Abstract
Background
Malaria transmission in Tanzania has declined significantly over the last 2 decades due to scaled-up control interventions. However, recent confirmation of artemisinin partial resistance (ART-R) in Kagera region in northwest Tanzania threatens the ongoing efforts to eliminate malaria in the country. This study was conducted according to the World Health Organization recommendation to generate evidence of malaria burden in areas with confirmed ART-R as the first step before developing a response strategy to the resistance.
Methods
We assessed the local burden of malaria in Kagera region by geospatial analysis, using data collected retrospectively from health facilities and community surveys from 2015 to 2023 to identify malaria hot spots.
Results
From 2017 to 2023, a total of 8 124 363 suspected malaria cases were reported by health facilities, and 2 983 717 (36.7% [95% range across wards, 22.7%–50.7%]) tested positive by rapid diagnostic tests. Test positivity rates were similar among patients aged <5 years (33.1% [95% range, 19.7%–46.5%]) and those aged ≥5 years (33.7% [21.0%–46.5%]). The malaria prevalence was 10.0% (95% range across wards, 5.1%–14.9% [n = 84 999 of 853 761]) in pregnant women and 26.1% (11.7%–40.6% [n = 3409 of 13 065]) in schoolchildren. Despite high temporal variations, we identified hot spots and cold spots, including persistently high burden in 69 of 192 wards (35.9%).
Conclusions
The malaria burden in Kagera exhibited high temporal and spatial heterogeneity, with schoolchildren showing the highest prevalence. This demographic pattern underlines the need for targeted interventions and provides evidence for developing an ART-R response for the region.
Keywords: artemisinin partial resistance, Kagera region, malaria, malaria hot spots, Tanzania
This study investigated the spatial and temporal patterns of malaria burden, using data from multiple sources to identify areas with the highest and lowest burdens and help develop a response strategy for confirmed artemisinin partial resistance in Kagera region, Tanzania.
Tanzania is one of the priority countries defined by the World Health Organization (WHO) as a high-burden high-impact country; it accounted for an estimated 3.2% of all malaria cases and 4.4% of deaths reported worldwide in 2022 [1]. In Tanzania, about 96% of all cases are caused by Plasmodium falciparum, and the remaining cases are due to other species (Plasmodium malariae and Plasmodium ovale) individually or in mixed infections [2]. The strategy of the National Malaria Control Programme (NMCP) is to reduce the burden and progress to control and eliminate malaria using a range of interventions. The main strategies include integrated malaria vector control (mainly with insecticide-treated nets [ITNs] and indoor residual spraying), case management, preventive therapies, and other supportive strategies [3]. However, progress to malaria elimination is under key biological threats, such as the emergence and spread of insecticide resistance [4], antimalarial drug resistance [5], emergence of parasites with histidine-rich protein 2/3 (hrp2/3) gene deletions [6], and invasive vector species (Anopheles stephensi) in some malaria-endemic countries in Africa [1].
Malaria transmission in Tanzania is heterogeneous, with 93% of the population living in areas where transmission occurs [3]. Regions with very low and low transmission risk are located in the central, northeastern, and southwestern parts surrounded on both sides by moderate transmission regions and high transmission in the northwestern, southern, and western parts of the country [7]. Various factors including mosquito vector species, environmental conditions, and other human-related factors are responsible for the current pattern and persistence of malaria transmission in Tanzania [8, 9].
Over the past 4 decades, malaria-endemic countries in sub-Saharan Africa have reported resistance of P falciparum to all major antimalarial drugs, including chloroquine, sulfadoxine-pyrimethamine [5], artemisinin derivatives, and the currently used partner drugs [10]. Nevertheless, artemisinin-based combination therapies are still highly effective against parasites of African origin [5]. However, artemisinin partial resistance (ART-R) has been confirmed in Rwanda [11, 12], Uganda [13], Tanzania [14] and Eritrea [15], and one particular genotype has only been detected in the Horn of Africa, in Ethiopia [16], Somalia [17], and Sudan [18]. In addition, the mutation that originated in Rwanda has also been reported in Kenya [19]. In Tanzania, little was known about ART-R until 2021, when a countrywide survey through the project on molecular surveillance of malaria in Tanzania (MSMT) reported a focus in Kagera region only, with a high frequency (0.077) of parasites with mutations in the pfkelch13 gene (k13) and as high as 0.22 in Karagwe District Council (DC), mutations that are linked to ART-R in Africa [20]. Based on WHO's recommendation [5], a follow-up therapeutic efficacy study conducted in 2022 confirmed the presence of ART-R in the region although the tested drugs (artemether-lumefantrine and artesunate-amodiaquine) still had high efficacy, exceeding 98.0% [14].
As per WHO's strategy to respond to ART-R in Africa, when resistance is confirmed, new strategies to contain, prevent, and mitigate the spread and consequences of ART-R must be considered urgently [21]. As the first step toward the development of a response strategy, WHO recommends mapping and providing a detailed profile of the disease burden and its spread in different areas [22]. Therefore, this study aimed to investigate the temporal trends and spatial patterns of malaria burden in Kagera region and identify areas at the highest or lowest risk of malaria transmission as an initial stage toward the development of a response to ART-R in Tanzania. The findings from this study provide the government and other stakeholders with evidence to support the designing, deployment, and implementation of interventions to monitor, prevent, and contain the spread of ART-R within the region and to other areas of Tanzania.
METHODS
Study Design and Site
This study used aggregated secondary data collected retrospectively from health facilities (HFs) and community surveys in Kagera region of northwestern Tanzania. The region consists of 8 councils, 192 wards, and 734 villages covering an area of approximately 35 868 km2 (Supplementary Figure 1; see the study site in the Supplementary materials for additional details).
Data Collation and Management
The data for this study were downloaded from the District Health Information System, version 2 (DHIS2), a web-based software platform for reporting, analyzing, storing, and disseminating health data, as described elsewhere [7]. This platform is an electronic format for the paper-based Health Management Information System of Tanzania's Ministry of Health, which is used by HFs to collect and report their routine data [3]. The description of how the information is entered and maintained as well as quality control of the data is provided elsewhere [23].
Statistical Analysis
The data used in this study included monthly malaria testing of pregnant women attending first antenatal care clinics, patients with symptomatic malaria seeking care at HFs, aged <5 and ≥5 years, both from 2017 to 2023, and schoolchildren through school malaria parasitological surveys of 2015, 2017, 2019, and 2021 from the 37 selected wards in all councils of Kagera region. The data were downloaded in Excel (Office 2013) and checked for completeness and identification of HFs missing the reports fully or partially, followed by confirmation of the results with the support of the NMCP team. Data cleaning and preprocessing were done to ensure accuracy and consistency, using both Excel and R software, version 4.4.1 (https://www.r-project.org/).
Monthly data from total malaria tests performed and positive tests (by rapid diagnostic tests [RDTs]) reported by all HFs in the region were aggregated to provide annualized estimates (see Supplementary materials). The malaria test-positivity rate (TPR) was defined as the proportion of positive malaria tests among all malaria tests (overall) and in patients aged <5 years (“under-5s”) and those aged ≥5 years. The prevalence of malaria infections was calculated for pregnant women and schoolchildren and was defined as the proportion of individuals in a cross-sectional sample—either pregnant women or schoolchildren—who were RDT positive for malaria. Using R software, analysis was done for overall, per year, per study group, by council, and at the ward level; results are presented in tables, figures, and text. Python plotting tools (pandas v2.2.0, matplotlib v3.8.2, and numpy v1.26.3; Python.org) were used to show temporal trends of TPRs and prevalence in the councils.
Kruskal-Wallis and Mann-Kendall tests were used to assess and detect any differences in malaria burden among councils and wards, and across years and for trend testing. Data on administrative boundaries of Tanzania at all levels and its neighboring countries were downloaded from various sources including the Tanzanian National Bureau of Statistics, gadm.org, and naturalearthdata.com, accessed via the R package rnaturalearth (v1.0.1). These were used to create the study sites and for choropleth mapping, spatial autocorrelation (SA), and hot-spot analysis. The spatial distribution of malaria burden in the region was mapped using R packages ggplot2 (v3.5.1) and sf (v1.0.15). To investigate the spatial and temporal patterns of malaria burden in the region, the global Moran index was calculated for each group and year. The value of this index ranges from −1 (negative SA) to +1 (positive SA).
For hot-spot analysis, local spatial statistical methods were applied through the spdep package (v1.3.3) found in R software. In this study, malaria hot spots were defined as wards whose malaria burden was noticeably higher than surrounding areas, and cold spots as those whose burden was lower than the surrounding areas. The formulas and illustration of how SA and hot-spot analysis were conducted can be found elsewhere [24] (also see Supplementary materials for details). The description of wards and HFs included in the analysis is provided in Supplementary Figure 2 and the Supplementary materials, while the cutoffs applied to characterize malaria prevalence were as follows: <1% (very low), 1% to <5% (low), 5% to <30% (moderate), and ≥30% (high), as described elsewhere [25, 26]. For malaria TPR, the cutoffs were <5% (very low), 5% to <15% (low), 15% to <30% (moderate), and ≥30% (high) (see Supplementary materials for details).
Patient Consent Statement
This study used aggregated secondary data; therefore, no patient consent was required.
RESULTS
Reported Malaria Cases From HFs
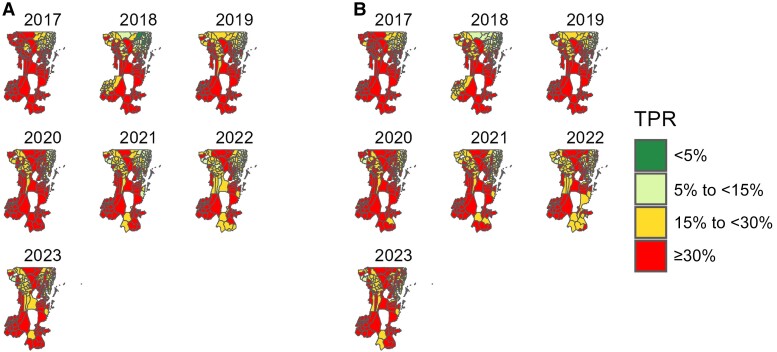
Among HF-reported cases in Kagera region, an average 36.7% of patients (95% range across wards, 22.7%–50.7% [n = 2 983 717 of 8 124 363]) with malarialike symptoms seeking care from 2017 to 2023, had RDT-positive results. The TPRs were similar among under-5s (33.1% [95% range, 19.7%–46.5%]) and those aged ≥5 years (33.7% [21.0%–46.5%]), with clear geographic and temporal variations (P < .001; Kruskal-Wallis). Among under-5s, most of the wards (43.2% [83 of 192]) in the high stratum (TPR ≥30%) were from rural areas, while wards in the very low stratum (TPR <5%) came from urban areas in all years (Supplementary Table 1A). Furthermore, 5 councils had >10% of the wards with consistently high TPR (≥30%) throughout the years. These included 6 of 17 wards (35.3%) in Biharamulo DC, 12 of 43 (27.9%) in Muleba DC, 5 of 22 (22.7%) in Ngara DC, 4 of 23 (17.4%) in Karagwe DC, and 4 of 29 (13.8%) in Bukoba DC. In Kyerwa DC, only 2 of 24 wards (8.3%) had consistently high TPRs, while only 1 ward (Kashai in Bukoba Municipal Council [MC]) recorded the lowest TPR (<5%) (Figure 1A and Supplementary Table 2A).
Figure 1.
Spatial distribution of malaria test-positivity rates (TPRs) in patients aged <5 (A) or ≥5 (B) years at the ward level in Kagera region from 2017 to 2023. White areas represent wards (conservation areas) excluded from the analysis.
Among patients aged ≥5 years, the pattern was similar to that of under-5s, with a large number of wards (48.4% [93 of 192]) observed in the high stratum throughout the years (Supplementary Table 1B). Similar to findings in the under-5s, the same 5 councils had >10% of the wards with consistently high TPRs (≥30%) over the years and these included 7 of 17 wards (41.2%) in Biharamulo DC, 7 of 23 (30.4%) in Karagwe DC, 11 of 43 (25.6%) in Muleba DC, 5 of 22 (22.7%) in Ngara DC, and 4 of 29 (13.8%) in Bukoba DC. Only 1 of 24 wards (4.2%) in Kyerwa DC had consistently high TPR (≥30%) (Figure 1B and Supplementary Table 2B).
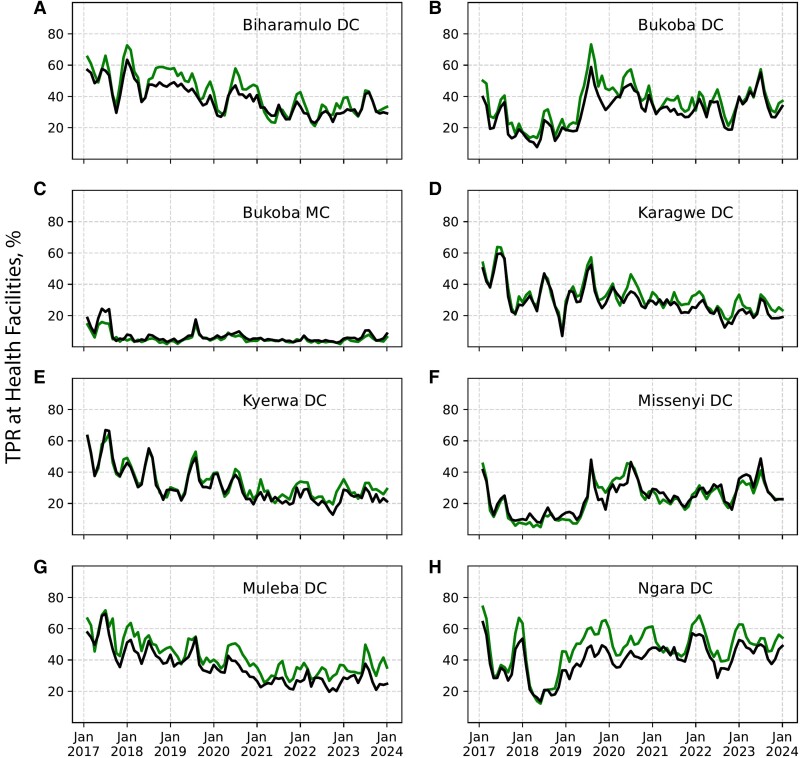
Monthly Variation of TPRs at the HF Level
Malaria TPR was high (≥30%) in patients of all ages (in Muleba DC, Ngara DC, Biharamulo DC, Bukoba DC, and Karagwe DC), and the pattern was similar in all years, except in Bukoba MC, which recorded the lowest TPR (<5%) in both age groups. In all councils except Bukoba MC and Missenyi DC, TPRs of <20% were rarely observed (Figure 2).
Figure 2.
Monthly test-positivity rates (TPRs) at health facility level in 8 district councils (DCs) in Kagera region in patients aged <5 (green) or ≥5 (black) years from 2017 to 2023. Part figures A to H show the arrangement of the 8 DCs in the region.
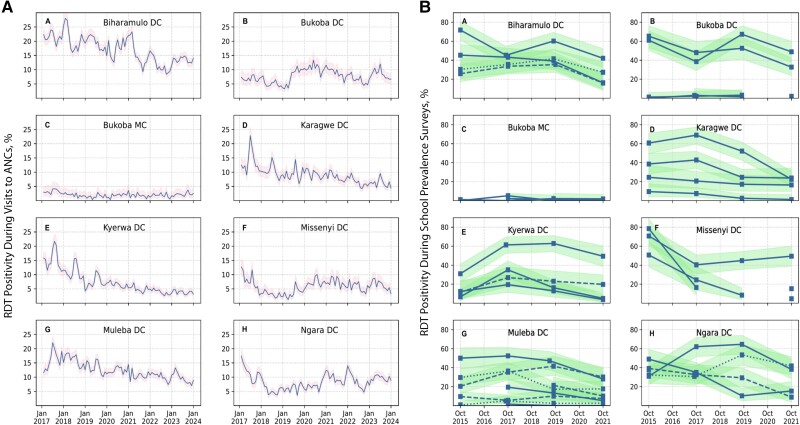
Malaria Prevalence Among Pregnant Women
From 2017 to 2023, the average prevalence of malaria in pregnant women was 10.0% (95% range across wards, 5.1%–14.9% [total n = 84 999 of 853 761]), with significant differences among wards and across years (P < .001; Kruskal-Wallis). The prevalence was consistently higher in Biharamulo DC, Karagwe DC, Muleba DC, and Kyerwa DC, and low in Bukoba MC (<1%) throughout the years (Figure 3A).
Figure 3.
A, Trends of malaria prevalence in pregnant women by district councils (DCs), in Kagera region from 2017 to 2023. Pink-shaded regions show Clopper-Pearson 95% confidence bands. B, Malaria prevalence trends for school-based malaria parasitological surveys conducted in 2015, 2017, 2019, and 2021. The y-axis shows the percentage of schoolchildren in a cross-sectional sample in a given school who were rapid diagnostic test (RDT) positive for malaria at the time of sampling. Sampling months were generally October and sometimes September. Data are separated into 8 panels to show school trends separately in Kagera region's 8 councils. In each DC subpanel, a single line represents an individual school. Some lines are drawn as dashed or dotted to distinguish separate schools more clearly from each other when the lines crowd around the same prevalence value. Light-green-shaded areas show Clopper-Pearson 95% confidence bands. Part figures A to H show the arrangement of the 8 DCs in the region.
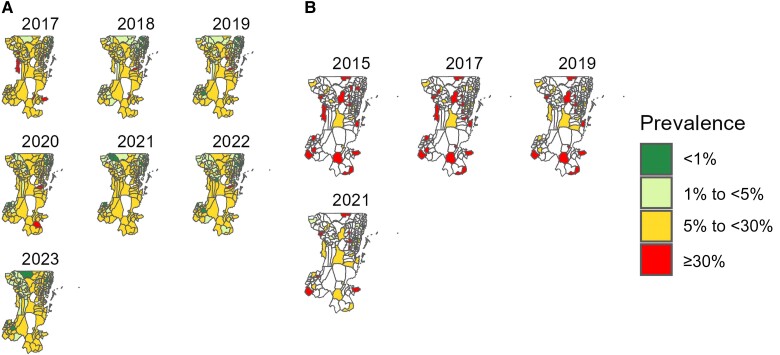
The prevalence in pregnant women showed that the largest number of wards, 116 of 151 (76.8%), were located in the moderate stratum in 2017, while only 1 of 159 was in the high-risk stratum in 2022 (Supplementary Table 3). In addition, only 3 of 8 (councils 37.5%) had ≥5% of the wards with high prevalence (≥30%) including 3 of 17 wards (17.6%) in Biharamulo DC, 4 of 43 (9.3%) in Muleba DC, and 2 of 23 (8.7%) in Karagwe DC, while Kyerwa DC (n = 1 of 24 [4.2%]) and Bukoba DC (n = 1 of 29 [3.4%]) had <5% of the wards with high prevalence. At least 5% of the wards from Bukoba DC (n = 2 of 29 [6.9%]) and Missenyi DC (n = 1 of 20 [5%]) were consistently recorded in the lowest-prevalence category (Figure 4A and Supplementary Table 4).
Figure 4.
Spatial distribution of malaria prevalence in pregnant women (A) from 2017 to 2023 and in schoolchildren (B) at the ward level in Kagera region for 2015, 2017, 2019, and 2021 surveys. White areas represent wards excluded (conservation areas) from the analysis or not selected for the survey.
Malaria Prevalence in Schoolchildren
The average prevalence of malaria in schoolchildren was 26.1% (95% range across wards, 11.7%–40.6% [n = 3409 of 13 065]) (see Supplementary materials for more details). The prevalence was high (≥30%) in some schools in all councils for all years, except in Bukoba MC (Figure 3B). More than half of the selected wards (19 of 34 [55.9%]) were located in the high stratum (≥30%), and only 1 of 34 wards (2.9%) was in the very-low-prevalence stratum (<1%) in 2017 (Supplementary Table 5). Furthermore, 3 wards from Ngara DC and 2 from Bukoba DC had persistently high prevalence (≥30%), while no ward was recorded in the lowest-prevalence category (<1%) for all years in these 2 councils, Ngara DC and Bukoba DC (Figure 4B and Supplementary Table 6).
Trends of Overall Malaria TPRs at the Ward Level
A nonsignificant declining trend (P = .38; Kendall test) was observed among under-5s and patients aged ≥5 years, and a shift from under-5s to older patients (due to high values of TPRs) was observed from 2020 onward (Supplementary Table 7). In both age groups, an increase in TPRs was observed in 2023 (Supplementary Figure 3).
Overall Malaria Prevalence at the Ward Level
Malaria prevalence was higher in schoolchildren than in pregnant women, a trend that was similar across years (Supplementary Figure 4). Furthermore, the prevalence in pregnant women showed a significant decreasing trend (P = .01; Kendall test) with minor variability before 2022, but the prevalence increased by approximately 15% in 2023 (Supplementary Table 8).
Global Spatial Autocorrelation Analysis of Malaria Burden
The malaria burden in Kagera region was clustered in under-5s, patients aged ≥5 years, and pregnant women. Councils/wards with high/low burden were consistently surrounded by other areas with high/low values as well for all years. The observed patterns were statistically significant (P < .05; Moran test) in all groups, except schoolchildren (Supplementary Tables 9 and 10).
Hot Spots and Cold Spots of Malaria Burden
Malaria hot spots were concentrated in the southern, central, and northwestern parts of Kagera region. Four councils (Ngara DC, Biharamulo DC, Muleba DC, and Bukoba DC) had >17% of wards classified as hot spots in under-5s, patients aged ≥5 years, and pregnant women; while Karagwe DC and Kyerwa DC had <17% of wards classified as hot spots in the same groups. The numbers of wards classified as hot spots in each group at 95% and 99% confidence levels (CLs) are presented in Table 1 (Supplementary Table 11).
Table 1.
Wards per Council Classified as Malaria Hot Spots or Cold Spots in Patients Aged <5 or ≥5 Years and Pregnant Women at 95% and 99% Confidence Levels
| Study Group | Malaria Hot Spots | Malaria Cold Spots | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Councils | No. of Wards/Total No. (%) |
χ
2
Value |
P Value | Councils | No. of Wards/Total No. (%) | χ 2 Value | P Value | |||
| 95% CL | 99% CL | 95% CL | 99% CL | |||||||
| TPR in patient aged <5 years | Ngara DC; Biharamulo DC; Muleba DC; Bukoba DC; Karagwe DC; Kyerwa DC | 19/22 (86.4); 11/17 (64.7); 15/43 (34.9); 9/29 (31.0); 2/23 (8.7); 1/24 (4.2) | 17/22 (77.3); 8/17 (47.1); 12/43 (27.9); 6/29 (20.7); 1/23 (4.3); … |
30 | .22 | Bukoba MC; Missenyi DC; Bukoba DC; Karagwe DC; Kyerwa DC; Muleba DC |
13/14 (92.9); 17/20 (85); 15/29 (51.7); 1/23 (4.3); 1/24 (4.2); 1/43 (2.3) | 12/14 (85.7); 14/20 (70); 12/29 (41.4); …; …; … | 12 | .06 |
| TPR in patients aged ≥5 years | Ngara DC; Biharamulo DC; Muleba DC; Bukoba DC; Kyerwa DC; Karagwe DC | 20/22 (90.9); 9/17 (52.9); 16/43 (37.2); 8/29 (27.6); 3/24 (12.5); 1/23 (4.3) | 16/22 (72.7); 7/17 (41.2); 13/43 (30.2); 5/29 (17.2); 1/24 (4.2); 1/23 (4.3) | 24 | .24 | Bukoba MC; Missenyi DC; Bukoba DC; Kyerwa DC; Muleba DC |
12/14 (85.7); 17/20 (85); 14/29 (48.3); 1/24 (4.2); 1/43 (2.3) |
11/14 (78.6); 14/20 (70); 11/29 (37.9); …; 1/43 (2.3) |
10 | .35 |
| ANC prevalence | Biharamulo DC; Ngara DC; Muleba DC; Bukoba DC; Karagwe DC; Kyerwa DC; Missenyi DC |
13/17 (76.5); 7/22 (31.8); 13/43 (30.2); 7/29 (24.1); 4/23 (17.4); 3/24 (12.5); 2/20 (10) | 11/17 (64.7); 4/22 (18.2); 10/43 (23.3); 7/29 (24.1); 3/23 (13.0); 1/24 (4.2); … |
28 | .26 | Bukoba MC; Bukoba DC; Missenyi DC; Kyerwa DC; Ngara DC | 10/14 (71.4); 11/29 (37.9); 8/20 (40); 4/24 (16.7); 1/22 (4.5) |
3/14 (21.4); 9/29 (31.0); 2/20 (10); …; … | 15 | .24 |
Abbreviations: ANC, antenatal care; CL, confidence level; DC, District Council; MC, Municipal Council; TPR, test-positivity rate.
... no ward(s) classified as malaria hot spots or cold spots for a particular council and confidence level.
On the other hand, malaria cold spots were situated in the northeast and a few in the remaining parts of the region. Three councils (Bukoba MC, Missenyi DC, and Bukoba DC) had >20% of wards classified as cold spots in under-5s, patients aged ≥5 years, and pregnant women, while Kyerwa DC and Muleba DC had <20% of the wards classified as cold spots. The numbers of wards classified as cold spots in each group at 95% and 99% CLs are also presented in Table 1 (Supplementary Table 12). The locations of hot and cold spots at 95% and 99% CLs based on TPRs (in under-5s and patients aged ≥5 years) and the prevalence in pregnant women are shown in Supplementary Figures 5A, 5B, and 6.
DISCUSSION
This study was conducted as an initial stage of a response to ART-R as recommended by WHO [21] and aimed to undertake an assessment to generate and provide evidence of the current burden of malaria in Kagera region, where ART-R was recently confirmed [14]. We used spatial statistical and disease mapping techniques to assess the local burden of malaria and identify potential malaria hot spots in Kagera region to support NMCP's efforts of developing a response strategy against ART-R. Using aggregated secondary data from multiple sources, we showed that 35.9% (69 of 192) of all wards in Kagera region, mostly from rural areas, had a high malaria burden, and these wards were clustered across years. The TPR was similar between patients aged ≥5 years and under-5s, which, however, is not comparable to findings in previous studies conducted in Tanzania, possibly due to different designs of the respective studies [9, 27]. The findings reported here provide important evidence to support the designing and implementation of mitigation and response strategies to recently confirmed ART-R in Kagera region and Tanzania in general.
Consistent with findings in previous studies [27], the current study reported high temporal and spatial heterogeneity of malaria burden at council and ward levels within the region. Most of the wards, especially from rural areas, recorded a high malaria burden for most of the years; while some wards mainly in urban areas or areas with both (mixed) rural and urban settings recorded the lowest burden (Supplementary Tables 2A, 2B, 4, and 6). This finding was consistent across years and study groups, possibly due to conditions supporting malaria transmission in rural compared to urban areas as previously reported [28, 29]. The higher burden of malaria in rural areas is normally attributed to several factors, including the presence of favorable conditions for malaria transmission in rural areas such as stagnant water bodies, which tend to create desirable breeding sites for mosquito vectors [29].
In addition, a large proportion of poor people tend to reside in rural areas and normally live in poorly constructed houses, which support higher mosquito biting rates and transmission of malaria [27, 30]. To reduce the burden in these areas, various interventions need to be implemented, such as distribution of ITNs, house improvement strategies, biolarviciding in areas surrounded by water bodies, and behavioral change and communication strategies, as these have been reported to significantly reduce malaria transmission and the disease burden [31, 32]. On the other hand, the low burden of malaria in urban areas observed in this study is consistent with previous studies [28, 29]. This is possibly due to the high socioeconomic status of the urban population and the availability, as well as access to malaria treatment and control measures when compared with rural areas [29].
A significant clustered spatial pattern of malaria burden reported in under-5s, patients aged ≥5 years, and pregnant women within the region aligns with results of other studies conducted elsewhere [24, 33]. The reports of areas with persistently high malaria burden in the southern, central, and northwestern parts of the region as reported in this study may be attributed to the presence of similar risk factors, such as environmental conditions, socioeconomic status, healthcare infrastructures, and the presence of suitable mosquito breeding sites that tend to create foci of malaria transmission [8, 27, 34]. Hence, future interventions in the region to reduce the local disease burden need to be designed based on these results to target the areas that have been identified to have a persistently high burden of malaria.
Malaria prevalence in Kagera region was higher in schoolchildren than in pregnant women, reaching as high as 78.4% in some wards. Although the high burden of malaria in schoolchildren has been attributed to their behavior and late evening activities, which potentially expose them to mosquito bites, the exact causes have not been established [35]. To reduce malaria transmission in schoolchildren, various initiatives have been undertaken by NMCP and other stakeholders to protect this risky group, including the distribution of ITNs through different channels like the school net program [3]. However, the prevalence of malaria infections in this asymptomatic group is still high [27, 36]. Future studies are needed to explore more reasons and factors for this recurring observation in the region and the whole country and determine whether this will support the spread of the reported ART-R.
On the other hand, pregnant women in Tanzania are normally given ≥2 doses of sulfadoxine-pyrimethamine for intermittent preventive treatment as well as ITNs during their first antenatal care visits to HFs, which might have significantly contributed to reducing the risk of malaria infections in this group [3]. However, the increasing prevalence of malaria in pregnant women observed in 2023 could be caused by increased malaria transmission, which occurred in all parts of Tanzania and might be attributed to climate change, particularly an increase in rainfall, as was previously reported in Tanga region [9]. The study in Tanga showed that an increase in rainfall over and above the average annual rainfall of 30 years was significantly associated with higher transmission, suggesting that changes in annual rainfall and possibly temperature can significantly affect the pattern and intensity of malaria transmission. Future studies are needed to uncover all possible reasons for this change and monitor the patterns, transmission intensity, and burden of malaria and how these changes may be related to the recently confirmed ART-R in the region.
Malaria transmission in Kagera region was observed throughout the year with some months recording higher TPRs than others. The overall yearly pattern suggests that malaria transmission in the region was not seasonal, an observation that can signify the presence of other factors as causes of changes in TPR, such as population movements, changes in malaria control, or unexpected increases or decreases in breeding sites or mosquito populations [27, 37]. Causal effects for these factors are in general difficult to establish, and future studies are needed to explore and uncover the key drivers of persistent malaria transmission in this malaria-endemic region of the country.
The findings presented here had some limitations that may be addressed in future studies. Since this study included HFs with a reporting rate of ≥50%, future studies may consider including all HFs during analysis and use different geostatistical methods to account for monthly missing data. In addition, the HF data did not include personal information such as age and sex, and this limited additional analysis. For instance, previous studies showed a high burden of malaria in schoolchildren (aged 5–15 years) and among males, but this could not be teased out because all patients aged ≥5 years were put in a single group, and data on sex were missing. Future studies may also include populations at risk and use incidence to track changes in the disease burden within the region.
Malaria infections in the region were high in >35% of the wards and the majority were from rural areas, compared with urban areas. The prevalence of malaria infections was also higher among schoolchildren compared with pregnant women, and TPRs were similar among patients aged ≥5 years and under-5s. The observed heterogeneity of malaria burden underscores prioritization of Kagera region for additional malaria control, mainly due to the currently circulating P falciparum parasites carrying the pfkelch13 R561H mutation in the region [14]. As early and preemptive action is preferred to late response [38], identifying specific resistance containment strategies [39] for 2025 and 2026—in addition to general malaria control strategies—will be critical for slowing down the spread of artemisinin-resistant parasites from Kagera region to other parts of Tanzania.
Supplementary Material
Acknowledgments
The authors thank the Ministry of Health (MoH) through the National Malaria Control Programme (NMCP) and all members of the Department of Mathematics, University of Dar es Salaam, and the molecular surveillance of malaria in Tanzania (MSMT) project team for their participation and invaluable support to make this work complete. Permission to publish this paper was sought and received from the Director General of the National Institute for Medical Research.
Contributor Information
Daniel A Petro, Department of Mathematics, University of Dar es Salaam, Dar es Salaam, Tanzania.
Nyimvua Shaban, Department of Mathematics, University of Dar es Salaam, Dar es Salaam, Tanzania.
Sijenunu Aaron, Office of the Chief Medical Officer, Ministry of Health, National Malaria Control Programme, Dodoma, Tanzania.
Frank Chacky, Office of the Chief Medical Officer, Ministry of Health, National Malaria Control Programme, Dodoma, Tanzania.
Samuel Lazaro, Office of the Chief Medical Officer, Ministry of Health, National Malaria Control Programme, Dodoma, Tanzania.
Maciej F Boni, Department of Biology, Institute for Genomics and Evolutionary Medicine, Temple University, Philadelphia, Pennsylvania, USA.
Deus S Ishengoma, Genomics Laboratory, National Institute for Medical Research, Dar es Salaam, Tanzania; Department of Biochemistry, Kampala International University in Tanzania, Dar es Salaam, Tanzania.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Author contributions. D. A. P. and D. S. I. designed the study, and S. A., F. C., and S. L. supported the acquisition and cleaning of the data. D. A. P. conducted the initial analysis, under the supervision of N. S. and D. S. I., and N. S., M. F. B., and D. S. I. were involved in interpreting the results. D. A. P. and M. F. B. performed final analysis of the data under the guidance of D. S. I., and D. A. P. drafted the initial manuscript. All coauthors contributed to the revision of the initial draft and subsequent versions of the manuscript, and all authors read and approved the final manuscript.
Data availability. The data used in this study are not publicly available and were obtained with a request from the MoH through NMCP. Restrictions apply to the availability of this data, and permission can be obtained with a reasonable request from the MoH of Mainland Tanzania.
Financial support. This work was supported by the Tanzania National Institute for Medical Research under the MSMT project, which is funded in whole by the Bill & Melinda Gates Foundation (grant INV 02202); by the Bill & Melinda Gates Foundation (grant INV-056612 to M. F. B.); and by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant R01AI153355 to M. F. B.). Under the grant conditions of the Bill & Melinda Gates Foundation, a Creative Commons Attribution 4.0 Generic License has already been assigned to the author of the accepted manuscript version that might arise from this submission.
References
- 1. World Health Organization (WHO) . World malaria report 2023. Geneva, Switzerland: World Health Organization; 2023. Available at: https://www.who.int/publications/i/item/9789240086173. Accessed June 20, 2024.
- 2. Popkin-Hall ZR, Seth MD, Madebe RA, et al. Malaria species positivity rates among symptomatic individuals across regions of differing transmission intensities in mainland Tanzania. J Infect Dis 2024; 229:959–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Malaria Control Programme (NMCP), Ministry of Health, Community Development, Gender, Elderly and Children . National Malaria Strategic Plan 2021-2025: transitioning to malaria elimination in phases. 2020. Available at: https://www.nmcp.go.tz/storage/app/uploads/public/643/916/391/6439163912677679591851.pdf. Accessed June 20, 2024.
- 4. World Health Organization (WHO) . Global report on insecticide resistance in malaria vectors: 2010–2016. Geneva, Switzerland: World Health Organization; 2018. Available at: https://www.who.int/publications/i/item/9789241514057. Accessed May 15, 2024.
- 5. World Health Organization (WHO) . Report on antimalarial drug efficacy, resistance and response: 10 years of surveillance (2010–2019). Geneva, Switzerland: World Health Organization; 2020. Available at: https://www.who.int/publications/i/item/9789240012813. Accessed May 5, 2024.
- 6. Watson OJ, Tran TNA, Zupko RJ, et al. Global risk of selection and spread of Plasmodium falciparum histidine-rich protein 2 and 3 gene deletions. MedRxiv [Preprint: not peer reviewed]. 1 January 2024. Available from: 10.1101/2023.10.21.23297352. [DOI]
- 7. Thawer SG, Golumbeanu M, Munisi K, et al. The use of routine health facility data for micro-stratification of malaria risk in mainland Tanzania. Malar J 2022; 21:345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rumisha SF, Shayo EH, Mboera LEG. Spatio-temporal prevalence of malaria and anaemia in relation to agro-ecosystems in Mvomero district, Tanzania. Malar J 2019; 18:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishengoma DS, Mmbando BP, Mandara CI, et al. Trends of Plasmodium falciparum prevalence in two communities of Muheza district North-eastern Tanzania: correlation between parasite prevalence, malaria interventions and rainfall in the context of re-emergence of malaria after two decades of progressively declining transmission. Malar J 2018; 17:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noedl H, Se Y, Schaecher K, et al. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med 2008; 359:2619–20. [DOI] [PubMed] [Google Scholar]
- 11. Uwimana A, Legrand E, Stokes BH, et al. Emergence and clonal expansion of in vitro artemisinin-resistant Plasmodium falciparum kelch13 R561H mutant parasites in Rwanda. Nat Med 2020; 26:1602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Uwimana A, Umulisa N, Venkatesan M, et al. Association of Plasmodium falciparum kelch13 R561H genotypes with delayed parasite clearance in Rwanda: an open-label, single-arm, multicentre, therapeutic efficacy study. Lancet Infect Dis 2021; 21:1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balikagala B, Fukuda N, Ikeda M, et al. Evidence of artemisinin-resistant malaria in Africa. N Engl J Med 2021; 385:1163–71. [DOI] [PubMed] [Google Scholar]
- 14. Ishengoma DS, Mandara CI, Bakari C, et al. Evidence of artemisinin partial resistance in northwestern Tanzania: clinical and molecular markers of resistance. Lancet Infect Dis 2024; 24(11):1225–33. doi: 10.1016/S1473-3099(24)00362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mihreteab S, Platon L, Berhane A, et al. Increasing prevalence of artemisinin-resistant HRP2-negative malaria in Eritrea. N Engl J Med 2023; 389:1191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fola AA, Feleke SM, Mohammed H, et al. Plasmodium falciparum resistant to artemisinin and diagnostics have emerged in Ethiopia. Nat Microbiol 2023; 8:1911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jalei AA, Na-Bangchang K, Muhamad P, Chaijaroenkul W. Monitoring antimalarial drug-resistance markers in Somalia. Parasites Hosts Dis 2023; 61:78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Molina-de la Fuente I, Benito MJS, Ousley J, et al. Screening for K13-propeller mutations associated with artemisinin resistance in Plasmodium falciparum in Yambio County (Western Equatoria State, South Sudan). Am J Trop Med Hyg 2023; 109:1072–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Westercamp N, Owidhi M, Otieno K, et al. Efficacy of artemether-lumefantrine and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria among children in western Kenya, 2016 to 2017. Antimicrob Agents Chemother 2022; 66:e0020722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Juliano JJ, Giesbrecht DJ, Simkin A, et al. Country wide surveillance reveals prevalent artemisinin partial resistance mutations with evidence for multiple origins and expansion of high level sulfadoxine-pyrimethamine resistance mutations in northwest Tanzania. MedRxiv [Preprint: not peer reviewed]. 30 November 2023. Available from: 10.1101/2023.11.07.23298207. [DOI]
- 21. World Health Organization (WHO) . Strategy to respond to antimalarial drug resistance in Africa 2022. Vol 04. Geneva, Switzerland: World Health Organization, 2022. Available at: https://www.who.int/publications/i/item/9789240060265. https://cdn.who.int/media/docs/default-source/malaria/who-antimalarial-drugresistance-strategy-for-consultation.pdf?sfvrsn=9d4eaa0_6. Date accessed: December 31, 2023.
- 22. Zupko RJ, Nguyen TD, Ngabonziza JCS, et al. Modeling policy interventions for slowing the spread of artemisinin-resistant pfkelch R561H mutations in Rwanda. Nat Med 2023; 29:2775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Malaria Control Programme (NMCP), Ministry of Health, Community Development, Gender, Elderly and Children . National guidelines for malaria diagnosis, treatment and preventive therapies. 2020. Available at: https://www.nmcp.go.tz/storage/app/uploads/public/643/90d/29e/64390d29ef4b0392189644.pdf. Accessed June 11, 2024.
- 24. Tewara MA, Mbah-Fongkimeh PN, Dayimu A, Kang F, Xue F. Small-area spatial statistical analysis of malaria clusters and hotspots in Cameroon; 2000–2015. BMC Infect Dis 2018; 18:636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. National Malaria Control Programme (NMCP), Consultative malaria expert meeting report 2018. Tanzania; Ministry of Health, Community Development, Gender, Elderly and Children; . 2018.
- 26. National Malaria Control Programme (NMCP), Supplementary malaria midterm strategic plan 2018–2020. Tanzania; Ministry of Health, Community Development, Gender, Elderly and Children; . 2018.
- 27. Mandai SS, Francis F, Challe DP, et al. High prevalence and risk of malaria among asymptomatic individuals from villages with high prevalence of artemisinin partial resistance in Kyerwa district of Kagera region, north-western Tanzania. Malar J 2024; 23:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nundu SS, Culleton R, Simpson SV, et al. Malaria parasite species composition of Plasmodium infections among asymptomatic and symptomatic school-age children in rural and urban areas of Kinshasa, Democratic Republic of Congo. Malar J 2021; 20:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mbah CE, Ambe LA, Ngwewondo A, et al. A comparative study of asymptomatic malaria in a forest rural and depleted forest urban setting during a low malaria transmission and COVID-19 pandemic period. Biomed Res Int 2022; 2022:2545830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tusting LS, Rek J, Arinaitwe E, et al. Why is malaria associated with poverty? Findings from a cohort study in rural Uganda. Infect Dis Poverty 2016; 5:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCann RS, van den Berg H, Diggle PJ, et al. Assessment of the effect of larval source management and house improvement on malaria transmission when added to standard malaria control strategies in southern Malawi: study protocol for a cluster-randomised controlled trial. BMC Infect Dis 2017; 17:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Orkoh E, Efobi U. Effects of behaviour change communication on knowledge and prevention of malaria among women in Ghana. Eval Rev 2024;48:1050–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saxena R, Nagpal BN, Das MK, et al. A spatial statistical approach to analyze malaria situation at micro level for priority control in Ranchi district, Jharkhand. Indian J Med Res 2012; 136:776–82. [PMC free article] [PubMed] [Google Scholar]
- 34. Dabaro D, Birhanu Z, Negash A, Hawaria D, Yewhalaw D. Effects of rainfall, temperature and topography on malaria incidence in elimination targeted district of Ethiopia. Malar J 2021; 20:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kihwele F, Gavana T, Makungu C, et al. Exploring activities and behaviours potentially increases school-age children's vulnerability to malaria infections in south-eastern Tanzania. Malar J 2023; 22:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chacky F, Runge M, Rumisha SF, et al. Nationwide school malaria parasitaemia survey in public primary schools, the United Republic of Tanzania. Malar J 2023; 17:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dida GO, Anyona DN, Abuom PO, et al. Spatial distribution and habitat characterization of mosquito species during the dry season along the Mara river and its tributaries, in Kenya and Tanzania. Infect Dis Poverty 2018; 7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boni MF, White NJ, Baird JK. The community as the patient in malaria-endemic areas: preempting drug resistance with multiple first-line therapies. PLoS Med 2016; 13:e1001984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Boni MF. Breaking the cycle of malaria treatment failure. Front Epidemiol 2022; 2:1041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.