ABSTRACT
Apoptosis, necroptosis and pro-inflammatory NF-κB-dependent signaling are repressed by receptor-interacting serine/threonine-protein kinase 1 (RIPK1). A recent paper in Immunity describes a small molecule inducing the proteolytic degradation of RIPK1. In preclinical experiments, this RIPK1 inhibitor improved the anticancer efficacy of radiotherapy, immunotherapy (with PD-1 blockade) and radioimmunotherapy (with CTLA-4 blockade).
KEYWORDS: Cancer immunotherapy, immune checkpoint inhibitor, immunogenic cell death, necroptosis, radiotherapy
Main text
In a recent study, Mannion J et al. investigated the role of receptor-interacting serine/threonine-protein kinase 1 (RIPK1) in cancer biology, highlighting its dual function as both a driver of cell survival and an orchestrator of immunogenic cell death (ICD).1 This builds upon a growing body of literature emphasizing the importance of ICD in enhancing anticancer immune responses, a concept that is increasingly seen as central to optimizing cancer therapies, particularly in the context of overcoming resistance to immunotherapy and radiotherapy.
ICD represents a critical mechanism in cancer immunosurveillance, wherein stressed or dying cancer cells release damage-associated molecular patterns (DAMPs) that stimulate an effective antitumor immune response.2,3 In this process, various signaling pathways, including those regulated by RIPK1, play crucial roles in determining whether a cell death event will be immunogenic, thereby influencing the ability of the immune system to recognize and eradicate cancer cells.
Mannion and colleagues extend these findings by focusing on the complex regulatory functions of RIPK1 in both cell death and survival, involving the NF-κB pathway, noting that cancer cells frequently exploit the scaffold function of RIPK1 to evade necroptosis, a form of regulated necrosis associated with robust immunogenicity.4 By developing a small-molecule proteolysis-targeting chimera (PROTAC) that selectively degrades RIPK1, they have identified a strategy to circumvent this cancer defense mechanism, triggering necroptosis and promoting ICD.
Their research reveals that degradation of RIPK1 not only sensitizes cancer cells to treatment-induced tumor necrosis factor (TNF) and interferons but also potentiates the immunostimulatory effects of radiotherapy, anti-PDCD1 (programmed cell death 1, best known as PD-1)-based immunotherapy, and anti-CTLA4-based radioimmunotherapy (Figure 1). This finding echoes previous reports showing that necroptotic cancer cells can enhance CD8+ T cell cross-priming and elicit durable antitumor immunity.5,6
Figure 1.
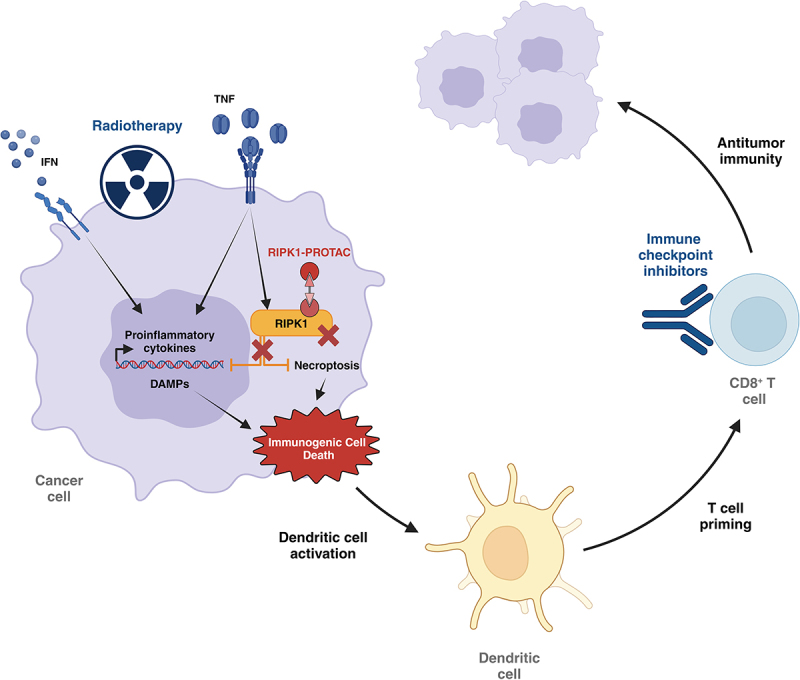
RIPK1-PROTAC improves the efficacy of radio- and immune-therapy. RIPK1 inhibits RIPK3/MLKL-triggered necroptosis and the production of proinflammatory factors. RIPK1-PROTAC degrades RIPK1 which sensitizes cancer cells treated by radiotherapy and/or immune checkpoint inhibitors (anti-PD-1, anti-CTLA-4) to immunogenic necroptotic cell death. The related emission/exposure of DAMPs accompanied by the secretion of inflammatory cytokines (e.g. chemokines, IFNs, TNFs) favor the recruitment and activation of dendritic cells and the subsequent priming of tumor-specific cytotoxic CD8+ T cells. DAMP, damage-associated molecular pattern; IFN, interferon; PROTAC, proteolysis-targeting chimera; TNF, tumor-necrosis factor.
However, at least at first glance, the role or RIPK1 in modulating the immunogenicity of necroptosis appears controversial. A prior report suggested that RIPK1 activation is required for necroptosis to be immunogenic because knockout of the Ripk1 gene reduced the capacity of polyinosinic-polycytidylic acid (poly I:C)-treated cells (which undergo necroptosis) to induced immune responses against the model antigen ovalbumin and to elicit protective immune responses in vaccination experiments.5 In sharp contrast, PROTAC-induced degradation of RIP1K enhances the immunogenicity of cell death.4 Future studies must investigate whether the knockout of the Ripk1 gene and the PROTAC-induced degradation of RIPK1 protein have exactly the same cell biological consequences or whether one of the two methods for inhibiting RIPK1 may have off-target effects explaining these discrepant conclusions. Alternatively, the duration of RIPK1 inhibition might affect the system. Indeed, acute pharmacological removal of RIPK1 may have rather distinct effects than knockout of the gene, because the latter can lead to long-term adaptation of the cells, as this has been suggested by Mannion et al.4
The work by Mannion et al. aligns with emerging approaches aimed at exploiting immunogenic forms of cell death, such as necroptosis and ferroptosis, to boost antitumor immune responses.7–9 Along this way, Zhang J et al. recently described a synthetic compound named necrocide-1 (NC1). NC1 operates as an inducer of TNF-independent necrosis accompanied with the release of DAMPs, underscoring the therapeutic potential of pharmacologically triggering necrotic cell death to enhance the immunogenicity of cancer cells.10
This work highlights not only the versatility of RIPK1 as a therapeutic target but also the far-reaching implications of RIPK1 degradation in driving lasting antitumor immunity. By disrupting cancer cells’ reliance on RIPK1 for survival and immune modulation, the PROTAC approach amplifies the inflammatory cascade associated with DAMP release, enhances dendritic cell recruitment, and strengthens CD8+ T cell priming, all of which are critical to effective antitumor responses. Mannion et al.’s findings provide a significant contribution to the field, bridging molecular targeting and immune modulation to potentiate current treatment modalities and present a promising pathway for translational cancer therapies that synergize with existing therapeutic frameworks. This could be transformative in clinical contexts where treatment-resistant malignancies necessitate innovative and multi-faceted therapeutic strategies.
Funding Statement
J.G.P is supported by the SIRIC Cancer Research and Personalized Medicine (CARPEM); Multi-Organism Institute (ITMO) Aviesan Cancer (National Alliance for Life Sciences and Health), Institut National du Cancer (INCa), and Fondation pour la Recherche Médicale (FRM). GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR-22-CE14-0066 VIVORUSH, ANR-23-CE44-0030 COPPERMAC, ANR-23-R4HC-0006 Ener-LIGHT); Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Joint Programme on Rare Diseases (EJPRD) Wilsonmed; European Research Council Advanced Investigator Award (ERC-2021-ADG, Grant No. 101052444; project acronym: ICD-Cancer, project title: Immunogenic cell death (ICD) in the cancer-immune dialogue); The ERA4 health Cardinoff Grant Ener-LIGHT; European Union Horizon 2020 research and innovation programmes Oncobiome (grant agreement number: 825410, Project Acronym: ONCOBIOME, Project title: Gut OncoMicrobiome Signatures [GOMS] associated with cancer incidence, prognosis and prediction of treatment response, Prevalung (grant agreement number 101095604, Project Acronym: PREVALUNG EU, project title: Biomarkers affecting the transition from cardiovascular disease to lung cancer: toward stratified interception), Neutrocure (grant agreement number 861878 : Project Acronym: Neutrocure; project title: Development of “smart” amplifiers of reactive oxygen species specific to aberrant polymorphonuclear neutrophils for treatment of inflammatory and autoimmune diseases, cancer and myeloablation); National support managed by the Agence Nationale de la Recherche under the France 2030 programme (reference number 21-ESRE-0028, ESR/Equipex+ Onco-Pheno-Screen); Hevolution Network on Senescence in Aging; Institut National du Cancer (INCa); Institut Universitaire de France; LabEx Immuno-Oncology ANR-18-IDEX-0001; a Cancer Research ASPIRE Award from the Mark Foundation; PAIR-Obésité INCa_1873, the RHUs Immunolife and LUCA-pi (ANR-21-RHUS-0017 and ANR-23-RHUS-0010, both dedicated to France Relance 2030); Seerave Foundation; SIRIC Cancer Research and Personalized Medicine (CARPEM). This study contributes to the IdEx Université de Paris Cité ANR-18-IDEX-0001. Views and opinions expressed are those of the author(s) only and do not necessarily reflect those of the European Union, the European Research Council or any other granting authority. Neither the European Union nor any other granting authority can be held responsible for them.
Disclosure statement
J.G.P. is the inventor of patents covering the diagnosis, prognosis, and treatment of cancers, including patents licensed to Turnstone Biologics and Therafast Bio. GK has been holding research contracts with Daiichi Sankyo, Eleor, Kaleido, Lytix Pharma, PharmaMar, Osasuna Therapeutics, Samsara Therapeutics, Sanofi, Sutro, Tollys, and Vascage. GK is on the Board of Directors of the Bristol Myers Squibb Foundation France. GK is a scientific co-founder of everImmune, Osasuna Therapeutics, Samsara Therapeutics and Therafast Bio. GK is in the scientific advisory boards of Hevolution, Institut Servier, Longevity Vision Funds and Rejuveron Life Sciences. GK is the inventor of patents covering therapeutic targeting of aging, cancer, cystic fibrosis and metabolic disorders. GK’s wife, Laurence Zitvogel, has held research contracts with Glaxo Smyth Kline, Incyte, Lytix, Kaleido, Innovate Pharma, Daiichi Sankyo, Pilege, Merus, Transgene, 9 m, Tusk and Roche, was on the on the Board of Directors of Transgene, is a cofounder of everImmune, and holds patents covering the treatment of cancer and the therapeutic manipulation of the microbiota. GK’s brother, Romano Kroemer, was an employee of Sanofi and now consults for Boehringer-Ingelheim. The funders had no role in the writing of the manuscript.
Data availability statement
This manuscript comments data published by Mannion J et al (Immunity 2024 Jul 9;57(7):1514–1532).
References
- 1.Mannion J, Gifford V, Bellenie B, Fernando W, Ramos Garcia L, Wilson R, John SW, Udainiya S, Patin EC, Tiu C, et al. A RIPK1-specific PROTAC degrader achieves potent antitumor activity by enhancing immunogenic cell death. Immunity. 2024;57(7):1514–1532.e15. doi: 10.1016/j.immuni.2024.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kroemer G, Chan TA, Eggermont AMM, Galluzzi L.. Immunosurveillance in clinical cancer management. CA A Cancer J Clinicians. 2024;74(2):187–3. doi: 10.3322/caac.21818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galluzzi L, Guilbaud E, Schmidt D, Kroemer G, Marincola FM. Targeting immunogenic cell stress and death for cancer therapy. Nat Rev Drug Discov. 2024;23(6):445–460. doi: 10.1038/s41573-024-00920-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang H, Ma Y, Chen G, Zhou H, Yamazaki T, Klein C, Pietrocola F, Vacchelli E, Souquere S, Sauvat A, et al. Contribution of RIP3 and MLKL to immunogenic cell death signaling in cancer chemotherapy. Oncoimmunology. 2016;5(6):e1149673. doi: 10.1080/2162402X.2016.1149673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yatim N, Jusforgues-Saklani H, Orozco S, Schulz O, Barreira da Silva R, Reise, Green DR, Reis e Sousa C, Oberst A, Albert ML. RIPK1 and nf-κB signaling in dying cells determines cross-priming of CD8 + T cells. Science. 2015;350(6258):328–334. doi: 10.1126/science.aad0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aaes TL, Kaczmarek A, Delvaeye T, De Craene B, De Koker S, Heyndrickx L, Delrue I, Taminau J, Wiernicki B, De Groote P, et al. Vaccination with necroptotic cancer cells induces efficient anti-tumor immunity. Cell Rep. 2016;15(2):274–287. doi: 10.1016/j.celrep.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 7.Meier P, Legrand AJ, Adam D, Silke J. Immunogenic cell death in cancer: targeting necroptosis to induce antitumour immunity. Nat Rev Cancer. 2024;24(5):299–315. doi: 10.1038/s41568-024-00674-x. [DOI] [PubMed] [Google Scholar]
- 8.Catanzaro E, Demuynck R, Naessens F, Galluzzi L, Krysko DV. Immunogenicity of ferroptosis in cancer: a matter of context? Trends In Cancer. 2024;10(5):407–416. doi: 10.1016/j.trecan.2024.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Kayagaki N, Webster JD, Newton K. Control of cell death in health and disease. Annu Rev Pathol Mech Dis. 2024;19(1):157–180. doi: 10.1146/annurev-pathmechdis-051022-014433. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Trojel-Hansen C, Wang J, Zhang Z, Wang X, Qiao Y, Jiao H, Michaud M, Kepp O, Jäättelä M, et al. Necrocide 1 mediates necrotic cell death and immunogenic response in human cancer cells. Cell Death Dis. 2023;14(4):238. doi: 10.1038/s41419-023-05740-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This manuscript comments data published by Mannion J et al (Immunity 2024 Jul 9;57(7):1514–1532).


