Abstract
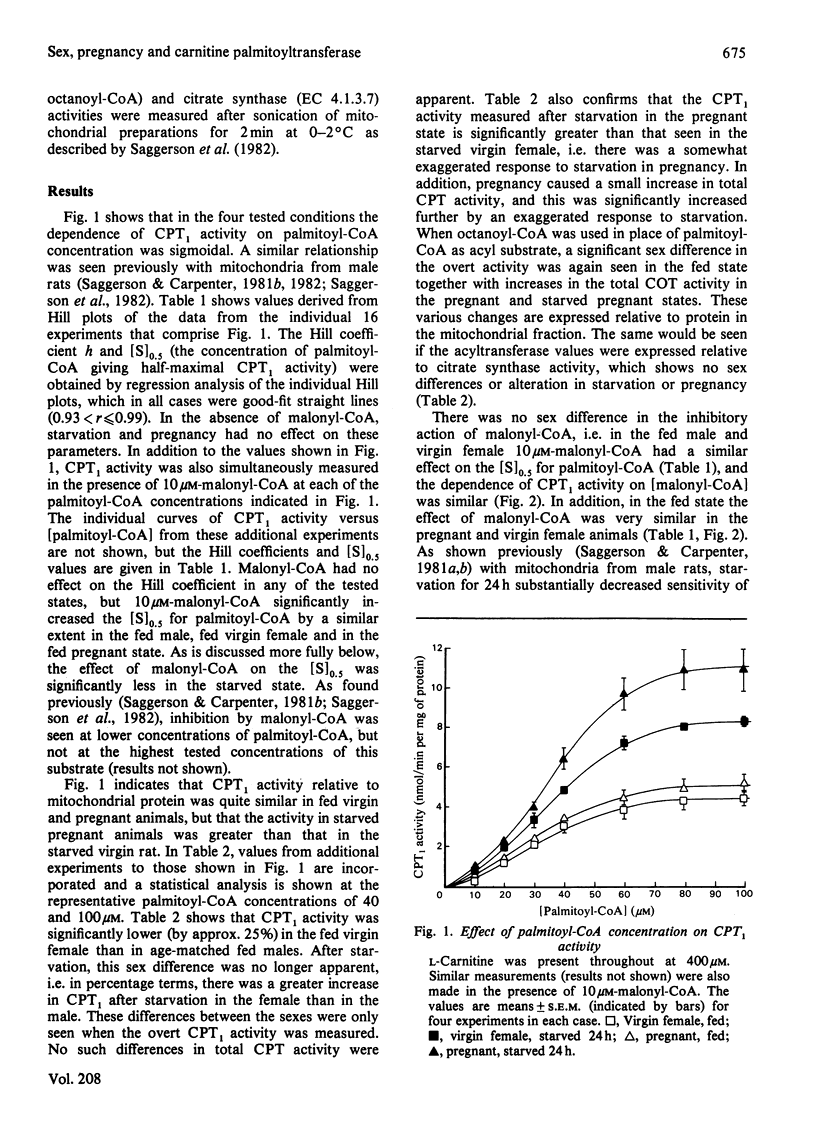
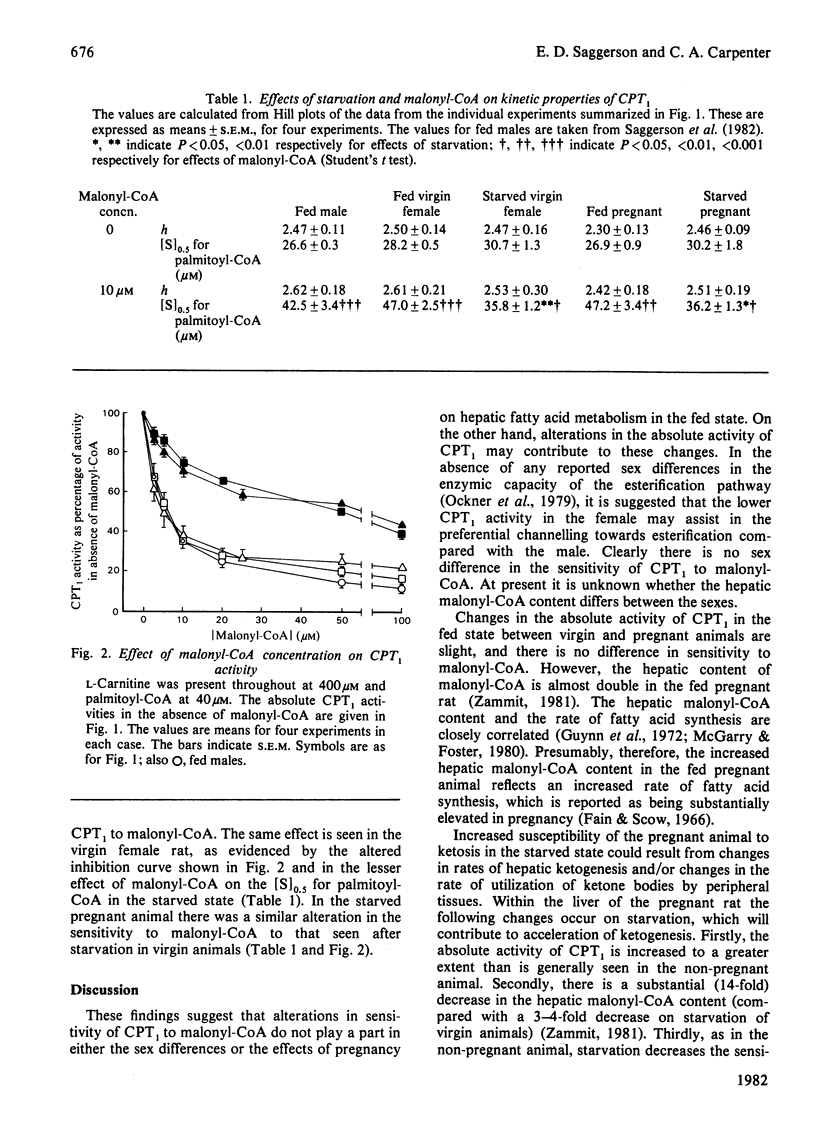
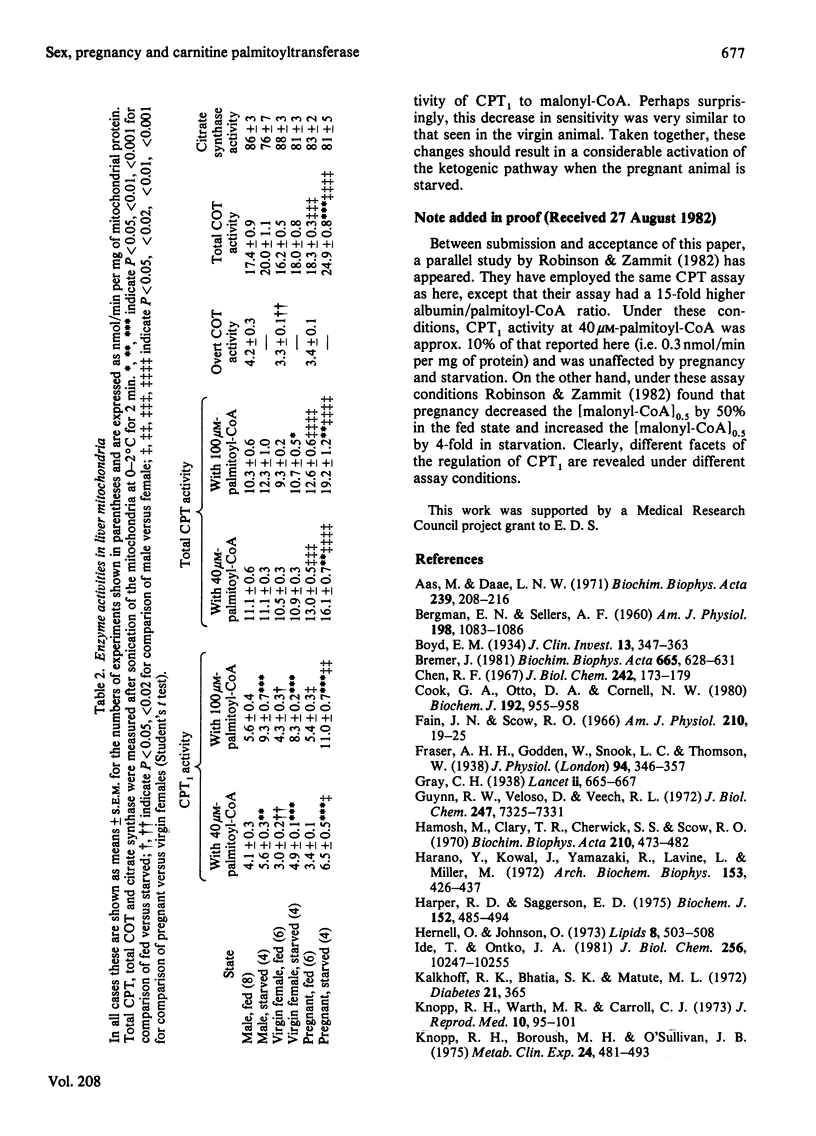
1. Hepatic carnitine palmitoyltransferase activity was measured over a range of concentrations of palmitoyl-CoA and in the presence of several concentrations of the inhibitor malonyl-CoA. These measurements were made in mitochondria obtained from the livers of fed and starved (24 h) virgin female and fed and starved pregnant rats. 2. In the fed state overt carnitine palmitoyltransferase activity was significantly lower in virgin females than in age-matched male rats. 3. Starvation increased overt carnitine palmitoyltransferase activity in both virgin and pregnant females. This increase was larger than in the male and was greater in pregnant than in virgin females. 4. In the fed state pregnancy had no effect on the Hill coefficient or the [S]0.5 when palmitoyl-CoA was varied as substrate. Pregnancy did not alter the sensitivity of the enzyme to inhibition by malonyl-CoA. 5. Starvation decreased the sensitivity of the enzyme to malonyl-CoA. The change in sensitivity was similar in male, virgin female and pregnant rats. 6. The possible relevance of these findings to known sex differences and changes with pregnancy in hepatic fatty acid oxidation and esterification are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aas M., Daae L. N. Fatty acid activation and acyl transfer in organs from rats in different nutritional states. Biochim Biophys Acta. 1971 Jul 13;239(2):208–216. doi: 10.1016/0005-2760(71)90166-4. [DOI] [PubMed] [Google Scholar]
- BERGMAN E. N., SELLERS A. F. Comparison of fasting ketosis in pregnant and nonpregnant guinea pigs. Am J Physiol. 1960 May;198:1083–1086. doi: 10.1152/ajplegacy.1960.198.5.1083. [DOI] [PubMed] [Google Scholar]
- Boyd E. M. THE LIPEMIA OF PREGNANCY. J Clin Invest. 1934 Mar;13(2):347–363. doi: 10.1172/JCI100590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J. The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-CoA. Biochim Biophys Acta. 1981 Sep 24;665(3):628–631. doi: 10.1016/0005-2760(81)90282-4. [DOI] [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Cook G. A., Otto D. A., Cornell N. W. Differential inhibition of ketogenesis by malonyl-CoA in mitochondria from fed and starved rats. Biochem J. 1980 Dec 15;192(3):955–958. doi: 10.1042/bj1920955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A. H., Godden W., Snook L. C., Thomson W. The influence of diet upon ketonaemia in pregnant ewes. J Physiol. 1938 Dec 14;94(3):346–357. doi: 10.1113/jphysiol.1938.sp003685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guynn R. W., Veloso D., Veech R. L. The concentration of malonyl-coenzyme A and the control of fatty acid synthesis in vivo. J Biol Chem. 1972 Nov 25;247(22):7325–7331. [PubMed] [Google Scholar]
- Hamosh M., Clary T. R., Chernick S. S., Scow R. O. Lipoprotein lipase activity of adipose and mammary tissue and plasma triglyceride in pregnant and lactating rats. Biochim Biophys Acta. 1970 Sep 8;210(3):473–482. doi: 10.1016/0005-2760(70)90044-5. [DOI] [PubMed] [Google Scholar]
- Harano Y., Kowal J., Yamazaki R., Lavine L., Miller M. Carnitine palmitoyltransferase activities (1 and 2) and the rate of palmitate oxidation in liver mitochondria from diabetic rats. Arch Biochem Biophys. 1972 Dec;153(2):426–437. doi: 10.1016/0003-9861(72)90360-8. [DOI] [PubMed] [Google Scholar]
- Harper R. D., Saggerson E. D. Some aspects of fatty acid oxidation in isolated fat-cell mitochondria from rat. Biochem J. 1975 Dec;152(3):485–494. doi: 10.1042/bj1520485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernell O., Johnson O. Effect of ethanol on plasma triglycerides in male and female rats. Lipids. 1973 Sep;8(9):503–508. doi: 10.1007/BF02531985. [DOI] [PubMed] [Google Scholar]
- Ide T., Ontko J. A. Increased secretion of very low density lipoprotein triglyceride following inhibition of long chain fatty acid oxidation in isolated rat liver. J Biol Chem. 1981 Oct 25;256(20):10247–10255. [PubMed] [Google Scholar]
- Knopp R. H., Boroush M. A., O'Sullivan J. B. Lipid metabolism in pregnancy. II. Postheparin lipolytic acitivity and hypertriglyceridemia in the pregnant rat. Metabolism. 1975 Apr;24(4):481–493. doi: 10.1016/0026-0495(75)90073-6. [DOI] [PubMed] [Google Scholar]
- Knopp R. H., Warth M. R., Carrol C. J. Lipid metabolism in pregnancy. I. Changes in lipoprotein triglyceride and cholesterol in normal pregnancy and the effects of diabetes mellitus. J Reprod Med. 1973 Mar;10(3):95–101. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Meier J. M., Foster D. W. The effects of starvation and refeeding on carbohydrate and lipid metabolism in vivo and in the perfused rat liver. The relationship between fatty acid oxidation and esterification in the regulation of ketogenesis. J Biol Chem. 1973 Jan 10;248(1):270–278. [PubMed] [Google Scholar]
- Norum K. R. Activation of palmityl-coA: carnitine palmityltransferase in livers from fasted, fat-fed, or diabetic rats. Biochim Biophys Acta. 1965 Jun 1;98(3):652–654. doi: 10.1016/0005-2760(65)90166-9. [DOI] [PubMed] [Google Scholar]
- Ockner R. K., Burnett D. A., Lysenko N., Manning J. A. Sex differences in long chain fatty acid utilization and fatty acid binding protein concentration in rat liver. J Clin Invest. 1979 Jul;64(1):172–181. doi: 10.1172/JCI109437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontko J. A. Effects of ethanol on the metabolism of free fatty acids in isolated liver cells. J Lipid Res. 1973 Jan;14(1):78–86. [PubMed] [Google Scholar]
- Ontko J. A., Johns M. L. Evaluation of malonyl-CoA in the regulation of long-chain fatty acid oxidation in the liver. Evidence for an unidentified regulatory component of the system. Biochem J. 1980 Dec 15;192(3):959–962. doi: 10.1042/bj1920959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otway S., Robinson D. S. The significance of changes in tissue clearing-factor lipase activity in relation to the lipaemia of pregnancy. Biochem J. 1968 Feb;106(3):677–682. doi: 10.1042/bj1060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otway S., Robinson D. S. The use of a non-ionic detergent (Triton WR 1339) to determine rates of triglyceride entry into the circulation of the rat under different physiological conditions. J Physiol. 1967 May;190(2):321–332. doi: 10.1113/jphysiol.1967.sp008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERS J. P., HEINEMANN M., MAN E. B. The lipids of serum in pregnancy. J Clin Invest. 1951 Apr;30(4):388–394. doi: 10.1172/JCI102455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTSON A., THIN C. A study of starvation ketosis in the ruminant. Br J Nutr. 1953;7(1-2):181–195. doi: 10.1079/bjn19530019. [DOI] [PubMed] [Google Scholar]
- Robinson I. N., Zammit V. A. Sensitivity of carnitine acyltransferase I to malonly-CoA inhibition in isolated rat liver mitochondria is quantitatively related to hepatic malonyl-CoA concentration in vivo. Biochem J. 1982 Jul 15;206(1):177–179. doi: 10.1042/bj2060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOW R. O., CHERNICK S. S., BRINLEY M. S. HYPERLIPEMIA AND KETOSIS IN THE PREGNANT RAT. Am J Physiol. 1964 Apr;206:796–804. doi: 10.1152/ajplegacy.1964.206.4.796. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D. Carnitine acyltransferase activities in rat liver and heart measured with palmitoyl-CoA and octanoyl-CoA. Latency, effects of K+, bivalent metal ions and malonyl-CoA. Biochem J. 1982 Feb 15;202(2):397–405. doi: 10.1042/bj2020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Malonyl CoA inhibition of carnitine acyltransferase activities: effects of thiol-group reagents. FEBS Lett. 1982 Jan 11;137(1):124–128. doi: 10.1016/0014-5793(82)80329-3. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A., Tselentis B. S. Effects of thyroidectomy and starvation on the activity and properties of hepatic carnitine palmitoyltransferase. Biochem J. 1982 Dec 15;208(3):667–672. doi: 10.1042/bj2080667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler-Argilaga C., Heimberg M. Comparison of metabolism of free fatty acid by isolated perfused livers from male and female rats. J Lipid Res. 1976 Nov;17(6):605–615. [PubMed] [Google Scholar]
- Tol V. A. Aspects of long-chain acyl-COA metabolism. Mol Cell Biochem. 1975 Apr 30;7(1):19–31. doi: 10.1007/BF01732160. [DOI] [PubMed] [Google Scholar]
- Veerkamp J. H., Van Moerkerk H. T. The effect of malonyl-CoA on fatty acid oxidation in rat muscle and liver mitochondria. Biochim Biophys Acta. 1982 Feb 15;710(2):252–255. doi: 10.1016/0005-2760(82)90157-6. [DOI] [PubMed] [Google Scholar]
- Warth M. R., Arky R. A., Knopp R. H. Lipid metabolism in pregnancy. II. Altered lipid composition in intermediage, very low, low and high-density lipoprotein fractions. J Clin Endocrinol Metab. 1975 Oct;41(4):649–655. doi: 10.1210/jcem-41-4-649. [DOI] [PubMed] [Google Scholar]
- Wasfi I., Weinstein I., Heimberg M. Hepatic metabolism of [1-14C]oleate in pregnancy. Biochim Biophys Acta. 1980 Sep 8;619(3):471–481. doi: 10.1016/0005-2760(80)90099-5. [DOI] [PubMed] [Google Scholar]
- Wasfi I., Weinstein I., Heimberg M. Increased formation of triglyceride from oleate in perfused livers from pregnant rats. Endocrinology. 1980 Aug;107(2):584–590. doi: 10.1210/endo-107-2-584. [DOI] [PubMed] [Google Scholar]
- Watkins M. L., Fizette N., Heimberg M. Sexual influences on hepatic secretion of triglyceride. Biochim Biophys Acta. 1972 Sep 7;280(1):82–85. doi: 10.1016/0005-2760(72)90214-7. [DOI] [PubMed] [Google Scholar]
- Weinstein I., Seltzer M., Belitsky R. The interrelation of glucagon and gonadectomy upon hepatic triglyceride metabolism in rats. Biochim Biophys Acta. 1974 Apr 26;348(1):14–22. doi: 10.1016/0005-2760(74)90088-5. [DOI] [PubMed] [Google Scholar]
- Whitelaw E., Williamson D. H. Effects of lactation of ketogenesis from oleate or butyrate in rat hepatocytes. Biochem J. 1977 Jun 15;164(3):521–528. doi: 10.1042/bj1640521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox H. G., Woodside W. F., Breen K. J., Knapp H. R., Jr, Heimberg M. The effect of sex on certain properties of the very low density lipoprotein secreted by the liver. Biochem Biophys Res Commun. 1974 Jun 18;58(4):919–926. doi: 10.1016/s0006-291x(74)80231-7. [DOI] [PubMed] [Google Scholar]
- Zammit V. A. Regulation of hepatic fatty acid metabolism. The activities of mitochondrial and microsomal acyl-CoA:sn-glycerol 3-phosphate O-acyltransferase and the concentrations of malonyl-CoA, non-esterified and esterified carnitine, glycerol 3-phosphate, ketone bodies and long-chain acyl-CoA esters in livers of fed or starved pregnant, lactating and weaned rats. Biochem J. 1981 Jul 15;198(1):75–83. doi: 10.1042/bj1980075. [DOI] [PMC free article] [PubMed] [Google Scholar]


