBO J. 24, 1546–1556 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takai K., Drain A. P., Lawson D. A., Littlepage L. E., Karpuj M., Kessenbrock K., Le A., Inoue K., Weaver V. M., Werb Z., Discoidin domain receptor 1 (DDR1) ablation promotes tissue fibrosis and hypoxia to induce aggressive basal-like breast cancers. Genes Dev. 32, 244–257 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hidalgo-Carcedo C., Hooper S., Chaudhry S. I., Williamson P., Harrington K., Leitinger B., Sahai E., Collective cell migration requires suppression of actomyosin at cell–cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat. Cell Biol. 13, 49–59 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagdas S., Kashatus J. A., Nascimento A., Hussain S. S., Trainor R. E., Pollock S. R., Adair S. J., Michaels A. D., Sesaki H., Stelow E. B., Bauer T. W., Kashatus D. F., Drp1 promotes KRas-driven metabolic changes to drive pancreatic tumor growth. Cell Rep. 28, 1845–1859.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen N. D., Yu M., Reddy V. Y., Acevedo-Diaz A. C., Mesarick E. C., Jaoude J. A., Yuan M., Asara J. M., Taniguchi C. M., Comparative untargeted metabolomic profiling of induced mitochondrial fusion in pancreatic cancer. Metabolites 11, 627 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer J. N., Leuthner T. C., Luz A. L., Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 391, 42–53 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youle R. J., Van Der Bliek A. M., Mitochondrial fission, fusion, and stress. Science 337, 1062–1065 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han B., Zhen F., Sun Y., Sun B., Wang H. Y., Liu W., Huang J., Liang X., Wang Y. R., Chen X. S., Li S. J., Hu J., Tumor suppressor KEAP1 promotes HSPA9 degradation, controlling mitochondrial biogenesis in breast cancer. Cell Rep. 43, 114507 (2024). [DOI] [PubMed] [Google Scholar]
- 46.Ngo J., Choi D. W., Stanley I. A., Stiles L., Molina A. J. A., Chen P.-H., Lako A., Sung I. C. H., Goswami R., Kim M., Miller N., Baghdasarian S., Kim-Vasquez D., Jones A. E., Roach B., Gutierrez V., Erion K., Divakaruni A. S., Liesa M., Danial N. N., Shirihai O. S., Mitochondrial morphology controls fatty acid utilization by changing CPT1 sensitivity to malonyl-CoA. EMBO J. 42, e111901 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seifert E. L., Hajnóczky G., Fuel oxidation under the control of mitochondrial shape and dynamics. EMBO J. 42, e114129 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hollestelle A., Elstrodt F., Nagel J. H. A., Kallemeijn W. W., Schutte M., Phosphatidylinositol-3-OH kinase or RAS pathway mutations in human breast cancer cell lines. Mol. Cancer Res. 5, 195–201 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Kashatus J. A., Nascimento A., Myers L. J., Sher A., Byrne F. L., Hoehn K. L., Counter C. M., Kashatus D. F., Erk2 phosphorylation of Drp1 promotes mitochondrial fission and MAPK-driven tumor growth. Mol. Cell 57, 537–551 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serasinghe M. N., Wieder S. Y., Renault T. T., Elkholi R., Asciolla J. J., Yao J. L., Jabado O., Hoehn K., Kageyama Y., Sesaki H., Chipuk J. E., Mitochondrial division is requisite to RAS-induced transformation and targeted by oncogenic MAPK pathway inhibitors. Mol. Cell 57, 521–536 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bajzikova M., Kovarova J., Coelho A. R., Boukalova S., Oh S., Rohlenova K., Svec D., Hubackova S., Endaya B., Judasova K., Bezawork-Geleta A., Kluckova K., Chatre L., Zobalova R., Novakova A., Vanova K., Ezrova Z., Maghzal G. J., Magalhaes Novais S., Olsinova M., Krobova L., An Y. J., Davidova E., Nahacka Z., Sobol M., Cunha-Oliveira T., Sandoval-Acuña C., Strnad H., Zhang T., Huynh T., Serafim T. L., Hozak P., Sardao V. A., Koopman W. J. H., Ricchetti M., Oliveira P. J., Kolar F., Kubista M., Truksa J., Dvorakova-Hortova K., Pacak K., Gurlich R., Stocker R., Zhou Y., Berridge M. V., Park S., Dong L., Rohlena J., Neuzil J., Reactivation of dihydroorotate dehydrogenase-driven pyrimidine biosynthesis restores tumor growth of respiration-deficient cancer cells. Cell Metab. 29, 399–416.e10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mathur D., Stratikopoulos E., Ozturk S., Steinbach N., Pegno S., Schoenfeld S., Yong R., Murty V. V., Asara J. M., Cantley L. C., Parsons R., PTEN regulates glutamine flux to pyrimidine synthesis and sensitivity to dihydroorotate dehydrogenase inhibition. Cancer Discov. 7, 380–390 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ozturk S., Mathur D., Zhou R. W., Mulholland D., Parsons R., Leflunomide triggers synthetic lethality in PTEN-deficient prostate cancer. Prostate Cancer Prostatic Dis. 23, 718–723 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jin R., Liu B., Liu X., Fan Y., Peng W., Huang C., Marcus A., Sica G., Gilbert-Ross M., Liu Y., Zhou W., Leflunomide suppresses the growth of LKB1-inactivated tumors in the immune-competent host and attenuates distant cancer metastasis. Mol. Cancer Ther. 20, 274–283 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamaguchi N., Weinberg E. M., Nguyen A., Liberti M. V., Goodarzi H., Janjigian Y. Y., Paty P. B., Saltz L. B., Kingham T. P., Loo J., de Stanchina E., Tavazoie S. F., PCK1 and DHODH drive colorectal cancer liver metastatic colonization and hypoxic growth by promoting nucleotide synthesis. eLife 8, e52135 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leflunomide in treating patients with previously treated HER2-negative metastatic breast cancer—NCI; https://cancer.gov/about-cancer/treatment/clinical-trials/search/v?id=NCI-2018-02927&r=1.
- 57.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Preibisch S., Rueden C., Saalfeld S., Schmid B., Tinevez J. Y., White D. J., Hartenstein V., Eliceiri K., Tomancak P., Cardona A., Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valente A. J., Maddalena L. A., Robb E. L., Moradi F., Stuart J. A., A simple ImageJ macro tool for analyzing mitochondrial network morphology in mammalian cell culture. Acta Histochem. 119, 315–326 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Bolger A. M., Lohse M., Usadel B., Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T. R., STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li B., Dewey C. N., RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 1–16 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.R Core Team, R: A language and environment for statistical computing [Preprint] (2023); https://R-project.org/.
- 63.Love M. I., Huber W., Anders S., Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu T., Hu E., Xu S., Chen M., Guo P., Dai Z., Feng T., Zhou L., Tang W., Zhan L., Fu X., Liu S., Bo X., Yu G., clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.G. Korotkevich, V. Sukhov, N. Budin, B. Shpak, M. N. Artyomov, A. Sergushichev, Fast gene set enrichment analysis. bioRxiv 060012 [Preprint] (2021). 10.1101/060012. [DOI]
- 66.Pang Z., Chong J., Zhou G., de Lima Morais D. A., Chang L., Barrette M., Gauthier C., Jacques P.-É., Li S., Xia J., MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 49, W388–W396 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mookerjee S. A., Gerencser A. A., Nicholls D. G., Brand M. D., Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements. J. Biol. Chem. 292, 7189–7207 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gao J., Aksoy B. A., Dogrusoz U., Dresdner G., Gross B., Sumer S. O., Sun Y., Jacobsen A., Sinha R., Larsson E., Cerami E., Sander C., Schultz N., Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 6, pl1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cerami E., Gao J., Dogrusoz U., Gross B. E., Sumer S. O., Aksoy B. A., Jacobsen A., Byrne C. J., Heuer M. L., Larsson E., Antipin Y., Reva B., Goldberg A. P., Sander C., Schultz N., The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foroutan M., Bhuva D. D., Lyu R., Horan K., Cursons J., Davis M. J., Single sample scoring of molecular phenotypes. BMC Bioinformatics 19, 404 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bhuva D. D., Cursons J., Davis M. J., Stable gene expression for normalisation and single-sample scoring. Nucleic Acids Res. 48, e113 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.T. Hothorn, maxstat: Maximally selected rank statistics [Preprint] (2017); https://CRAN.R-project.org/package=maxstat.
- 73.T. M. Therneau, A package for survival analysis in R [Preprint] (2023); https://CRAN.R-project.org/package=survival.
- 74.A. Kassambara, M. Kosinski, P. Biecek, survminer: Drawing Survival Curves using “ggplot2” [Preprint] (2021); https://CRAN.R-project.org/package=survminer.
- 75.Hänzelmann S., Castelo R., Guinney J., GSVA: Gene set variation analysis for microarray and RNA-Seq data. BMC Bioinformatics 14, 7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S7