Abstract
Background:
The scientific literature has studies that assess the influence of erosive challenges with citric acidic drinks and substances on the adhesive bond strength to enamel and dentin, but does not contain information about the influence of regional components of an acidic diet on this process. Thus, this study evaluated the erosive influence of Amazonian tucupi on enamel and dentin microshear bond strength.
Materials and Methods:
One hundred and sixty-eight healthy bovine incisors teeth were used, divided into 12 groups (n = 14). For erosive cycling, distilled water (negative control), cola-based soft drink (positive control), or tucupi were used, followed by adhesive strategies of (1) etch-and-rinse (conventional) (Adper™ Single Bond 2) and (2) self-etching (Clearfil SE Bond). All specimens were subjected to erosive cycling for 5 days and, after 24 h, composite resin cylinders were built up for the microshear bond strength test. The data showed normal distribution and were analyzed by two-way ANOVA, followed by the Tukey post and test (P ≤ 0.05).
Results:
There were no significant differences in enamel (P > 0.05). In dentin, only the groups exposed to cola-based soft drink showed significant differences (P < 0.01). The failure mode showed that Type II (mixed) was predominant (95%).
Conclusion:
The erosive challenge with tucupi did not influence the bond strength to enamel and dentin, regardless of the adhesive strategy used.
Keywords: Dentin, enamel, Manihot esculenta, shear strength, tooth erosion
Introduction
Dental erosion can be defined as the irreversible loss of hard structures due to a chemical process, with no involvement of bacteria.[1,2] Its etiology can be associated with frequent exposure of teeth endogenous (intrinsic) factors, or even related to professional occupation, practice of sports, ingestion of medications and oral hygiene products, as well as the consumption of acidic foods and beverages (exogenous/extrinsic factors).[2,3,4,5]
The increased prevalence of these lesions seems to be strongly related to changes in lifestyle and worldwide elevated consumption of acidic foods and beverages with high titratable acidity, and little to no amount of calcium, fluoride, and phosphate ions in its composition.[1,2,3,4,5]
Erosive wear initially causes enamel dissolution, which can reach dentin due to the permanent loss of mineralized tissue.[1,2,3,4] Still, after identifying and stabilizing this erosive process, the dentist may need to recover, among others, the functional and aesthetic of the dental elements involved (through restorative procedures). However, dental substrates (enamel and dentin) can undergo morphological changes due to erosion processes, compromising the adhesive bond to these tissues.[4,5,6,7]
Aware of the significant increase in the prevalence of dental erosion worldwide, several products belonging to a potentially acidic diet have been studied.[2,8,9] However, regional components of an acidic diet can also be considered erosive and deserve attention. In Brazil, cassava (Manihot esculenta crantz) is highly consumed, occupying a prominent place in the population’s diet. From it, several by-products can be obtained, like cassava flour and tucupi, and the latter presenting a pH ≈3.5.[10] In this scenario, Martins et al.,[11] in their pioneering study, demonstrated the erosive potential of tucupi through the microhardness test on bovine dental enamel, highlighting that it was not possible to prevent tooth erosion even with the topical application of fluorides. Loretto et al.[12] evaluated the microhardness, surface roughness, mass variation, and ultrastructure of bovine enamel exposed to tucupi, which was able to reduce microhardness, increasing surface roughness and causing loss of enamel mass.
The scientific literature has studies that assess the influence of erosive challenges with citric acid, hydrochloric acid, isotonic drinks, cola-based soft drinks, orange juice, on the adhesive bond strength to enamel and dentin,[4,5,9,13] but does not contain information about the influence of regional components of an acidic diet on this process. Therefore, the present study aimed to evaluate the erosive influence of tucupi on the bond strength of enamel and dentin.
Materials and Methods
This study was analyzed and approved by the Ethics Committee on Animal Use of the Federal University of Para (CEUA/UFPA) in the meeting of December 22, 2020 under the n°1358240920). One hundred and sixty-eight healthy bovine incisor teeth of the species Bos taurus indicus (with an average age of 24 months) were obtained from animals slaughtered at the Cooperative of the Agricultural Industry of Pará (SOCIPE-Belém, Pará, Brazil). Bovine teeth that had erupted in the oral cavity, with an intact crown and complete root formation, were included in the research. The sample was initially immersed for 1 week in a 0.1% thymol solution for disinfection. Afterward, the teeth were analyzed with a stereoscopic magnifying glass (×40) to evaluate the vestibular enamel of the middle coronal portion, and specimens with cracks on the enamel surface were discarded. Then, they were stored in distilled water (4°C).
The sample size was calculated based on the results of a pilot study and taking as reasonable a bond strength of 20 MPa ± 20%. G Power software was used to analyze the data and estimate a sample of 14 cylinders per experimental group.
Characterization of tucupi acidity and pH determination
All evaluations were made in triplicate were carried out over 5 days, performed on a 2-day interval, as described below.
The values were determined by direct reading in a pH meter (Tecnal, TEC-51, China) properly calibrated with buffer solutions of pH 7.0 and 4.0, at 20°C, according to the method number 981.12 of the Association of Official Agricultural Chemists (AOAC).[14] For the evaluations, 20 mL of tucupi were used at room temperature, inserted in an Erlenmeyer flask, followed by the pH’s note in each analysis.
Total titratable acidity determination
The total titratable acidity of the sample was determined using a 0.1 M NaOH solution, according to method number 942.15 of the AOAC[14] with the result expressed in g/100 mL.
Initially, 50 mL of tucupi were used, being submitted to a boiling process. Later, having tucupi at room temperature, the process was carried out in triplicate, with 5 mL of tucupi being pipetted, diluted with 50 mL of distilled water in Erlenmeyer flasks, followed by the addition of 3 drops of phenolphthalein in each flask. After this step, a burette was loaded with a 0.1 M NaOH solution to start the titration of the samples, until reaching a light pink color (for at least 30 s), with the annotation of the spent volume of NaOH being made.
After the above procedures, the data were tabulated and applied to the formula:
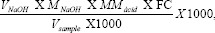 thus, obtaining the results in g/100 mL, of each made titration.
thus, obtaining the results in g/100 mL, of each made titration.
Obtaining the Tooth fragments and preparation of specimens
The dental crowns went through two cross sections with a double-faced diamond disc with constant refrigeration (DIN 862; Mitutoyo, São Paulo, SP, Brazil), thus obtaining samples of the middle portion of the dental crown 10 mm in height.
All dental fragments had their buccal surface inserted in a n°7 wax and were embedded in chemically activated acrylic resin (JET Classico, Campo Limpo Paulista, SP, Brazil), using 1 cm high PVC matrices. After 24 h, the specimen surface was flattened using aluminum oxide sandpaper with #180, #400, and #600 grains under refrigeration (Buehler Ltd., Lake Buff, IL, USA – FEPA Standard). The 84 specimens destined for dentin evaluations were first flattened with sandpaper #180 until exposing superficial dentin (5 mm in diameter), followed by sandpapers #400 and #600. Eighty-four enamel specimens received a smooth planning/polishing with #400 and 600 sandpapers. After using a sandpaper, specimens were washed in an ultrasonic bath (TD30 Plus, Bio-Art, São Carlos, São Paulo, Brazil) with distilled water for 20 min.[15]
Group division
A total of 168 specimens were randomly divided into 12 groups (n = 14), being that the groups destined to enamel were G1, G3, G5, G7, G9, G11 and to dentin were G2, G4, G6, G8, G10, G12. The groups were divided for the treatments according to Table 1.
Table 1.
Description of experimental groups
| Enamel | Dentin |
|---|---|
| G1: Distilled water cycling+etch and rinse (conventional) adhesive strategy | G2: Distilled water cycling+etch and rinse (conventional) adhesive strategy |
| G3: Cola-based soft drink cycling+etch and rinse (convencional) adhesive strategy | G4: Cola-based soft drink cycling+etch and rinse (convencional) adhesive strategy |
| G5: Tucupi cycling+etch and rinse (conventional) adhesive strategy | G6: Tucupi cycling+etch and rinse (conventional) adhesive strategy |
| G7: Distilled water cycling+self-etching adhesive strategy | G8: Distilled water cycling+self-etching adhesive strategy |
| G9: Cola-based soft drink cycling+self-etching adhesive strategy | G10: Cola-based soft drink cycling+self-etching adhesive strategy |
| G11: Tucupi cycling+self-etching adhesive strategy | G12: Tucupi cycling+self-etching adhesive strategy |
Erosive challenges
The pH of the solutions was obtained using a pHmeter with glass electrode and digital display (K39-1014B, Kavsi, São José dos Pinhais, PR, Brazil), before immersing the specimens. The specimens were immersed in 50 mL of distilled water (negative control), cola-based soft drink (positive control), or tucupi in sterile Becker-type flasks. The immersion cycles took place under slight agitation, with the aid of a magnetic stirrer (Quimis, Diadema, SP, Brazil), for 20 min daily,[16] during 5 days. After each immersion cycle, the specimens were washed with distilled water for 1 min at approximately a 5 cm distance, dried with absorbent paper, immersed in 50 mL artificial saliva, and kept in a biological oven at 37°C for 24 h, until the next immersion cycle. Artificial saliva was daily renewed in all groups. Solutions were used at room temperature and were discarded after each cycle.
Composite resin cylinders build-up
Twenty-four hours after the 5th day of erosive cycling, composite resin cylinders were built up on the vestibular surface of the specimens. The bonding area was delimited using a double-sided adhesive tape (Tectape®, Manaus, AM, Brazil), perforated in a circular shape (0.8 mm in diameter). After fixing the tape, the adhesive strategies were carried out as follows:
Adhesive strategy 1: Etch and rinse (conventional) adhesive strategy
Etching was performed using 37% phosphoric acid gel (Condac 37%, FGM Produtos Odontológicos, Joinville, SC, Brazil) on the enamel or dentin surface for 15 s, followed by washing for 20 s. After drying the surface with an air jet for 5 s and 2 absorbent paper discs,[9] Adper™ Single Bond 2 adhesive system (3M ESPE, St. Paul, Minnesota, USA) was actively applied (rubbing it) for 20 s, followed by a light blast of air for 5 s, and then it was light-activated for 10 s with a Radii-Cal LED device (SDI Limited, Victoria, Australia) (1200 mW/cm2). The light intensity was measured, with a radiometer, every 10 specimens.
Adhesive strategy 2: Self-etching adhesive strategy
An active application of the acidic primer of the Clearfil SE Bond adhesive (Kuraray Medical, Osaka, Japan) (rubbing it) on the enamel or dentin surfaces was performed for 20 s, followed by the active application of the bond solution for 10 s, which was photoactivated for 10 s with Radii-Cal LED device (SDI Limited, Victoria, Australia) (1200 mW/cm2). As performed before, light intensity was measured, with a radiometer, every 10 specimens.
After specimens’ hybridization, the first layer of adhesive tape was removed, and composite resin cylinders (Filtek Z350XT, 3M ESPE, St. Paul, Minnesota, USA) (A2B) were built up with the aid of a special catheter tube (Tygon®) (0.8 mm in internal diameter and 0.5 mm in height). Two composite resin cylinders were built up on the surface of each specimen. Light-curing was performed for 20 s with a Radii-Cal LED device (SDI Limited, Victoria, Australia) (1200 mW/cm2).
All products or solutions used are described in Table 2.
Table 2.
Description of the materials used in the experiment, containing their trade names, manufacturers and composition (according to the respective manufacturers)
| Name | Manufacturer | Composition |
|---|---|---|
| Adper™ single bond 2 | 3M ESPE, St. Paul, MN, USA | Water, ethanol, HEMA, Bis-GMA, other dimethacrilate resins and copolymers of polycarboxylic acids and modified dimethacrylate and photoinitiator system |
| Clearfil SE bond | Kuraray Medical, Osaka, Japão | Primer: HEMA, hydrophilic dimethacrylate, 10-MDP, N, Ndietanolptoluidina, CQ, water Adhesive: Silanized silica, Bis-GMA, HEMA, hydrophilic dimethacrylate, 10-MDP, CQ, toluidine |
| Distilled water | Asfer Indústria Química Ltda, São Caetano do Sul, SP, Brazil | Demineralized water |
| Coca-Cola® | The Coca-Cola Company, Rio de Janeiro, RJ, Brazil | Carbonated water, sugar, cola nut extract, caffeine, IV caramel dye, phosphoric acid acidulant, natural aroma |
| Condac 37% | FGM Produtos Odontológicos, Joinville, SC, Brazil | 37% phosphoric acid, thickener, dye and deionized water |
| Filtek Z350XT | 3M ESPE, St. Paul, MN, USA | TEGDMA, PEGDMA and Bis-EMA, treated silanized ceramics, silica treated silane |
| Artificial Saliva | A Fórmula - Compounding Pharmacy, Belém, PA, Brazil | Baking soda 2190 mg, potassium phosphate 1270 mg, magnesium chloride 125 mg, calcium chloride 441 mg, potassium chloride 820 mg, sodium fluoride 4.5 mg, nipazol 100 mg, sorbitol 24 mg, carboxymethylcellulose 8 mg, distilled water 3000 mL |
| Vovó da Floresta Tucupi | Agroindústria São Francisco do Itá Ltda. Santa Isabel do Pará, PA, Brazil | Yellow cassava sap, water, garlic vine, chicory, salt and alfavaca |
HEMA: Hydroxyethyl-methacrylate; MDP: Methacryloyloxydecyl dihydrogen phosphate; GMA: Glycidyl Methacrylate; PEGDMA: Poly ethylene glycol dimethacrylate; TEGDMA: Triethylene glycol dimethacrylate; EMA: Ethylene methyl acrylate; CQ: Camphorquinone
Microshear bond strength test
24 hours after manufacturing the composite resin cylinders, the specimens were individually fixed in the universal testing machine (Kratos KE®, Cotia, SP, Brazil). A 0.2 mm diameter metallic wire (Morelli®, Sorocaba, SP, Brazil) was used to tie the load cell extension and the composite resin cylinder. The microshear mechanical test was carried out with a crosshead speed of 0.5 mm/min, and the results were obtained in MPa.
Classification of failure mode
After the mechanical test, specimens were analyzed using a stereoscopic magnifying glass (×40), to classify failures that occurred on enamel and dentin surfaces, being: type I (adhesive), Type II (mixed), Type III (cohesive in composite resin), and Type IV (cohesive in enamel or dentin). The number of failures for each type was determined in percentages.
Statistical analysis
The results obtained in MPa presented normal distribution (Shapiro–Wilk test) and were evaluated by two-way ANOVA and Tukey’s post test (P ≤ 0.05). Data analysis was performed using the BioEstat 5.3 statistical software (Instituto Mamirauá, Tefé, AM, Brazil).
Results
Table 3 can be observed stability of pH values and total titratable acidity of tucupi over the 5 days, where the average pH was 4.4 and the total titratable acidity was 0.084 (g/100 mL).
Table 3.
Tucupi mean pH values and total titratable acidity over 5 days of evaluation
| Evaluation | 1° day | 3° day | 5° day |
|---|---|---|---|
| pH | 4.4 | 4.4 | 4.4 |
| Total titratable acidity (g/100 mL) | 0.083 | 0.085 | 0.084 |
The enamel and dentin groups exposed to distilled water had the highest bond strength values, followed by the groups exposed to tucupi. The groups exposed to the cola-based soft drink obtained the lowest bond strength values. The results in Table 4 showed that there were no statistical differences for the enamel groups studied, regardless of the solution and adhesive strategy used (P > 0.05). However, in dentin, there were significant differences only in the groups exposed to cola-based soft drink (P < 0.01).
Table 4.
Means±standard deviation of the values of union resistance (MPa) to enamel and dentin submitted to 5 days of exposure to the tested solutions using 2 adhesive strategies
| Distilled water | Cola-based soft drink | Tucupi | |
|---|---|---|---|
| Enamel | |||
| Adper Single Bond 2 | 13.12±2.28a | 11.06±3.06a | 12.76±3.24a |
| Clearfil SE bond | 12.48±1.52a | 10.55±1.49a | 11.76±4.25a |
| Dentin | |||
| Adper Single Bond 2 | 12.62±1.80a | 7.20±1.25b | 11.25±2.56a |
| Clearfil SE bond | 13.52±2.87a | 8.96±1.60b | 12.26±3.40a |
ANOVA two-way test (P≤0.05). Different letters indicate statistical difference on the same line
Considering the failure mode, Type II (mixed) (95%) was predominant.
Discussion
The process of erosive tooth wear, associated with the consumption of acidic foods and beverages, has been increasingly prevalent in the world population.[2,3,16] Although the literature present studies that explore the bond strength of restorative materials to previously eroded surfaces in enamel and dentin with acid drinks.[4,9,13] This study was the first one to provide experimental evidence of the exposure of regional diet’s components, such as tucupi, in the bond strength to enamel and dentin. In this sense, our results suggest that the Amazonian delicacy (tucupi) did not interfere with the bond strength to enamel and dentin (P > 0.05), regardless of the adhesive strategy used.
Analyzes of pH and total titratable acidity of tucupi were carried out to characterize the acidity of this regional delicacy. The pH of a beverage influences its erosive potential while being consumed. After ingestion, the total titratable acidity becomes responsible for the time that the salivary pH is kept at a low level in the oral cavity, because the greater the amount of base needed to reach a neutral pH, the greater the amount of saliva (alkaline) necessary for acid neutralization.[17]
The values (means) of total titratable acidity and pH were respectively 0.084 g/100 mL and 4.4. Loretto et al.[12] obtained pH values and total titratable acidity, respectively, of 4.3 and 0.090 g/mL, that are very close to those found in the present study. Furthermore, the tucupi used was a widely marketed brand, due to the ease of acquisition, standardization of samples, and for being registered by Agricultural Defense Agency of the State of Pará (ADEPARÁ). In addition, the choice for tucupi is justified by the fact that it is a popularly consumed food in Brazil, with a characteristically low pH (ranging from 3.0 to 4.3)[10] and because its erosive influence is still little explored in the scientific literature.[11]
The cola-based soft drink (Coca-Cola®) was used as a positive control, due to its high erosive potential (pH ≈ 2.5) and total titratable acidity (around 0.57 g/mL),[17,18] with phosphoric acid being the main acidic compound present in its composition.[9,19] Likewise, distilled water was used as a negative control for presenting a neutral, or close to neutral, acidic behavior (pH ranging from 5.7 to 7.0).[18]
Erosive cycling protocols vary in the literature.[4,9,13,16] To simulate dental erosion, erosive cycles were performed with distilled water, cola-based soft drink, and tucupi, under light agitation, for 20 min a day, for 5 days.[16] Thus, this study worked with an erosive cycling time (5 days) based on those previously established for phosphoric and citric acid.
According to the results of the present study, no difference was found between groups submitted to erosive cycling with tucupi and distilled water on enamel, as well as dentin bond strength, regardless of the adhesive strategy used. Considering enamel, this absence of differences can be attributed to the greater mineral content of the substrate, which did not allow this tissue to be severely compromised by these 2 solutions of less acidic pH (distilled water and tucupi). Even the cola-based soft drink, which has a lower pH and higher total titratable acidity, did not show significant differences on enamel bond strength, which suggests again that the high mineral content of this substrate responds to these findings.
In addition, the thickness of the “softened” enamel layer caused by an erosive challenge can be between 0.2 and 3 μm.[19] Thus, Wang et al.[13] and Giacomini et al.[9] evaluated the influence of erosive/abrasive challenges on the bond strength to enamel using in situ/in vivo protocol with cola-based soft drink and orange juice, respectively, for 5 days, and found that a possible interference from the previous erosive challenge would be minimized by the use of phosphoric acid, as the depth of demineralization by the acid will overcome the “softened” enamel layer by the erosive process. For this reason, no erosion effect on adhesion would be expected for conventional adhesives, which agrees with our results when using Adper Single Bond 2 on enamel bonding. Nonetheless, it is reasonable to admit that the effect of eroded enamel on adhesion is not well understood in the literature.[13,20]
In dentin, considering distilled water and tucupi again, no statistical differences were also observed in groups restored with a conventional adhesive system, and it can be attributed to the infiltration capacity of this material into the collagen fiber network. In that regard, the aggressiveness of the erosive challenge with these solutions possibly may not have resulted in a collagen network thick enough to prevent monomers penetration.[21,22]
Our study shows that dentin groups restored with conventional adhesive system had, numerically, lower bond strength values, with statistical difference only for group exposed to cola-based soft drink (P < 0.01). In the latter (cola-based soft drink), a high degree of demineralization in eroded dentin tends to form a deeper demineralized layer, which after penetration of the adhesive system, allows the formation of a thicker hybrid layer than in healthy (noneroded) dentin. This layer contains structural imperfections and porosities that, consequently, cause areas of hydrophilic predominance and demineralized zones. As such, this may contribute to lower bond strength values for the groups previously eroded with cola-based soft drink, because resin monomers may not penetrate as deeply. Furthermore, greater collagen exposure tends to form areas that are more prone to degradation over time, limiting its clinical longevity. Besides, in severe erosive cases, dentinal exposure can lead to a common sclerotic dentin substrate with a hypermineralized shiny surface layer performed by tubular occlusion.[3,4,5,21,23]
Distilled water and tucupi did not interfere with enamel and dentin bond strenght when using the self-etching adhesive. These solutions (distilled water and tucupi) have a not-so-acidic pH, and it may lead to a non or little morphological change on the structure of dental substrates. This fact, combined with the possible advantages of a 10-Methacryloyloxydecyl dihydrogen phosphate (10-MDP) self-etching adhesive, probably can explain these results. In fact, 10-MDP monomer is known for promoting a stable chemical interaction with hydroxyapatite, which is more resistant than the other functional monomers found in bonding agents,[24,25] also related to the formation of calcium monomer salts. According to Yoshihara et al.[26] the greater bonding effectiveness of 10-MDP-based adhesives must be attributed to their greater conditioning potential to provide surface microretention with a stable chemical interaction.
Amazonian products have gained prominence over the years and are products that are now being consumed on a large scale.[11] Therefore, more studies must be carried out to shed light on the impact of these products on oral health as they are mostly acidic in origin.
Conclusion
Within the limitations of this in vitro study, it is concluded that the erosive cycling with tucupi did not influence the bond strength to enamel and dentin, regardless of the adhesive strategy used.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledment
The Faculty of Food Engineering, The Federal University of Pará and the University Center of the State of Pará for using their respective laboratories to carry out this research.
References
- 1.Ganss C, Lussi A, Schlueter N. The histological features and physical properties of eroded dental hard tissues. Monogr Oral Sci. 2014;25:99–107. doi: 10.1159/000359939. [DOI] [PubMed] [Google Scholar]
- 2.Saads Carvalho T, Lussi A. Chapter 9: Acidic beverages and foods associated with dental erosion and erosive tooth wear. Monogr Oral Sci. 2020;28:91–8. doi: 10.1159/000455376. [DOI] [PubMed] [Google Scholar]
- 3.Lussi A, Schlueter N, Rakhmatullina E, Ganss C. Dental erosion –An overview with emphasis on chemical and histopathological aspects. Caries Res. 2011;45(Suppl 1):2–12. doi: 10.1159/000325915. [DOI] [PubMed] [Google Scholar]
- 4.Cruz JB, Lenzi TL, Tedesco TK, Guglielmi Cde A, Raggio DP. Eroded dentin does not jeopardize the bond strength of adhesive restorative materials. Braz Oral Res. 2012;26:306–12. doi: 10.1590/s1806-83242012005000009. [DOI] [PubMed] [Google Scholar]
- 5.Cruz JB, Bonini G, Lenzi TL, Imparato JC, Raggio DP. Bonding stability of adhesive systems to eroded dentin. Braz Oral Res. 2015;29:S1806–83242015000100284. doi: 10.1590/1807-3107BOR-2015.vol29.0088. [DOI] [PubMed] [Google Scholar]
- 6.Yabuki C, Rikuta A, Murayama R, Akiba S, Suzuki S, Takamizawa T, et al. Effect of acid erosion on enamel bond strength of self-etch adhesives and sonic velocity measurement of enamel. Dent Mater J. 2018;37:542–8. doi: 10.4012/dmj.2017-117. [DOI] [PubMed] [Google Scholar]
- 7.Yoshihara K, Yoshida Y, Hayakawa S, Nagaoka N, Irie M, Ogawa T, et al. Nanolayering of phosphoric acid ester monomer on enamel and dentin. Acta Biomater. 2011;7:3187–95. doi: 10.1016/j.actbio.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Zanatta RF, Lungova M, Borges AB, Torres C, Sydow HG, Wiegand A. Microleakage and shear bond strength of composite restorations under cycling conditions. Oper Dent. 2017;42:E71–80. doi: 10.2341/16-132-L. [DOI] [PubMed] [Google Scholar]
- 9.Giacomini MC, Casas-Apayco LC, Machado CM, Freitas MC, Atta MT, Wang L. Influence of erosive and abrasive cycling on bonding of different adhesive systems to enamel: An in situ study. Braz Dent J. 2016;27:548–55. doi: 10.1590/0103-6440201600940. [DOI] [PubMed] [Google Scholar]
- 10.Chisté RC, Cohen KO, Oliveira SS. Study of tucupi physicochemical properties. Ciênc Tecnol Aliment. 2007;27:437–40. [Google Scholar]
- 11.Martins LM, Francisconi-dos-Rios LF, Meira Gde F, Bertocco VP, Silva LM, Rebelo MA. Amazonian delicacy tucupi is as erosive as a cola-based soft drink. Arch Oral Biol. 2016;61:84–8. doi: 10.1016/j.archoralbio.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 12.Loretto SC, Sousa NW, Ribeiro ME, Carneiro RV, Chisté R, Souza Júnior MH. Influence of tucupi on enamel surface roughness, microhardness, ultramorphology and mass variation. Clin Cosmet Investig Dent. 2023;15:63–70. doi: 10.2147/CCIDE.S394661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Casas-Apayco LC, Hipólito AC, Dreibi VM, Giacomini MC, Bim Júnior O, et al. Effect of simulated intraoral erosion and/or abrasion effects on etch-and-rinse bonding to enamel. Am J Dent. 2014;27:29–34. [PubMed] [Google Scholar]
- 14.Association of Official Anatytical Chemists. Official Methods of Analysis of AOAC International. 3rd rev. Gaitherburg: Association of Official Anatytical Chemists; 1997. [Google Scholar]
- 15.Baia JC, Oliveira RP, Ribeiro ME, Lima RR, Loretto SC, Silva E Souza Junior MH. Influence of prolonged dental bleaching on the adhesive bond strength to enamel surfaces. Int J Dent. 2020;2020:2609359. doi: 10.1155/2020/2609359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rios D, Honório HM, Magalhães AC, Silva SM, Delbem AC, Machado MA, et al. Scanning electron microscopic study of the in situ effect of salivary stimulation on erosion and abrasion in human and bovine enamel. Braz Oral Res. 2008;22:132–8. doi: 10.1590/s1806-83242008000200007. [DOI] [PubMed] [Google Scholar]
- 17.Tenuta LM, Fernández CE, Brandão AC, Cury JA. Titratable acidity of beverages influences salivary pH recovery. Braz Oral Res. 2015;29:S1806–83242015000100234. doi: 10.1590/1807-3107BOR-2015.vol29.0032. [DOI] [PubMed] [Google Scholar]
- 18.Furtado JR, Freire VC, Messias DC, Turssi CP. Physicochemical aspects related to the erosive potential of acid beverages. RFO. 2010;15:323–8. [Google Scholar]
- 19.Peumans M, De Munck J, Mine A, Van Meerbeek B. Clinical effectiveness of contemporary adhesives for the restoration of non-carious cervical lesions. A systematic review. Dent Mater. 2014;30:1089–103. doi: 10.1016/j.dental.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Lenzi T, Hesse D, Guglielmi C, Anacleto K, Raggio DP. Shear bond strength of two adhesive materials to eroded enamel. J Contemp Dent Pract. 2013;14:700–3. doi: 10.5005/jp-journals-10024-1387. [DOI] [PubMed] [Google Scholar]
- 21.Wiegand A, Lechte C, Kanzow P. Adhesion to eroded enamel and dentin: Systematic review and meta-analysis. Dent Mater. 2021;37:1845–53. doi: 10.1016/j.dental.2021.09.014. [DOI] [PubMed] [Google Scholar]
- 22.Perdigão J, Reis A, Loguercio AD. Dentin adhesion and MMPs: A comprehensive review. J Esthet Restor Dent. 2013;25:219–41. doi: 10.1111/jerd.12016. [DOI] [PubMed] [Google Scholar]
- 23.Attin T, Wegehaupt FJ. Impact of erosive conditions on tooth-colored restorative materials. Dent Mater. 2014;30:43–9. doi: 10.1016/j.dental.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Yoshihara K, Yoshida Y, Nagaoka N, Fukegawa D, Hayakawa S, Mine A, et al. Nano-controlled molecular interaction at adhesive interfaces for hard tissue reconstruction. Acta Biomater. 2010;6:3573–82. doi: 10.1016/j.actbio.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 25.Yoshihara K, Yoshida Y, Nagaoka N, Hayakawa S, Okihara T, De Munck J, et al. Adhesive interfacial interaction affected by different carbon-chain monomers. Dent Mater. 2013;29:888–97. doi: 10.1016/j.dental.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Yoshihara K, Hayakawa S, Nagaoka N, Okihara T, Yoshida Y, Van Meerbeek B. Etching efficacy of self-etching functional monomers. J Dent Res. 2018;97:1010–6. doi: 10.1177/0022034518763606. [DOI] [PubMed] [Google Scholar]


