Abstract
Anterior cruciate ligament (ACL) injury rates are on the rise, despite improved surgical techniques and prevention programs. While traditional rehabilitation emphasizes the restoration of motion, strength, and physical performance, emerging research highlights the importance of addressing neurocognitive deficits that can persist after injury. These deficits, including altered proprioception, impaired motor control and muscle recruitment, as well as heightened reliance on visual feedback, can significantly increase the risk of re-injury and impede return to sport. The purpose of this clinical commentary is to outline a proposed comprehensive approach to rehabilitation that challenges the neurocognitive system to optimize rehabilitation outcomes and reduce reinjury risk. Thus, this clinical commentary discusses the rationale for integrating neurocognitive training into all phases of ACLR rehabilitation, from initial injury to eight weeks post-surgery. It details the neurophysiological changes caused by ACL injury and presents evidence supporting the use of exercises that challenge visual attention, decision-making, and motor planning. A comprehensive rehabilitation framework incorporating both physical and neurocognitive components is proposed, aiming to improve long-term outcomes and reduce re-injury risk.
Level of Evidence: 5
Keywords: Anterior cruciate ligament reconstruction, neurocognitive training, neuroplasticity, proprioception, motor control
Introduction
Each year, an estimated 300,000 ACL injuries occur annually in the United States, resulting in about 225,000 to 250,000 surgeries.1 Approximately 70% of ACL injuries occur from a non-contact mechanism, such as running and pivoting, cutting, or landing from a jump.2–4 Unfortunately, between 10 and 25% of individuals will sustain a second ACL injury.5–7
Female athletes in sports like soccer, basketball, and volleyball, as well as gymnasts, and male football players, face a heightened risk of injury. These injuries are often triggered by unanticipated or decelerating movements, and the injury that is sustained disrupts the body’s normal sensory input and motor control, leading to neuroplastic changes in the brain.8 Research has shown that these changes can manifest as decreased reaction times, processing speeds, and proprioception, further contributing to the risk of injury.9–13
Neuroplasticity and ACL injury
Neuroplasticity, the brain’s ability to form new neural connections, plays a crucial role in recovery from ACL injury. This process can involve modification of cognitive strategies, recruitment of new and different neural networks (neural patterning), or adjusting the strength of existing connections in brain areas in charge of carrying out particular tasks.14 Neuroplasticity can be both beneficial and deleterious. While positive effects can enhance motor control, negative effects can lead to inefficient and compensatory movement patterns.14 This central nervous system re-wiring combined with biomechanical alterations in loading strategies could explain the reason some ACL patients are unable to recruit their quadriceps muscle leading to a quadriceps avoidance gait. As Wexler et al. describe, this maladaptation or negative neuroplastic change involves walking with a slightly flexed knee, marked by overactive hamstring musculature and inhibited quadriceps.15 If not corrected, this could lead to a delay in the return of quadriceps muscle strength, and poorer knee function.
Neuroplastic changes that occur following ACL injury are likely due to altered afferent input which affects the efferent output. The alteration of afferent input is often a result of a loss of somatosensory signal from the ruptured ligament and increased nociceptor activity associated with pain, swelling, and inflammation.11 The altered efferent output leads to a disruption in gamma-motor neuron feedback loops,16 delayed long latency reflexes,17 and altered spinal excitability.18 Therefore, increased cortical excitability is required to generate a muscle contraction after ACLR.19,20 This mechanism results in the shift from what used to be a feedforward or semi-automatic movement, to movements that now require increased volitional control. Grooms et al. demonstrated this paradigm utilizing fMRI with individuals following ACLR, finding that ACLR patients demonstrated increased cortical drive, visual dependency, and decreased neuromuscular control compared to controls during a simple supine knee extension exercise triggered by a visual prompt.10
Often, the regions of the brain most affected following ACL injury are those involved in visual and cognitive function.21 This results in an overreliance on visual processing/feedback and motor planning to maintain neuromuscular control and dynamic stabilization of the knee. Baumeister et al, utilizing electroencephalography, demonstrated that individuals following ACLR had greater brain activation in attention and sensory areas, however this did not correlate to improvements in proprioceptive performance.22 They concluded that the increased activation may be attributed to decreased efficiency and/or increased excitability required to complete the same task. Each of these studies highlight the complexity of neuroplastic changes that occur after ACL injury, therefore it is important to address these factors in rehabilitation.
Neurocognitive Rehabilitation: A Comprehensive Approach
Given the prevalence of non-contact, unanticipated injuries and their impact on the brain, the authors suggest implementing early neurocognitive training into ACL rehabilitation immediately following injury and/or surgery. This approach aims to improve neuromuscular control and dynamic stabilization, with the ultimate goal of reducing reinjury risk.
Neurocognitive rehabilitation can be thought of as any task that occupies the patient/athlete’s attention through visual, mental, auditory, verbal and/or kinesthetic stimuli, while simultaneously performing a movement, task, exercise, or skill.12 As the patient demonstrates more automatic control with decreased response time and proper mechanics, the complexity of the task is increased. The neurocognitive variables could include the following: speed, dual tasking, verbal, visual tracking, perturbations, directions, obstacles, and surfaces.
Traditional ACLR rehabilitation often focuses on physical aspects such as limb symmetry, range of motion, and strength, with less emphasis on neuromuscular and neurocognitive adaptations. Additionally, there is a reliance on an internal focus of control (i.e. “squeeze your quad”, “don’t let your knee go in” or “bend your knee”) as opposed to an external control (i.e. responding to a visual stimulus, hitting a target or catching a ball). While this may be necessary in the early stages for focusing attention to complete activation of specific muscles, it doesn’t fully prepare athletes for the dynamic and reactive environment of their sport. Internal focus can overload cognitive resources, leading to decreased neuromuscular control during complex tasks. Shifting towards an external focus, where athletes respond to environmental stimuli, promotes more automatic movements and better prepares them for the demands of their sport.
Principles of Neurocognitive Rehabilitation
Sports are filled with neurocognitive challenges that expand far beyond just the physical demands placed on an individual. Chaput et al. explain that visual-cognitive processes in sport require components of both divided and selective visual attention.21 Divided attention is described as the ability to simultaneously provide attention to or switch between multiple stimuli (i.e. dual tasking). Divided attention can further be described by the limited or multiple resource theory.23,24 When both stimuli rely on the same sense, such as auditory, they become competitive in nature (limited resource theory) versus when two stimuli affect different senses, such as vision and auditory, it is easier to multi-task (multiple resource theory). Selective attention is being able to ignore irrelevant stimuli to focus on a singular task which can occur by volitional control (top-down) or reactionary due to an un-anticipatory event (bottom-up). It has been mentioned that neurocognitive challenges for athletes do not have to be sport specific if they challenge the same underlying neurocognitive demands of sport (i.e. working memory, rapid decision making, visual processing, or postural perturbations).12,21
With increased task complexity, neuromuscular control deteriorates in those following ACLR as compared to healthy controls, possibly due to an overload of the motor planning resources.11 Sherman et al. found that individuals had greater cortical inhibition and committed more decision accuracy errors to maintain reaction time following ACLR, compared to healthy controls during a Go/No Go task stimulated by a visual target.25 Recently Chaput et al.21 have described implementing the Visual-Cognitive Control Chaos Continuum (VC-CCC) within ACLR rehabilitation which is an expansion from the original Control Chaos Continuum described by Taberner et al.26 In the VC-CCC, the authors proposed a five-phase progression beginning at high control and stability and ending at a high chaotic environment mimicking the visual cognitive demands of sport. As task complexity increases, if performance is errorless then the challenge may not be difficult enough to elicit learning.27
In the authors’ experience, a comprehensive approach that includes neurocognitive training is necessary to address neuroplasticity changes affecting proprioception and motor control deficits post-ACLR. The senior author has found that incorporating a combination of neuromuscular and neurocognitive training has led to significantly improved outcomes for ACL patients. Therefore, the purpose of this clinical commentary is to outline a proposed comprehensive approach to rehabilitation that challenges the neurocognitive system to optimize rehabilitation outcomes and reduce reinjury risk.
ACL Rehabilitation and the inclusion of Neurocognitive Strategies
It is imperative to include exercises that address these deficits both immediately after injury and throughout the continuum of care to prevent the deleterious effects of neuroplasticity. Performing a motor task while altering visual input through external factors (e.g., blindfolds/goggles, targets, balls, defenders, visual signals) has been shown to negatively impact neuromuscular control in ACLR patients compared to performing motor tasks alone.20 In addition, vibration can positively impact efferent motor output by enhancing muscle activation and proprioception.28,29 Moreover, many studies indicate no difference at one and two-year follow up with the addition of neuromuscular training alone,28,29 indicating the need for a cognitive component to be included during the exercise. Finally, deficits in both unilateral and contralateral limb proprioception and neuromuscular control30 exist after injury; thus it is important to include education and training for the entire neurocognitive system. The authors’ detailed program including variables and progressions is outlined in Table 1.
Table 1. ACLR Rehab Initial Phase (0-8 weeks) Neurocognitive Drills.
| Exercise/Drill | Variables/progressions to enhance neuroplasticity | Neurocognitive Demand | Examples: |
|---|---|---|---|
| Quad sets Straight leg raises (3 way) Knee extensions (90-40 deg) |
NMES EMG biofeedback Reactive cognitive motor task (BlazePods) |
Visual Cognitive Efferent firing Cognitive Dual task External focus of control Reaction time |
Quad set with NMES Straight leg raises while using UE to react to indicated BlazePod color Knee extensions using visual EMG biofeedback Virtual Reality Glasses |
| Mini squats Standing TKEs |
Surface (i.e. foam, rocker board) Resistance Dual task (i.e ball toss or HecoStix) Reactive cognitive motor task (BlazePods, Neurocognitive Sensory station) Perturbations Cognitive task (counting, memory, etc.) Altered vision Contralateral limb/UE task |
Visual Cognitive Decision making Memory Dual task External focus of control Reaction time |
Mini squats on rocker board with external perturbations while throwing/catching a ball Standing TKE while using UE to react to indicated quickboard target |
| Gait/stepping drills: Weight shifts Cone stepping Lateral lunges Lateral stepping (slides) Quick Board stepping drills |
Speed Direction (ant/post/lateral) Dual task (i.e ball toss or HecoStix) Cognitive task (counting, memory, etc.) Reactive cognitive-motor task |
Visual Cognitive Decision making Memory Dual task External focus of control Reaction time |
Weight shifts on force plates with visual feedback Multi-directional cone stepping counting backward by 7’s and tossing HecoStix Lateral lunges with sport cord perturbation and ball toss, landing on foam surface Lateral slides with resistance band around knees, reacting to verbal cues (direction, speed) and catching ball Rapid stepping in place with visual target, reacting to correct location on the board or surface (Quickboard) |
| CKC neuromuscular exercises: Anterior/lateral step downs Multi-directional lunges |
Resistance Surface Dual task (i.e ball toss or HecoStix) Perturbation/resistance from CLX band Cognitive task (counting, memory, etc.) Reactive cognitive motor task (BlazePods) |
Visual Cognitive Decision making Memory Dual task External focus of control Reaction time |
Anterior step down from box with CLX band pulling into femoral IR and dynamic LE valgus (figure 8) Lateral lunges with sport cord perturbation and ball toss toward indicated direction. Athlete must react to indicated direction using light up pods (i.e. antero-lateral or postero-lateral) |
| Single leg balance: SL RDL with UE task SL balance with band perturbations |
Resistance Surface Dual task (i.e. ball toss) Direction (star drill on floor) Visual targets Perturbation (KB exchange/resistance band) UE task (i.e cable row, med ball chop, reach) |
Visual Cognitive Decision making Memory Dual task External focus of control Reaction time |
SL RDL standing on foam while catching a ball and performing RDL to the indicated cone (5 cones set up in front of the patient) Single-leg balance with band resistance and valgus perturbations: The patient stands on a rocker board while resisting external forces, dribbling a basketball, and catching Hecostix with contralateral UE (figure 9) |
| Dynamic shuffling | Speed Direction Visual targets (with/without distraction targets) Auditory cues (i.e direction/speed) Height/location of target Sport specific task (i.e catching basketball or lacrosse ball with stick) |
Visual Cognitive Decision making Memory Dual task External focus of control Reaction time |
Lateral Shuffles with BlazePod and Sport Specific Ball Catching (figure 11) |
NMES= neuromuscular electrical stimulation, EMG=Electromyographic, UE= upper extremity, CLX= continuous loop Theraband; IR= internal rotation, LE= lower extremity, SL= single limb, RDL= Romanian dead lift, KB= kettle bell
Pre-operative Phase
The pre-operative phase of ACL reconstruction is crucial for preparing the patient physically and mentally for surgery and subsequent rehabilitation and is ideally incorporated immediately following injury. The primary goals during this phase include reducing inflammation and pain to achieve a “quiet knee” prior to surgery, normalizing ROM (especially extension and flexion), quadriceps muscle activation, preventing significant quadriceps atrophy, and normalizing gait. A critical component of this phase is the incorporation of neurocognitive exercises to decrease early motor compensations and quadriceps muscle inhibition. The specific exercises are recommended as soon as possible.
Motor and cognitive dual-tasking exercises are implemented, where patients perform functional and cognitive tasks simultaneously. This approach promotes agonist/antagonist muscle coactivation while stimulating brain centers responsible for motor planning and proprioception.20 Patients who initiate gait training early have been shown to have decreased compensations and increased functional outcomes compared to those who initiate gait training later in rehab.31 Gait training drills such as stepping over cones is initiated to develop symmetry between the lower extremities. Symmetrical hip, knee, and ankle flexion over the cone, quadriceps and hip control during weight acceptance, gastroc/soleus symmetry during push-off, and equal stance time between limbs are all vital components in this exercise. This drill is progressed to tax the neurocognitive system by performing at various speeds, in multiple directions (forward, backward, lateral), (Figure 1a, 1b) adding serial counting/memory recall drills, ball tossing/catching, or using strobe goggles.
Figure 1a. Forward/Backward Cone Stepping: Patient steps over cones, focusing on symmetry and 90-degree hip/knee/ankle flexion.
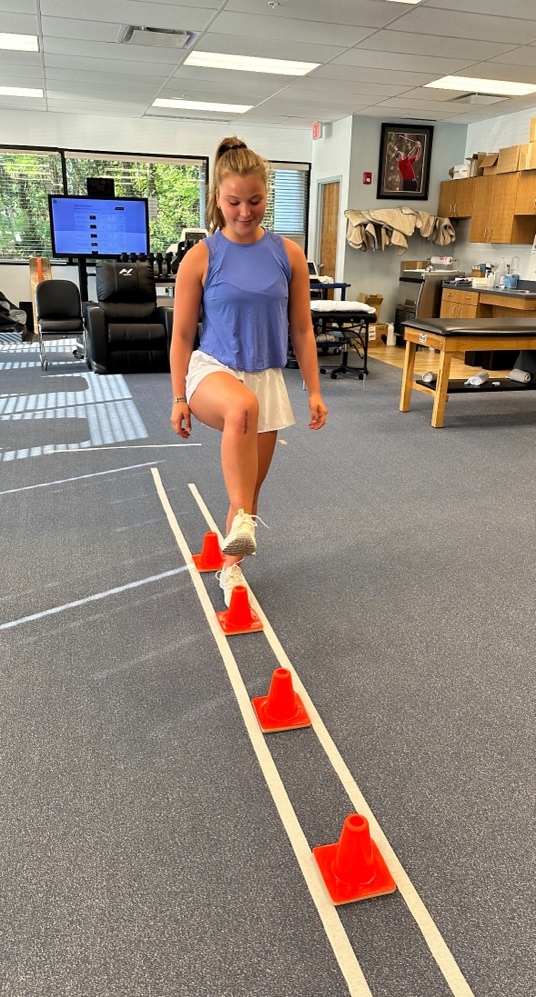
Figure 1b. Lateral Cone Stepping: Patient steps laterally over cones, emphasizing controlled movement and core engagement.

Additional exercises include squats on a tilt board while throwing/catching a ball or balancing on an unstable surface while catching a randomly colored stick and reacting to the correct color. These exercises target balance, reaction time, and visual processing. Furthermore, active joint repositioning drills can be performed with eyes closed to enhance proprioception. These early intervention drills are designed to minimize and prevent negative neuroplastic adaptations. Consistent implementation of these exercises prior to surgery is crucial to optimize beneficial neuroplastic changes and prevent maladaptive patterns.
Immediate Post-operative Phase (Days 1-7)
The immediate post-operative phase echoes the goals of the pre-operative phase and focuses on achieving a “quiet knee” quickly to facilitate early quadriceps activation, protect the surgical graft, and aid in regaining extension ROM. From day one, quadriceps activation exercises are implemented using neuromuscular electrical stimulation (NMES) and, for those with difficulty in efferent firing, electromyographic (EMG) visual biofeedback devices. These techniques attempt to override quadriceps inhibition by increasing action potentials in motor units (NMES)32 or enhancing real time visual feedback (EMG), allowing patients to adjust their muscle activation patterns during exercise. A limitation of using NMES alone is that, while many patients experience improved quadriceps contraction during individual sessions, it does not directly improve knee extensor strength. Additionally, there is often minimal carryover (voluntary control) to the next session, indicating limited beneficial neuroplasticity. Grooms et al. found that increased demands on the central nervous system during complex, high-stakes activities can override normal efferent inhibition mechanisms.11 To accomplish this, the authors advocate for the use of exercises such as quad sets (QS), straight leg raises (SLR), and knee extensions from 90-40 degrees with NMES to assist patients in quadriceps activation. These can be also performed with the patient visualizing EMG data for biofeedback to improve contraction or combined with a reactive cognitive-motor task, such as performing a QS while throwing/catching a ball or tapping light-up pods (BlazePods, Blazepod, San Diego, CA) with the upper extremity (Figure 2/Supplemental File 1). Patients can also perform knee extensions with NMES from 90 to 40 degrees with light resistance while performing hand taps with Blazepods (Figure 3/Supplemental File 2). Finally, standing terminal knee extensions with a resistance band behind the knee can be implemented using BlazePods to reinforce neural patterning (Figure 4/Supplemental File 3).
Figure 2. BlazePod Quad Set: Patient performs isometric quadriceps contraction with NMES while tapping corresponding BlazePods (San Diego, CA).
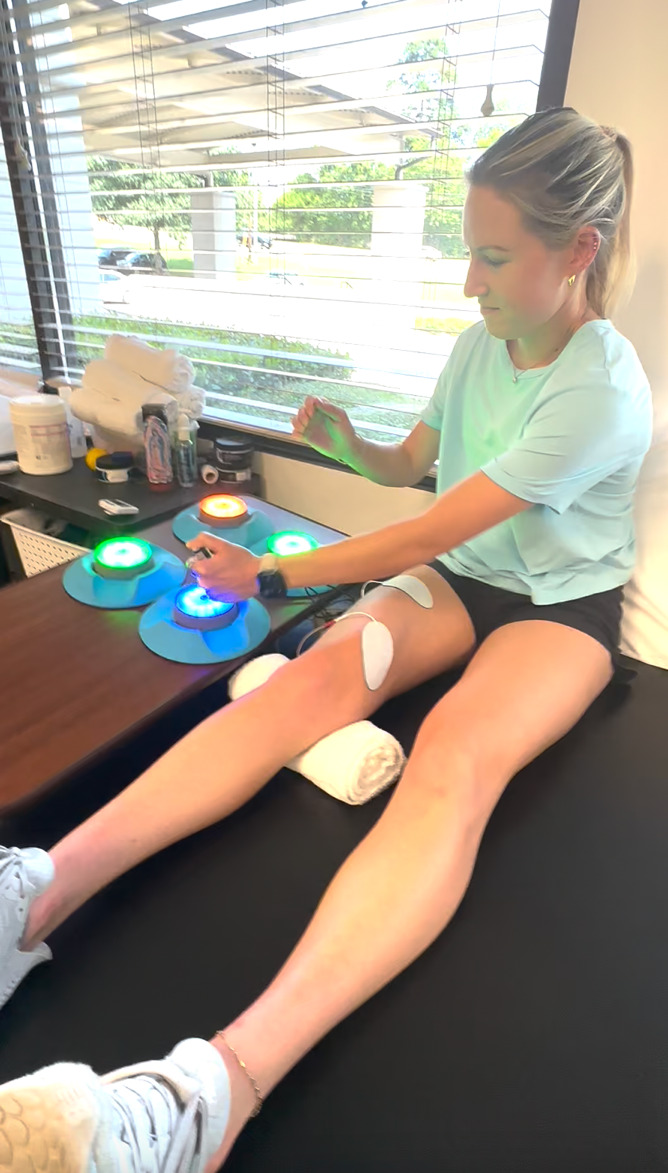
See Supplemental File 1 for video.
Figure 3. BlazePod Knee Extension: Patient performs assisted knee extension while tapping corresponding BlazePods (San Diego, CA).
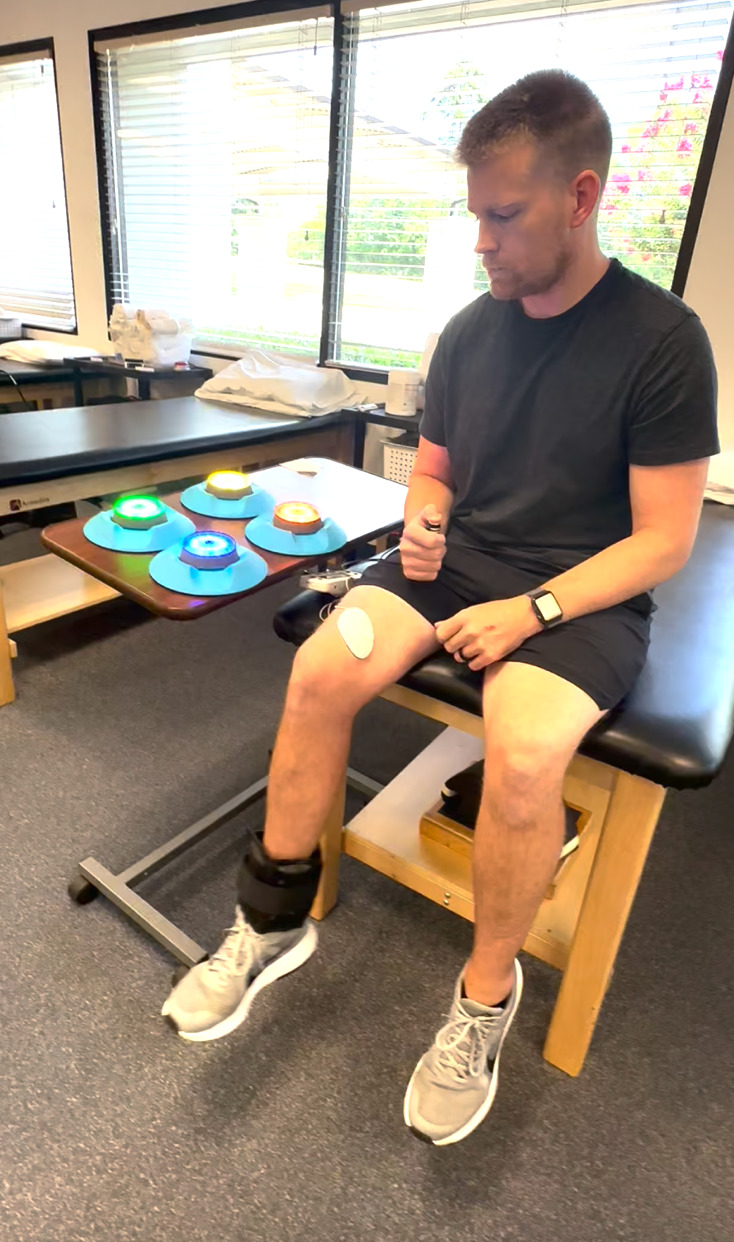
See Supplemental File 2 for video.
Figure 4. Blazepod TKE: Patient performs standing terminal knee extensions with band resistance while tapping BlazePods (San Diego, CA).

See Supplemental File 3 for video.
Acute Phase (days 8-21)
The acute phase of rehab builds upon the immediate post-operative phase, focusing on managing residual inflammation, progressing range of motion, gait training, optimizing quadriceps firing. During this time, proprioceptive/balance training is introduced to increase neuromuscular control and prepare for more advanced stages of rehabilitation. An external focus of control is utilized to reinforce motor learning, as this has been shown to increase intracortical inhibition, which plays a role in increasing quadriceps firing.9
Double leg exercises such as mini squats and weight shifts are performed first advancing to single leg exercises such as balance and stepping drills as a progression. The mini squat is performed from 0 to 45 degrees of flexion for maximal quadriceps and hamstring co-activation. A neurocognitive challenge can easily be added by introducing an unstable surface such as foam or a tilt board under the patient’s feet. The clinician can perturbate the surface so that the patient must react and right the rocker board (Figure 5). As proprioception improves, the patient can catch objects or perform cognitive tasks such as counting, math problems, or responding to visual stimuli during the task. If the patient continues to demonstrate quad avoidance gait, standing terminal knee extension (TKE) exercises with Blazepods can be performed.
Figure 5. Rocker Board Squats: Patient performs mini squats on rocker board while clinician provides perturbations.

Reinforcing proper gait patterns in this phase is vital as soon as possible as the patient will be transitioning off crutches and utilizing the drop-lock brace only for ambulation. Gait compensations that commonly occur after ACLR include reduced knee flexion, increased hip flexion/circumduction, reduced knee extension moment, altered quadriceps-hamstring co-activation during stance, along with reduced ipsilateral stance time.33–35 Stepping drills over cones can be utilized again here to challenge the neurocognitive system and reinforce proper mechanics.
Once the patient demonstrates appropriate gait mechanics and control with double leg exercises, lunging and stepping movements can be implemented to work towards single leg activity. Lunges are performed as the patient steps to the side and lands on a flexed knee to approximately 30-45 degrees for maximal stability. Each of these tasks are initiated straight in the frontal plane on a solid surface. In this phase, a ball toss or slight external perturbation may be added to incorporate additional core and lower extremity musculature with feedforward mechanisms.
Single leg exercises begin with eyes open, solid surface, and static positions, progressing with visual disturbances, varied surfaces, and dynamic UE tasks. Examples include single leg balance on the floor with the knee slightly flexed to 30-45 degrees. This can be performed on various unstable surfaces with external perturbations, and/or a reactive sensorimotor task on a television screen in front of them (ex: using Senaptec Sensory station [Senaptec, Beaverton, OR], reacting to animals or solving math problems on the screen – Figure 6). Each of these exercises are vital to include early in the rehabilitation process in order to reinforce fundamental movement patterns and prepare the patient for more complex movements in the later stages.
Figure 6. Neurocognitive Single Leg Balance: Patient balances on surgical leg on rocker board while solving math problems on Senaptec Sensory Station (Beaverton, OR) and maintaining balance during perturbations.

Intermediate Phase (weeks 4-8)
The intermediate phase of ACLR rehabilitation focuses on restoring full knee range of motion (ROM) to 0°-135°-145°, advancing lower extremity strength/endurance, balance, and neuromuscular control. Most patients will unlock and discharge their knee immobilizer in this phase, so it is imperative to prepare the neuromuscular system for dynamic environments in their daily life while focusing on limb confidence and closed kinetic chain (CKC) function.
CKC neuromuscular control exercises in the sagittal plane are initiated with an anterior step down on level ground. The patient is instructed to place their hands on their hips and tap the heel of the uninvolved leg on the ground in front of them while controlling dynamic lower extremity (LE) valgus and pelvic pronation. Initially, a mirror is used as an external focus of control. This can be progressed to an elevated surface using weights, and eventually with spiral band resistance to enhance the control of rotational patterns (Figure 7). The front step-down exercise can also be enhanced with the utilization of blazepods for reactive stability. These progressions will be described in greater detail in Part 2 of this commentary.
Figure 7. Single leg step downs with spiral band resistance: Patient performs step-downs with resistance band applying adduction and internal rotation force.

Frontal plane movements are progressed with lateral lunges and lateral stepping drills. Lateral stepping is introduced once the patient demonstrates appropriate sagittal plane control. This is performed with a resistance band placed around the knees while the patient steps laterally at the assigned speed (slow, medium, or fast) and direction. A ball is then thrown for additional neurocognitive engagement as the patient must react to both the verbal direction and direction of the ball. Faster speeds and reactive directions are reserved for the later stages.
Lunges are progressed to diagonal lunges in both the anterior and posterior direction and upon unstable surfaces. Rotation may be added when the patient is further along in the rehab process. Multi-directional lunges are progressed with resistance using a sport cord or manual external perturbation. A visual stimulus on the ground can also be added using target lights or the QuickBoard Device (Figure 8a). The patient can lunge onto the indicated surface, reacting to the correct position on the board (Figure 8b).
Figure 8a. Quickboard (Memphis, TN) Lateral Step: Patient performs lateral step onto Quickboard, focusing on knee stability and target reaction.

See Supplemental File 4 for video.
Figure 8b. Reactive Lateral Lunge with Catch: Patient performs lateral lunge towards Blazepod (San Diego, CA) while catching a ball.

See Supplemental File 5 for video.
Stepping exercises can be advanced in this stage to emphasize speed, coordination, and endurance. Patients perform in-place stepping drills while reacting to visual targets on the Quick Board (Figure 9a/Supplemental File 6). This can also be performed with a reaction target (Figure 9b/Supplemental File 7). The Quick Board may facilitate various single and dual-leg coordination exercises while assessing accuracy, reaction time, and limb symmetry.
Figure 9a. Quickboard (Memphis, TN) Foot Fire: Patient performs rapid stepping in place while tapping targets on Quickboard.

See Supplemental File 6 for video.
Figure 9b. Quickboard (Memphis, TN) Foot Fire with Reactive Taps: Patient performs rapid stepping in place while reacting to visual targets on the screen.

See Supplemental File 7 for video.
Finally, single leg tasks can also be combined into a functional task such as a single leg Romanian Deadlift (SL RDL). This can be performed on various surfaces, unweighted or weighted with kettlebells (KB). Tasks such as SL RDL with KB exchanges may be incorporated, where the patient performs an RDL holding the bottom position while exchanging a weighted KB from hand to hand to challenge the limits of stability. Another option is the SL RDL with star drill, where the patient catches a ball and touches a series of 5 cones placed on the floor while in the bottom of the RDL position, allowing for incorporation of UE, visual system, and SL stability. SL RDL with CLX tubing wrapped around the thigh is used for proprioceptive input to control dynamic LE valgus, adduction and internal rotation. These single-leg balance exercises with extremity movement are designed to engage multiple muscle groups while enhancing dynamic stabilization.
As exercise complexity increases, patients often exhibit deteriorating movement patterns, which can heighten their risk of injury. For those demonstrating significant pelvic drop and hip internal rotation on the stance leg during functional tasks, a targeted exercise using a resistance band can be implemented. The band is strategically placed around the athlete’s limb to replicate adduction and internal rotation forces commonly experienced during dynamic movements. The athlete then performs sport-specific tasks, such as dribbling a basketball or throwing/kicking a soccer ball, while balancing on an unstable surface (e.g., rocker board). Simultaneously, the practitioner applies external perturbations by pulling on the resistance band and tapping the board (Figure 10). Alternatively, the practitioner can pull the band medially to recreate knee valgus forces. To increase the neurocognitive challenge, BlazePods can be introduced on the floor for the athlete to tap with their contralateral extremity during the task.
Figure 10. Rocker Board Balance with Ball Catches and Valgus Resistance: Patient balances on rocker board while catching a ball and resisting valgus force from a band.

To introduce dynamic lateral movements focused on rapid visual processing and change of direction movements, lateral shuffling is introduced with BlazePods. Four Blazepods are placed in a straight line in front of the patient on top of cones for enhanced visibility. As the BlazePod lights up, the athlete performs a lateral shuffle towards the illuminated pod while staying low and maintaining balance. Simultaneously, the clinician tosses a ball towards the athlete. The athlete must catch the ball mid-shuffle, continue moving towards the BlazePod, and tap the lit pod with the ball before shuffling back to the starting position. This drill can be progressed by placing the pods on the floor so that the athlete must maintain a lower stance and with quicker speeds (Figure 11/Supplemental File 8). The exercise is designed to enhance reaction time, hand-eye coordination, lateral speed, and agility while keeping the athlete engaged in multiple tasks simultaneously. Each progressive variation simulates the visual-cognitive demands of sport, training the athlete to maintain control and react effectively to external stimuli while executing complex movements.
Figure 11. Reactive Lateral Shuffle Drill: Patient performs lateral shuffles towards illuminated BlazePods (San Diego, CA) while catching and tapping the pod with a ball.

See Supplemental File 8 for video.
Conclusion
In conclusion, incorporating neurocognitive training into ACL rehabilitation is essential to reduce re-injury rates and promote optimal recovery. Traditional rehabilitation methods that focus on physical aspects such as strength and range of motion, are missing a key element. Addressing neuromuscular control and cognitive function is equally crucial. Neurocognitive exercises that focus on dual-tasking and reactive/feed forward activities enhance proprioception, dynamic stability, and motor planning, and better mimic the real world and sporting environment, which is when most ACL injuries and re-injuries occur. These exercises not only improve the immediate functional outcomes, but also foster beneficial neuroplasticity, ensuring better long-term joint stability and movement efficiency. Clinically, it is vital to understand that neuro-cognitive training is a dynamic principle and should not be isolated to the beginning stages of rehabilitation. Instead, integrating neurocognitive training from the pre-operative phase through full recovery with sport specific drills is vital and provides a comprehensive approach, addressing the multifaceted demands of returning to both sport and competition, but it does not end here. It is essential to use neurocognitive training as an injury prevention mechanism and should be incorporated in both injured and non-injured athletes at any level of competition. Ultimately, the authors’ goal with this strategy is to bridge the gap between rehabilitation and performance, while enhancing the long-term resilience of the knee joint. Overall, the authors believe by including neuromuscular and neurocognitive exercises early and throughout the rehabilitation process, it reduces the likelihood of re-injury and supports patients in achieving a higher quality of life post-ACLR.
COI Statement
No funding was provided for this manuscript. Kevin Wilk serves on the medical advisory board for BlazePods and receives educational grant funds from Quickboard. No other relevant author disclosures.
Supplementary Material
Blazepod Quad Set: Patient performs isometric quadriceps contraction with NMES while tapping corresponding Blazepods.
Blazepod Knee Extension: Patient performs assisted knee extension while tapping corresponding Blazepods
Blazepod TKE: Patient performs standing terminal knee extensions with band resistance while tapping Blazepods
Quickboard (Memphis, TN) Lateral Step: Patient performs lateral step onto Quickboard, focusing on knee stability and target reaction.
Reactive Lateral Lunge with Catch: Patient performs lateral lunge towards Blazepod (Miami, FL) while catching a ball.
Quickboard (Memphis, TN) Foot Fire: Patient performs rapid stepping in place while tapping targets on Quickboard.
Quickboard (Memphis, TN) Foot Fire with Reactive Taps: Patient performs rapid stepping in place while reacting to visual targets on the screen.
Reactive Lateral Shuffle Drill: Patient performs lateral shuffles towards illuminated BlazePods (Miami, FL) while catching and tapping the pod with a ball.
References
- Anterior cruciate ligament injury prevention & rehabilitation: Let’s get it right. Wilk K. E. 2015J Orthop Sports Phys Ther. 45(10):729–730. doi: 10.2519/jospt.2015.0109. [DOI] [PubMed] [Google Scholar]
- Noncontact anterior cruciate ligament injuries: Risk factors and prevention strategies. Griffin L. Y., Albohm M. J., Arendt E. A., Dick R. W., Garrett W. E., Garrick J. G.., et al. 2000J Am Acad Orthop Surg. 8(3):141–150. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- Noncontact anterior cruciate ligament injuries: mechanisms and risk factors. Boden B.P., Sheehan F.T., Torg J.S., Hewett T.E. 2010J Am Acad Orthop Surg. 18(9):520–527. doi: 10.5435/00124635-201009000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanisms of anterior cruciate ligament injury. Boden B. P., Dean G. S., Feagin J. A., Jr., Garrett W. E., Jr. 2000Orthopedics. 23(6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- Incidence of contralateral and ipsilateral anterior cruciate ligament (ACL) injury after primary ACL reconstruction and return to sport. Paterno M. V., Rauh M. J., Schmitt L. C., Ford K. R., Hewett T. E. 2012Clin J Sport Med. 22(2):116–21. doi: 10.1097/JSM.0b013e318246ef9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risk of secondary injury in younger athletes after anterior cruciate ligament reconstruction: A systematic review and meta-analysis. Wiggins A. J., Grandhi R. K., Schneider D. K., Stanfield D., Webster K. E., Myer G. D. 2016Am J Sports Med. 44(7):1861–76. doi: 10.1177/0363546515621554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- One in 5 athletes sustain reinjury upon return to high-risk sports after ACL reconstruction: A systematic review in 1239 athletes younger than 20 years. Barber-Westin S., Noyes F. R. 2020Sports Health. 12(6):587–597. doi: 10.1177/1941738120912846. doi: 10.1177/1941738120912846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The relationship between neurocognitive function and noncontact anterior cruciate ligament injuries. Swanik C. B., Covassin T., Stearne D. J., Schatz P. 2007Am J Sports Med. 35(6):943–8. doi: 10.1177/0363546507299532. [DOI] [PubMed] [Google Scholar]
- Principles of motor learning to support neuroplasticity after ACL injury: Implications for optimizing performance and reducing risk of second ACL injury. Gokeler A., Neuhaus D., Benjaminse A., Grooms D.R., Baumeister J. 2019Sports Med. 49(6):853–865. doi: 10.1007/s40279-019-01058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuroplasticity associated with anterior cruciate ligament reconstruction. Grooms D. R., Page S. J., Nichols-Larsen D. S., Chaudhari A. M. W., White S. E., Onate J. A. 2017J Orthop Sports Phys Ther. 47(3):180–189. doi: 10.2519/jospt.2017.7003. [DOI] [PubMed] [Google Scholar]
- Brain activation for knee movement measured days before second anterior cruciate ligament injury: Neuroimaging in musculoskeletal medicine. Grooms D. R., Page S. J., Onate J. A. 2015J Athl Train. 50(10):1005–10. doi: 10.4085/1062-6050-50.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Integrating neurocognitive challenges into injury prevention training: A clinical commentary. Walker J. M., Brunst C. L., Chaput M., Wohl T. R., Grooms D. R. 2021Phys Ther Sport. 51:8–16. doi: 10.1016/j.ptsp.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuromuscular performance characteristics in elite female athletes. Huston L., Wojtys E. M. 1996Am J Sports Med. 24(4):427–36. doi: 10.1177/036354659602400405. [DOI] [PubMed] [Google Scholar]
- Neural plasticity and its contribution to functional recovery. Sharma N., Classen J., Cohen L. G. 2013Handb Clin Neurol. 110:3–12. doi: 10.1016/B978-0-444-52901-5.00001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Functional gait adaptations in patients with anterior cruciate ligament deficiency over time. Wexler G., Hurwitz D. E., Bush-Joseph C. A., Andriacchi T. P., Bach B. R., Jr. 1998Clin Orthop Relat Res. (348):166–175. doi: 10.1097/00003086-199803000-00026. [DOI] [PubMed]
- Gamma loop dysfunction in the quadriceps femoris of patients who underwent anterior cruciate ligament reconstruction remains bilaterally. Konishi Y., Aihara Y., Sakai M., Ogawa G., Fukubayashi T. 2007Scand J Med Sci Sports. 17:393–399. doi: 10.1111/j.1600-0838.2006.00573.x. [DOI] [PubMed] [Google Scholar]
- Neuromuscular responses in individuals with anterior cruciate ligament repair. Madhavan S., Shields R.K. 2011Clin Neurophysiol. 122:997–1004. doi: 10.1016/j.clinph.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corticomotor excitability associated with unilateral knee dysfunction secondary to anterior cruciate ligament injury. Héroux M. E., Tremblay F. 2006Knee Surg Sports Traumatol Arthrosc. 14:823–833. doi: 10.1007/s00167-006-0063-4. [DOI] [PubMed] [Google Scholar]
- Persistent neuromuscular and corticomotor quadriceps asymmetry after anterior cruciate ligament reconstruction. Kuenze C. M., Hertel J., Weltman A., Diduch D., Saliba S. A., Hart J. M. 2015J Athl Train. 50:303–312. doi: 10.4085/1062-6050-49.5.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuroplasticity following anterior cruciate ligament injury: a framework for visual-motor training approaches in rehabilitation. Grooms D. R., Onate J. A. 2016J Orthop Sports Phys Ther. 46(5):384–393. doi: 10.2519/jospt.2015.5549. [DOI] [PubMed] [Google Scholar]
- From control to chaos: Visual-cognitive progression during recovery from ACL reconstruction. Chaput M., Simon J., Taberner M., Grooms D. 2024J Orthop Sports Phys Ther. 54:1–26. doi: 10.2519/jospt.2024.12443. doi: 10.2519/jospt.2024.12443. [DOI] [PubMed] [Google Scholar]
- Altered electrocortical brain activity after ACL reconstruction during force control. Baumeister J., Reinecke K., Schubert M., Weiss M. 2011J Orthop Res. 29(9):1383–1389. doi: 10.1002/jor.21380. doi: 10.1002/jor.21380. [DOI] [PubMed] [Google Scholar]
- Multiple resources and mental workload. Wickens C. D. 2008Hum Factors. 50(3):449–455. doi: 10.1518/001872008X288394. doi: 10.1518/001872008X288394. [DOI] [PubMed] [Google Scholar]
- Dual-task interference in simple tasks: Data and theory. Pashler H. 1994Psych Bull. 116(2):220–244. doi: 10.1037/0033-2909.116.2.220. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- Inhibition of motor planning and response selection after anterior cruciate ligament reconstruction. Sherman D. A., Baumeister J., Stock M. S., Murray A. M., Bazett-Jones D. M., Norte G. E. 2023Med Sci Sports Exerc. 55(3):440–449. doi: 10.1249/MSS.0000000000003072. [DOI] [PubMed] [Google Scholar]
- Progressing rehabilitation after injury: Consider the ‘control-chaos continuum.’. Taberner M., Allen T., Cohen D. D. 2019Br J Sports Med. 53(18):1132–1136. doi: 10.1136/bjsports-2018-100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updates in motor learning: Implications for physical therapist practice and education. Leech K. A., Roemmich R. T., Gordon J., Reisman D. S., Cherry-Allen K. M. 2022Phys Ther. 102(1) doi: 10.1093/ptj/pzab250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuromuscular training versus strength training during first 6 months after anterior cruciate ligament reconstruction: a randomized clinical trial. Risberg M. A., Holm I., Myklebust G., Engebretsen L. 2007Phys Ther. 87(6):737–750. doi: 10.2522/ptj.20060041. [DOI] [PubMed] [Google Scholar]
- Individuals with an anterior cruciate ligament-deficient knee classified as noncopers may be candidates for nonsurgical rehabilitation. Moksnes H., Snyder-Mackler L., Risberg M. A. 2008J Orthop Sports Phys Ther. 38(10):586–595. doi: 10.2519/jospt.2008.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilateral deficit of voluntary quadriceps muscle activation after unilateral ACL tear. Urbach D., Nebelung W., Weiler H. T., Awiszus F. 1999Med Sci Sports Exerc. 31(12):1691–1696. doi: 10.1097/00005768-199912000-00001. [DOI] [PubMed] [Google Scholar]
- Return of normal gait as an outcome measurement in ACL reconstructed patients. A systematic review. Gokeler A., Benjaminse A., van Eck C. F., Webster K. E., Schot L., Otten E. 2013Int J Sports Phys Ther. 8(4):441–451. [PMC free article] [PubMed] [Google Scholar]
- Neuromuscular electrical stimulation is effective in strengthening the quadriceps muscle after anterior cruciate ligament surgery. Hauger A. V., Reiman M. P., Bjordal J. M., Sheets C., Ledbetter L., Goode A. P. 2018Knee Surg Sports Traumatol Arthrosc. 26(2):399–410. doi: 10.1007/s00167-017-4669-5. [DOI] [PubMed] [Google Scholar]
- The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Lewek M. D., Rudolph K. S., Axe M. J., Snyder-Mackler L. 2002Clin Biomech. 17(1):56–63. doi: 10.1016/S0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Paterno M. V., Schmitt L. C., Ford K. R.., et al. 2010Am J Sports Med. 38(10):1968–1978. doi: 10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walking gait asymmetries 6 months following anterior cruciate ligament reconstruction predict 12-month patient-reported outcomes. Pietrosimone B., Blackburn J. T., Padua D. A.., et al. 2018J Orthop Res. 36(11):2932–2940. doi: 10.1002/jor.24056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Blazepod Quad Set: Patient performs isometric quadriceps contraction with NMES while tapping corresponding Blazepods.
Blazepod Knee Extension: Patient performs assisted knee extension while tapping corresponding Blazepods
Blazepod TKE: Patient performs standing terminal knee extensions with band resistance while tapping Blazepods
Quickboard (Memphis, TN) Lateral Step: Patient performs lateral step onto Quickboard, focusing on knee stability and target reaction.
Reactive Lateral Lunge with Catch: Patient performs lateral lunge towards Blazepod (Miami, FL) while catching a ball.
Quickboard (Memphis, TN) Foot Fire: Patient performs rapid stepping in place while tapping targets on Quickboard.
Quickboard (Memphis, TN) Foot Fire with Reactive Taps: Patient performs rapid stepping in place while reacting to visual targets on the screen.
Reactive Lateral Shuffle Drill: Patient performs lateral shuffles towards illuminated BlazePods (Miami, FL) while catching and tapping the pod with a ball.


