Abstract
Background:
Stainless steel (SS) orthodontic brackets may have varying corrosion resistance when used with mouthwashes during orthodontic treatment. Studying their effects on orthodontic brackets will be beneficial.
Aim:
The study’s objective was to analyze the surface characteristics of SS orthodontic brackets and their resistance to corrosion, exposed to chlorhexidine and povidone-iodine mouth rinses – an in vitro and in vivo study.
Materials and Methods:
The in vitro test: MBT 0.022” slot SS orthodontic brackets were immersed in three groups – Group A – Modified Meyer-Fusayama artificial saliva (AS), Group B – Chlorhexidine, and Group C – Povidone-iodine mouthwash. The in vivo test: Brackets were conventionally bonded on the patient’s teeth and divided into Group I – control group, Group II – patients used chlorhexidine, and Group III used povidone-iodine mouth rinse. The corrosion resistance and surface characteristics of SS brackets were determined using scanning electron microscope (SEM), electrochemical impedance spectroscopy, and X-ray photoelectron spectroscopy.
Results:
Higher corrosion resistance was obtained for brackets immersed in chlorhexidine mouth rinse. The polarization resistance value of the orthodontic SS bracket was 109 MΩ, 1383 MΩ, and 769 MΩ immersed in AS, chlorhexidine, and povidone-iodine mouth rinse, respectively. After surface chemical analysis, XPS data showed the largest intensity peak of metallic chromium (CrO) fresh sample and in the sample immersed in chlorhexidine mouthwash. According to SEM, brackets immersed in chlorhexidine showed a relatively smooth surface.
Conclusion:
In this study, chlorhexidine was found to be less corrosive followed by povidone-iodine.
Keywords: Chlorhexidine mouth rinse, corrosion resistance, orthodontic brackets, povidone-iodine mouth rinse, surface characteristics
Introduction
Orthodontists are concerned about the degradation of orthodontic appliances in the oral environment. Citrus fruits, juices, pickles, and carbonated soft drinks with high acidity may reduce salivary pH to 2 or lower, while soups, salty nuts, and fluoride mouthwashes with high alkalinity may raise pH to 8.5 or higher.[1] Changes in temperature and pH fluctuations caused by food, nutritional breakdown, oral microorganisms, and associated by-products are all important factors that need to be considered while assessing the clinical efficacy of orthodontic devices that stay in the oral cavity for months or years.[2]
Corrosion on the surface of the orthodontic brackets affects both static and kinetic friction. Static friction is the force required to begin the movement, whereas kinetic friction is the force required to keep the movement going once it has begun.[3] The corrosion resistance of stainless steel (SS), cobalt–chromium, and titanium alloys used in orthodontic appliances is due to the passivating effect of the chromium oxide (Cr2O3) surface layer.[4] Nickel facilitates resistance to corrosion by competing with chromium to generate salts, to free up sufficient chromium for passivation.[1]
The frictional force between the bracket and the wire reduces about 40% of the available force, resulting in an anchorage loss. Kusy and Whitley were the first to show the effect of wire surface topography on the coefficient of friction using laser spectroscopy. Their findings demonstrated that lower surface roughness would not be a necessary prerequisite for low frictional coefficients.[3] However, the surface roughness of the bracket slots was not taken into account in these investigations. Previous studies have shown that mouth rinse containing chlorhexidine has a long-lasting antibacterial impact and is perhaps the most efficient chemical technique in preventing plaque build-up to date.[5] When chlorhexidine comes into direct contact with SS brackets because of the presence of chlorhexidine gluconate, the brackets show increased chromium content release. After immersion in a chlorhexidine-containing mouth rinse, the surface roughness of orthodontic SS brackets and the frictional force between SS archwires and brackets were substantially enhanced.[6] Surface changes may impact the dimensional accuracy and frictional resistance of the slot, which may impair bracket performance, such as torque and tip expression, and rotation control.[7]
The use of povidone-iodine as a mouth rinse against coronavirus has increased in recent years. The synergistic effect of iodine and the water-soluble polymer polyvinylpyrrolidone called povidone-iodine (PVP-I), has been shown to be beneficial against coronavirus, according to the evidence available.[8] No previous studies have been performed to assess the influence and clinical implications of povidone-iodine mouth rinse on orthodontic brackets. Thus, the aim of the present study was to compare the corrosion resistance and surface characteristics of SS orthodontic bracket materials, in chlorhexidine and povidone-iodine mouth rinses with a reference solution of Fusayama-Meyer artificial saliva (AS).
Materials and Methods
This study was a pilot study with a minimum of 57 samples since there is no previous evidence on the effect of povidone-iodine mouth rinses on corrosion resistance and surface characteristic SS orthodontic brackets. The 0.022” slot MBT bracket system was the most widely recognized bracket type used during the study (American Orthodontics Mini Master upper premolar bracket) [Figure 1].
Figure 1.

0.022” slot MBT bracket system (AO mini master upper premolar bracket)
The in vitro test (n = 45) includes three groups; (i) Group A (n = 15) served as the control group containing modified Meyer-Fusayama AS was used as the base solution; (ii) Group B (n = 15) consisted of 9% base solution mixed ultrasonically with 91% chlorhexidine mouthwash (0.2% chlorhexidine digluconate and 13.65% ethanol); and (iii) Group C (n = 15) consisted of 9% base solution mixed ultrasonically with 91% povidone-iodine mouthwash (2% povidone-iodine and 8.38% ethanol).[9] The pH of each solution was measured using pH meter. Each group was subdivided into four subgroups based on the time period of immersion; day 0, day 1, day 7, and day 28.[10] The entire set of SS orthodontic brackets was submerged in a 2 ml microcentrifuge tube of the test solution at 37°C ± 1°C.[11]
In the in vivo test (n = 12), all the brackets were conventionally bonded to the patient’s teeth using the “etch and bond technique.” Patients were divided into three groups; (i) Group I (n = 4) patients served as a control group without any mouth rinses, (ii) Group II (n = 4) patients were instructed to rinse with 10 ml chlorhexidine mouth rinse for 30 s thrice daily,[12] and (iii) Group III (n = 4) patients were instructed to rinse with 10 ml povidone-iodine mouth rinse for 30 s thrice daily.[13] After 90 days of exposure period, all the brackets were debonded with bracket removing plier with force applied only to the bracket base and were kept in receptacles filled with distilled water. They were rinsed with distilled water to remove any loosely attached debris and were kept in self-sealed sterilizing packs until analysis. Figure 2 shows the study flowchart.
Figure 2.
Flow chart of in vitro and in vivo study setup
Assessment of corrosion resistance
Electrochemical impedance spectroscopy (EIS) measurements were carried out with an electrochemical analyzer that was computer-controlled. Three electrodes were set up in a double-wall, one-compartment cell. A 2 cm2 platinum sheet serves as the auxiliary electrode. Ag/AgCl (3 M KCl) serves as the reference electrode. This electrode will be used to refer to all potential data presented in this investigation. The working electrode utilized in the experiment was SS orthodontic brackets.[9] The cleaning process involved washing the samples sequentially with bidistilled water and ethanol and dried with filter paper.
During the corrosion test, the whole orthodontic brackets were immersed in 20 ml of electrolyte solution and a temperature of 37°C ± 1°C. The EIS studies were carried out in the frequency range of 100 kHz–0.003 Hz at open circuit potential after immersion in the test solutions, by applying an alternating current signal with a peak value of 0.005 V.
Assessment of surface morphology
The surface morphology of slot surfaces of orthodontic SS brackets was analyzed with a scanning electron microscope (SEM) after 1, 7, and 28 days of immersion period in test solutions and 90 days of intraoral exposure and was compared to their controls. When imaging nonconductive specimens, the SEM was powered up at a 15 kV accelerating voltage and a low vacuum chamber pressure. With a working distance of 9 mm, no conductive coating is required. The bracket slot surface could be examined at ×100 magnification; after that, the surface was meticulously examined at higher magnifications of ×500 and ×1000 to identify any surface irregularity.
Assessment of surface chemical analysis
After EIS measurement, the specimens were removed from the electrolyte and dried by argon gas blasting. X-ray photoelectron spectroscopy (XPS, PHI 5000 VersaProbe II, ULVAC-PHI Inc., USA) with a micro-focused (100 m, 15 kV) monochromatic Al-K X-Ray source (hv = 1486.6 eV) was used to determine the surface chemical analyses of the specimens. Recorded were survey and narrow scan (high resolution) spectra. An X-ray source power of 50 W and a pass energy of 187.85 eV were used to record survey scans. At a pass energy of 46.95 eV, high-resolution spectra of the principal elements were captured. The Multipak program from PHI was used to process the XPS data.[14]
Statistical analysis
All the statistical analyses were done using IBM SPSS Statistics 20 (IBM, Armonk, New York, United States). Data were presented as median with interquartile range. Kruskal–Wallis test followed by multiple comparison Dun Boneferroni test was applied to compare the median parameters among groups. Wilcoxon signed-rank test was applied to compare the median parameters from baseline to different periods.
Results
pH measurement
To investigate whether the pH of the mouthwash had any impact on the degree of corrosion from the SS brackets, the pH of each electrolyte solution was evaluated in this investigation using a pH meter. The study found that modified Fusayama-Meyer AS has a pH of 7; chlorhexidine mouth rinse has a pH of 7; and povidone-iodine mouth rinse has a pH of 5.
Estimation of corrosion resistance
EIS gives a resistance (R) value at each frequency of current passing through the sample. The resistance value is a direct representation of corrosion resistance [Table 1].
Table 1.
The resistance value (R) of the As-received Commercial Stainless-Steel Brackets compared between control group, chlorhexidine, and povidone iodine mouth rinses at different time points
| Control group | Chlorhexidine group | Povidone iodine group | P -Value | Pairwise comparision | |
|---|---|---|---|---|---|
|
| |||||
| Median (Q1 - Q3) |
Median (Q1 - Q3) |
Median (Q1 - Q3) |
|||
| Day 0 | 124.19 (83.57- 155.95) |
404.25 (301.71 - 557.43) |
233.14 (159.63-373.20) |
<0.001 | 1 vs 2 - <0.001 2 vs 3 - <0.001 3 vs 2 - <0.001 |
| Day 1 | 101.06 (57.49- 123.93) |
521.67 (395.84 - 770.67) |
195.16 (160.55-274.53) |
<0.001 | 1 vs 2 - <0.001 2 vs 3 - <0.001 3 vs 2 - <0.001 |
| Day 7 | 121.84 (76.51 - 151.34) |
417.04 (365.87 - 595.15) |
155.60 (113.68-211.61) |
<0.001 | 1 vs 2 - <0.001 2 vs 3 - <0.001 3 vs 2 - <0.001 |
| Day 28 | 149.06 (110.21 -226.94) |
1482.61 ( 1343.70-1947.93) |
830.87 (718.51 - 1032.58) |
<0.001 | 1 vs 2 - <0.001 2 vs 3 - <0.001 3 vs 2 - <0.001 |
| Day 90 | 132.72 (97.75- 167.08) |
1742.40 ( 1399.95-2619.34) |
567.89 (221.34-947.89) |
<0.001 | 1 vs 2 - <0.001 2 vs 3 - <0.001 3 vs 2 - <0.001 |
On days 0, 1,7 28, and 90 of in vitro study evaluation, the corrosion resistance of orthodontic SS brackets was observed to be higher for the chlorhexidine group, with a resistance value (R) of 404.25 Ω, 521.67 Ω, 417.04 Ω,1482.61 Ω, and 1742.40 Ω, respectively. On days 0, 1, 7, 28, and 90 the corrosion resistance for the control group was observed to be the lowest, with a resistance value (R) of 124.19 Ω, 101.06 Ω, 121.84 Ω, 149.06 Ω, and 132.72 Ω, respectively. With a resistance value (R) of 233.14 Ω, 195.16 Ω, 155.60 Ω, 830.87 Ω, and 567.89 Ω on day 0, day 1, day 7, day 28, and day 90, respectively, the povidone-iodine group was found to be intermediate between chlorhexidine and the control group. The corrosion resistance of orthodontic SS brackets was observed to be highest on day 28. This was statistically significant between-group comparison and pairwise comparison, with a P < 0.001 [Table 2].
Table 2.
The resistance value (R) of the As-received Commercial Stainless-Steel Brackets compared within control group, chlorhexidine, and povidone iodine mouth rinses at different time points
| Day 0 | Day 1 | Day 7 | Day 28 | P.value | |
|---|---|---|---|---|---|
|
| |||||
| Median (Q1 - Q3) |
Median (Q1 -Q3) |
Median (Q1 - Q3) |
Median (Q1 - Q3) |
||
| Control group | 124.19 (83.57- 155.95) |
101.06 (57.49-123.93) |
121.84 (76.51 -151.34) |
149.06 (110.21 -226.94) |
<0.001 |
| Chlorhexidine group | 404.25 (301.71 -557.43) |
521.67 (395.84 - 770.67) |
417.04 (365.87 - 595.15) |
1482.61 (1343.70 -1947.93) |
<0.001 |
| Povidone iodine group | 233.14 (159.63 - 373.20) |
195.16 (160.55-274.53) |
155.60 (113.68-211.61) |
830.87 (718.51 - 1032.58) |
<0.001 |
EIS consists of plotting Nyquist plots that represent impedance expressions [Graph 1]. Each point on the Nyquist plot is an impedance value at a frequency point. At the X-axis, the impedance at the right side of the plot is conducted with low frequency, while, at the higher frequencies, their generated impedances are exerted on the left. At the high-frequency intercept, or near the plot origin, the real axis value gives the solution resistance. The low-frequency intercept of the real axis provides a summation of the polarization resistance (Rp) and the solution resistance. Therefore, the semicircle diameter will equal the Rp. The slope of the potential versus the current density at the corrosion potential in the linear polarization curves is the definition of the Rp, which is inversely proportional to the corrosion rate. The RP values are given in Table 3.
Graph 1.
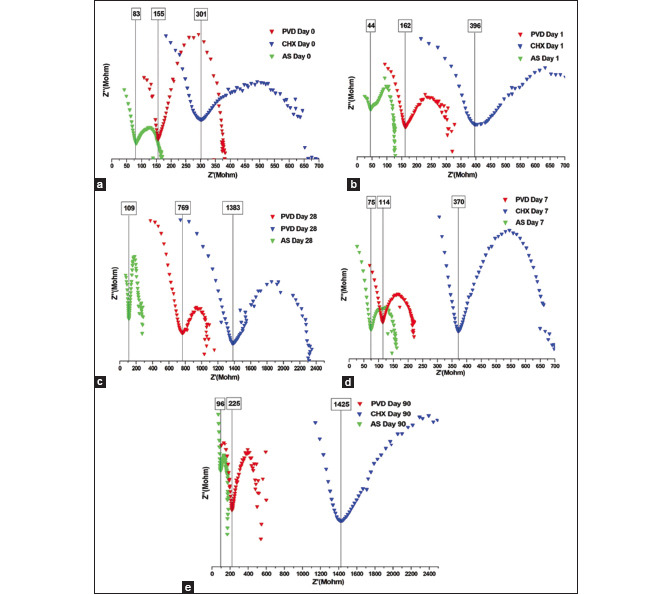
Electrochemical impedance spectroscopy Nyquist Plot for (a) Day 0, (b) day 1, (c) day 7, (d) day 28, (e) day 90. AS: Artificial saliva, CHX: Chlorhexidine, PVD: Povidone iodine
Table 3.
Polarization resistance (Rp) of the as-received commercial stainless-steel brackets immersed in artificial saliva, chlorhexidine, and povidone iodine mouth rinses on different time points
| Artificial saliva (Rp) |
Chlorhexidine + Artificial saliva (Rp) |
Povidion iodine+ Artificial saliva (Rp) |
|
|---|---|---|---|
| Day 0 | 83 | 301 | 155 |
| Day 1 | 44 | 396 | 162 |
| Day 7 | 75 | 370 | 114 |
| Day 28 | 109 | 1383 | 769 |
| Day 90 | 96 | 1425 | 225 |
Analysis of surface chemical composition
Survey spectra of the SS brackets were analyzed for an as-received bracket and after exposure to three different electrolyte solutions such as AS solution, chlorhexidine mouth rinse, and povidone-iodine mouth rinse for durations of 7 days incubated at 37°C ± 1°C. The samples were mechanically polished before each electrochemical experiment to expose a fresh surface to the solution. The survey scan Graph 2 only shows signals of the main alloy constituents of SS such as Iron (Fe), Chromium (Cr), Nickel (Ni), and Cobalt (Co). Apart from the transition metals, Carbon (C) and Oxygen (O) were also detected. Auger electrons of Iron and Nickel are also present in the survey spectra.
Graph 2.

XPS Survey spectra of orthodontic stainless-steel brackets immersed in electrolyte solution for 7-day duration (a) fresh sample, (b) artificial saliva, (c) chlorhexidine mouth rinse, (d) povidone-iodine mouth rinse. AS: Artificial saliva, CHX: Chlorhexidine, PVD: Povidone-iodine
Peaks from the XPS spectra give a relative number of electrons with a specific binding energy. The shorter the peak, the less electrons are represented. The peak intensities give information about the percent composition. The high-resolution spectra of Cr2 2p3/2, Fe2 2p3/2, Co2 2p3/2, and Ni2 2p3/2 obtained on the samples exposed for 7 days to the electrolyte solution are presented. Graph 3 all the transition metals showed 2p3/2 and 2p1/2 peaks, however, for clarity, only 2p3/2 peaks are presented here.
Graph 3.

High-resolution XPS spectra of Cr2 2p3/2, Fe 2p3/2, Co 2p3/2, Ni2 2p3/2. (a) Fresh sample, (b) artificial saliva, (c) chlorhexidine mouth rinse, (d) povidone-iodine mouth rinse
Surface characteristics evaluation
SEM analysis of as received fresh bracket on day 0 showed very less cracks, pits, scratches, and grooves. Analysis of SS orthodontic brackets after immersion revealed a steady deterioration of the brackets’ slot surface over time, which may have been caused by the solution’s corrosive activity. All three groups’ bracket slot surfaces had pits, grooves, and deformations of varied extensions visible on them.
The in vivo test to analyze of SS orthodontic brackets exposed to the povidone-iodine and chlorhexidine mouth rinse showed increased surface topography with an increased number of pits, cracks, and scratches. Brackets exposed to chlorhexidine showed a lesser surface irregularity with pits, groves, and cracks whereas the SS brackets without any mouth rinse exposure showed greater pits and grooves on the slot surface followed by povidone-iodine group. SEM images of SS orthodontic bracket immersed in AS, chlorhexidine (CHX), and povidone-iodine (PVD) comparison between day 0 and day 1, day 7, day 28, and day 90 at (a) ×100, (b) ×500, and (c) ×1000 are shown in Figures 3.
Figure 3.

Scanning electron microscope images of stainless steel orthodontic bracket immersed in artificial saliva, chlorhexidine mouth rinse, and povidone-iodine comparison between day 0 and day 1, day 7, day 28, and day 90 at (a) ×100, (b) ×500, and (c) ×1000. CHX: Chlorhexidine, PVD: Povidone iodine
Discussion
Corrosion is an electrochemical process that results in the loss of the essential metallic properties of a metal.[4] SS, which has a Fe-Cr-Ni composition, is one of the most widely used materials for orthodontic brackets due to its inherent mechanical characteristics and corrosion resistance traits. The Fe, Cr, or Ni (or all) ions may still be liberated from the metal surface in the acidic oral environment due to corrosion processes even though the SS alloy has a protective passive coating covering it.[1] Orthodontic treatment has been discovered to be affected by the corrosion of orthodontic brackets because it increases surface roughness, which impacts sliding mechanics by increasing friction.[7]
In this study, a corrosion test was done using an EIS under computer control. In this investigation, the Rp could be quickly determined and utilized as a parameter for evaluating corrosion resistance from the linear polarization test, which was taken as a nondestructive, quick, and precise electrochemical approach. After the investigation was finished, data revealed that there was a fluctuation in RP values in all three groups [Table 3], indicating that the corrosion process on the SS brackets had started. Corrosion is an electrochemical reaction on the metal surface in which the metal creates an ion release, according to Eliades et al.[15] and Eliades et al.[16] According to the Rp, AS is the most corrosive substance, followed by povidone-iodine mouthwash and chlorhexidine mouthwash. This was consistent with earlier studies that mention how the pH of the solution affects how much metal ion release occurs.[17]
Regarding the Fe-Cr-Ni-based SS alloy, it is well known that the Cr element can create a thin and adherent passive layer based on Cr2O3, which gives a substrate alloy its corrosion resistance. This is in accordance with the findings of the XPS investigation, which revealed that Cr2O3 was the outermost surface structure of the passive layer on all SS brackets.[18] This Cr2O3 layer intensity is varying among the different groups [Graph 3] which is correlating also with the RP value as well. Regarding their corrosion rate, a direct relationship between the Cr content of the SSs and corrosion rate.
Among all orthodontic materials, brackets stay in the patient’s mouth for the longest time. An in-vitro study revealed an early stage of supragingival plaque accumulation consisting of cocci on all the experimental surfaces of SS metal bracket surface by 1 week. With time plaque maturation included rods and filaments, as well as an increasing amount of inter-microbial matrix.[19]
Chlorhexidine (CHX) reduces the accumulation of plaque due to its bacteriostatic or bactericide properties and so maintains periodontal health.[20] Coating salivary bacteria with chlorhexidine molecules also alters the mechanisms of the adsorption of bacteria to the tooth. Because of its high cationic nature, chlorhexidine has a great affinity for the cell wall of microorganisms and changes the surface structures.[21]
In recent years, the usage of povidone-iodine (PVP-I) as a mouthwash to prevent coronavirus has become more widely used. Most obviously, in people with chronic lung/respiratory infections, gargling PVP-I can reduce the incidences of Staphylococcus aureus, Pseudomonas aeruginosa, and Haemophilus influenzae infections by approximately 50%.[8]
To find out whether the quantity of corrosion from the SS brackets had any impact on the pH of the mouthwash, this study additionally evaluated the pH of each mouthwash using a pH meter.[22] The study found that Modified Fusayama-Meyer AS has a pH of 7; Chlorhexidine mouth rinse has a pH of 7; povidone-iodine mouth rinse has a pH of 5. Kwon et al. reported that by increasing the acidity (reducing pH), increased elements released from the alloy causing corrosion, which increased friction between titanium-containing wires and steel brackets,[23] while according to Harris et al., the acidity of the environment did not have any effect on the properties of the alloy.[24] Report based on the study results despite the acidic pH corrosive nature of povidone-iodine is lesser than AS. This is because of the ethanol content in the povidone which causes inhibition of corrosion in the alloy.[25] This is supported by a previous study by Bhola et al. where they found as the concentration of povidone-iodine increases, the corrosion rate decreases, suggested by decreased RP values.[26]
SS orthodontic brackets were subjected to SEM analysis to determine the difference in the surface characteristics of brackets before and after immersion in AS, chlorhexidine, and povidone-iodine mouth rinse for different time periods. More surface alterations on the in vivo test brackets likely indicated surface degeneration brought on by treatment with orthodontics and oral exposure. The results of in-vitro studies cannot be applied to the clinical situation, since factors such as temperature, quantity, quality of saliva, plaque, physical and chemical properties of food and liquids, and oral health conditions can influence the results.[27] The majority of brackets are now made using metal injection molding, which is more affordable than earlier methods but produces surface irregularities.[28] These anomalies aid in the retention of plaque. SEM pictures of orthodontic alloys revealed dark pits and crevices, which are characteristics associated with various corrosion types, including pitting, crevice corrosion, and stress corrosion.[29] These findings are in accordance with previous reports that have also evaluated the performance of orthodontic materials after their exposure to the oral environment or to different mediums that simulated in vivo conditions.[12,30]
The influence of chlorhexidine and povidone-iodine mouthwash on the corrosion resistance and surface characteristics of SS orthodontic brackets is important clinically as it can impact the structural integrity and longevity of these brackets. Changes in the surface characteristics due to the interaction with mouthwashes may affect the brackets’ performance within the oral cavity, potentially altering their durability, biocompatibility, and overall effectiveness during orthodontic treatment. Thus this study results emphasize the significance of meticulous choice and proper utilization of mouthwashes during orthodontic treatments.
Conclusion
Metal ions are released from SS brackets as a result of the corrosive nature of the mouthwash, which is caused by the mouthwash’s chemical composition. It might be recommended to avoid prolonged use of povidone-iodine as mouthwash instead it can be used as a mouth gargle. Considering corrosion resistance, chlorhexidine mouthwashes would be a better alternative than povidone-iodine for orthodontic patients. However, extensive in vivo investigations are needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.House K, Sernetz F, Dymock D, Sandy JR, Ireland AJ. Corrosion of orthodontic appliances –Should we care? Am J Orthod Dentofacial Orthop. 2008;133:584–92. doi: 10.1016/j.ajodo.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Nanjundan K, Vimala G. Evaluation of frictional resistance and surface characteristics after immersion of orthodontic brackets and wire in different chemical solutions: A comparative in vitro study. Indian J Dent Res. 2016;27:513–20. doi: 10.4103/0970-9290.195641. [DOI] [PubMed] [Google Scholar]
- 3.Kusy RP, Whitley JQ. Effects of surface roughness on the coefficients of friction in model orthodontic systems. J Biomech. 1990;23:913–25. doi: 10.1016/0021-9290(90)90356-8. [DOI] [PubMed] [Google Scholar]
- 4.Barrett RD, Bishara SE, Quinn JK. Biodegradation of orthodontic appliances. Part I. Biodegradation of nickel and chromium in vitro. Am J Orthod Dentofacial Orthop. 1993;103:8–14. doi: 10.1016/0889-5406(93)70098-9. [DOI] [PubMed] [Google Scholar]
- 5.Gehlen I, Netuschil L, Berg R, Reich E, Katsaros C. The influence of a 0.2% chlorhexidine mouthrinse on plaque regrowth in orthodontic patients. A randomized prospective study. Part I: Clinical parameters. J Orofac Orthop. 2000;61:54–62. doi: 10.1007/BF02340932. [DOI] [PubMed] [Google Scholar]
- 6.Razavi EE, Nik TH, Hooshmand T, Farazdaghi H, Arefi AH. Surface characterization and frictional force between stainless steel brackets and archwires in orthodontic patients using chlorhexidine- and persica-containing mouthrinses: A randomized controlled trial. Dent Res J (Isfahan) 2021;18:21. [PMC free article] [PubMed] [Google Scholar]
- 7.Doshi UH, Bhad-Patil WA. Static frictional force and surface roughness of various bracket and wire combinations. Am J Orthod Dentofacial Orthop. 2011;139:74–9. doi: 10.1016/j.ajodo.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 8.Kirk-Bayley J, Sunkaraneni S, Challacombe S. The use of povidone iodine nasal spray and mouthwash during the current COVID-19 pandemic may reduce cross infection and protect healthcare workers. Available at SSRN 3563092. 2020 May 4. doi: 10.2139/ssrn.3563092. [Google Scholar]
- 9.Nalbantgil D, Ulkur F, Kardas G, Culha M. Evaluation of corrosion resistance and surface characteristics of orthodontic wires immersed in different mouthwashes. Biomed Mater Eng. 2016;27:539–49. doi: 10.3233/BME-161607. [DOI] [PubMed] [Google Scholar]
- 10.Huang HH, Chiu YH, Lee TH, Wu SC, Yang HW, Su KH, et al. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials. 2003;24:3585–92. doi: 10.1016/s0142-9612(03)00188-1. [DOI] [PubMed] [Google Scholar]
- 11.Danaei SM, Safavi A, Roeinpeikar SM, Oshagh M, Iranpour S, Omidkhoda M. Ion release from orthodontic brackets in 3 mouthwashes: An in vitro study. Am J Orthod Dentofacial Orthop. 2011;139:730–4. doi: 10.1016/j.ajodo.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Hosseinzadeh Nik T, Hooshmand T, Farazdaghi H, Mehrabi A, Razavi ES. Effect of chlorhexidine-containing prophylactic agent on the surface characterization and frictional resistance between orthodontic brackets and archwires: An in vitro study. Prog Orthod. 2013;14:48. doi: 10.1186/2196-1042-14-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chopra A, Sivaraman K, Radhakrishnan R, Balakrishnan D, Narayana A. Can povidone iodine gargle/mouthrinse inactivate SARS-CoV-2 and decrease the risk of nosocomial and community transmission during the COVID-19 pandemic?An evidence-based update. Jpn Dent Sci Rev. 2021;57:39–45. doi: 10.1016/j.jdsr.2021.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang HH. Surface characterizations and corrosion resistance of nickel-titanium orthodontic archwires in artificial saliva of various degrees of acidity. J Biomed Mater Res A. 2005;74:629–39. doi: 10.1002/jbm.a.30340. [DOI] [PubMed] [Google Scholar]
- 15.Eliades T, Eliades G, Athanasiou AE, Bradley TG. Surface characterization of retrieved NiTi orthodontic archwires. Eur J Orthod. 2000;22:317–26. doi: 10.1093/ejo/22.3.317. [DOI] [PubMed] [Google Scholar]
- 16.Eliades T, Pratsinis H, Kletsas D, Eliades G, Makou M. Characterization and cytotoxicity of ions released from stainless steel and nickel-titanium orthodontic alloys. Am J Orthod Dentofacial Orthop. 2004;125:24–9. doi: 10.1016/j.ajodo.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 17.Kuhta M, Pavlin D, Slaj M, Varga S, Lapter-Varga M, Slaj M. Type of archwire and level of acidity: Effects on the release of metal ions from orthodontic appliances. Angle Orthod. 2009;79:102–10. doi: 10.2319/083007-401.1. [DOI] [PubMed] [Google Scholar]
- 18.Shintcovsk RL, Knop LA, Gandini LG, Jr, Martins LP, Pires AS. Comparison surface characteristics and chemical composition of conventional metallic and nickel-free brackets. Braz Oral Res. 2015;29:1–8. doi: 10.1590/1807-3107BOR-2015.vol29.0022. [DOI] [PubMed] [Google Scholar]
- 19.Sukontapatipark W, el-Agroudi MA, Selliseth NJ, Thunold K, Selvig KA. Bacterial colonization associated with fixed orthodontic appliances. A scanning electron microscopy study. Eur J Orthod. 2001;23:475–84. doi: 10.1093/ejo/23.5.475. [DOI] [PubMed] [Google Scholar]
- 20.Brecx M, Netuschil L, Reichert B, Schreil G. Efficacy of listerine, meridol and chlorhexidine mouthrinses on plaque, gingivitis and plaque bacteria vitality. J Clin Periodontol. 1990;17:292–7. doi: 10.1111/j.1600-051x.1990.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 21.Lang Niklaus P, Brecx MC. Chlorhexidine digluconate-an agent for chemical plaque control and prevention of gingival inflammation. J Periodontal Res. 1986;21:74–89. [Google Scholar]
- 22.Ahn HS, Kim MJ, Seol HJ, Lee JH, Kim HI, Kwon YH. Effect of pH and temperature on orthodontic NiTi wires immersed in acidic fluoride solution. J Biomed Mater Res B Appl Biomater. 2006;79:7–15. doi: 10.1002/jbm.b.30505. [DOI] [PubMed] [Google Scholar]
- 23.Kwon YH, Cheon YD, Seol HJ, Lee JH, Kim HI. Changes on NiTi orthodontic wired due to acidic fluoride solution. Dent Mater J. 2004;23:557–65. doi: 10.4012/dmj.23.557. [DOI] [PubMed] [Google Scholar]
- 24.Harris EF, Newman SM, Nicholson JA. Nitinol arch wire in a simulated oral environment: Changes in mechanical properties. Am J Orthod Dentofacial Orthop. 1988;93:508–13. doi: 10.1016/0889-5406(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 25.Matějovský L, Macák J, Pleyer O, Straka P, Staš M. Efficiency of steel corrosion inhibitors in an environment of ethanol-gasoline blends. ACS Omega. 2019;4:8650–60. doi: 10.1021/acsomega.8b03686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhola R, Bhola SM, Mishra B, Olson DL. Effect of povidone-iodine addition on the corrosion behavior of cp-Ti in normal saline. J Mater Sci Mater Med. 2010;21:1413–20. doi: 10.1007/s10856-010-4001-0. [DOI] [PubMed] [Google Scholar]
- 27.Zabel DD, Brown SA, Merritt K, Payer JH. AES analysis of stainless steel corroded in saline, in serum and in vivo. J Biomed Mater Res. 1988;22:31–44. doi: 10.1002/jbm.820220105. [DOI] [PubMed] [Google Scholar]
- 28.Siargos B, Bradley TG, Darabara M, Papadimitriou G, Zinelis S. Galvanic corrosion of metal injection molded (MIM) and conventional brackets with nickel-titanium and copper-nickel-titanium archwires. Angle Orthod. 2007;77:355–60. doi: 10.2319/0003-3219(2007)077[0355:GCOMIM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Fors R, Persson M. Nickel in dental plaque and saliva in patients with and without orthodontic appliances. Eur J Orthod. 2006;28:292–7. doi: 10.1093/ejo/cji091. [DOI] [PubMed] [Google Scholar]
- 30.Kao CT, Huang TH. Variations in surface characteristics and corrosion behaviour of metal brackets and wires in different electrolyte solutions. Eur J Orthod. 2010;32:555–60. doi: 10.1093/ejo/cjp146. [DOI] [PubMed] [Google Scholar]



