Abstract
Epilepsy and migraine without aura (MWoA) are often comorbid, but the exact mechanisms are unclear. Magnetic resonance spectroscopy (1H-MRS) may help to understand the neurometabolic mechanisms in patients with epilepsy comorbid with MWoA (EWM). In this prospective cross-sectional study, we recruited 64 female patients, including 24 with EWM, 20 with epilepsy, and 20 with MWoA, as well as 20 age-level-matched and educational-level-matched female healthy controls from our hospital between August 2021 and November 2022. A single-voxel point-resolved spectroscopy sequence was used to acquire spectra of the bilateral dorsolateral prefrontal cortices (DLPFCs). Metabolites were quantified by linear combination model software, and the values were corrected for the partial volume effect of cerebrospinal fluid. MRS data comparisons were performed with multivariate analyses of variance. Correlation analyses were calculated between metabolites and main clinical data. The results showed that N-acetyl aspartate (NAA) was asymmetrical between the bilateral DLPFCs. Both NAA and myoinositol were significantly reduced in EWM than in healthy controls. Choline-containing compounds (Cho) were higher in MWoA than in the other three groups. Correlation analyses revealed that NAA of the right DLPFC and Cho of the bilateral DLPFCs in EWM were negatively related to migraine frequency. In addition, glutamate and glutamine (Glu and Gln, Glx) of the right DLPFC in EWM were negatively correlated with migraine severity. Our findings suggested that comorbid epilepsy and MWoA in female patients can lead to a synergistic reduction of both NAA and myoinositol, reflecting more serious injuries of neurons and glial cells.
Keywords: comorbidity, dorsolateral prefrontal lobe, epilepsy, migraine without aura, proton magnetic resonance spectroscopy
Introduction
Epilepsy is a condition characterized by sudden, short-lived, and repetitive brain malfunctions due to abnormal discharge of brain neuron activity [1]. It can last for years or decades, and prolonged seizures are often accompanied by many comorbidities [1,2]. Migraine, one of the most common comorbidities, is a chronic neurovascular disorder characterized by unilateral or bilateral episodic headaches [3,4]. Although epilepsy and migraine are two heterogeneous diseases, they are both recurrent, transient, and paroxysmal chronic neurological disorders that often occur simultaneously or successively in the same patient and have certain correlations in epidemiology, clinical characteristics, pathogenetic mechanisms, and treatments [3,5–7]. Patients with epilepsy comorbid with migraine were more likely to have symptoms such as depression and anxiety than those without migraine [8], which further increases the disease burden. Migraine includes two main clinical subtypes: migraine with aura (MWA) and migraine without aura (MWoA) [9]. Of these, MWoA is more common [9], and when comorbid with epilepsy, it is easily misdiagnosed because of the absence of aura symptoms. However, the specific mechanisms of epilepsy comorbid with MWoA (EWM) remain unclear. Therefore, it is necessary to explore the mechanisms of EWM.
To date, with the help of neuroimaging methods, a few structural and functional abnormalities in epilepsy comorbid with migraine have been reported [10,11]. For example, structural impairments in the white matter tracts of the brain stem, uncinate fasciculus, and fornix may contribute to migraine attacks following epileptic seizures [10]. A functional study revealed that dysfunction of the periaqueductal gray is closely associated with epilepsy comorbid with migraine [11]. However, migraine types in these two studies were mixed, and there have been no studies of brain metabolites in vivo in patients with both epilepsy and MWoA. Magnetic resonance spectroscopy (1H-MRS) is a noninvasive technique that can quantify metabolites in specific brain regions in vivo and detect subtle neurodegenerative changes that are not visible in regular MRI scans [12]. The application of 1H-MRS may help us to better understand the neurobiochemical mechanisms of EWM. The dorsolateral prefrontal cortex (DLPFC) plays an important role in cognition, affection, and other functions [13]. It is involved in cognitive and affective processes in response to painful stimuli and provides active control of pain perception through top-down modulation [14]. In addition, seizures that originate from epileptic foci may spread and affect the DLPFC [15–17]. Several studies have revealed abnormal changes in the DLPFC in patients with epilepsy, such as metabolite changes [16,17] or abnormal functional connectivity [15]. There is nearly no sex predominance in patients with epilepsy, whereas female patients make up more than 75% of migraineurs [18]. Thus, to eliminate sex predominance in migraineurs, we mainly focused on female patients in this study.
Therefore, we hypothesized that female patients with epilepsy and with MWoA may cause metabolic alterations in the bilateral DLPFCs, and when they are comorbid, there may be an interaction effect in the metabolite changes. In this study, our goal was to investigate potential metabolic alterations in the bilateral DLPFCs of female epilepsy patients with MWoA (referred to as EWM) by using 1H-MRS. We expected that this study would offer additional insights into the underlying mechanisms of the comorbidity between epilepsy and MWoA from the perspective of neurobiochemistry.
Materials and methods
Participants
From August 2021 to November 2022, a total of 64 patients, including 20 with MWoA, 20 with epilepsy, and 24 patients with epilepsy and MWoA (EWM) were consecutively included in the 3.0 T 1H-MRS research conducted by expert neurologists at the Epilepsy Center of West China Hospital. Moreover, 20 age-level-matched and educational-level-matched healthy controls were also enrolled with the same protocol. The diagnosis of epilepsy relied mainly on the symptoms of epilepsy with or without EEG findings as per the International League Against Epilepsy Classification and Terminology (ILAE 2017) [19]. The diagnosis of MWoA was based on the criteria of the International Classification of Headache Disorders (ICHD 3) [9]. The diagnosis of EWM was based on a combination of ILAE 2017 and ICHD 3, and symptoms of migraine in EWM appeared after the diagnosis of epilepsy. Subjects were excluded if they had positive brain MRI results, were under 18 or over 60 years old, had a history of other organic or neuropsychiatric diseases, had other types of headaches or had MRI contraindications like inability to cooperate or claustrophobia. Migraine types in EWM and MWoA are all MWoA. All participants were female and right-handed. All patients were scanned in the interictal period of diseases and stopped taking any medication for 72 h before the scan.
We also collected demographic and clinical data, including the age and education level of all subjects. The disease duration, attack frequency, and other characteristics of all patients were collected. Patients with EWM and MWoA also completed the Visual Analog Scale (VAS, with zero being no pain and 10 being the most intense pain imaginable) and the Headache Impact Test (HIT-90) to evaluate the severity and impact of migraine, respectively. The research was authorized by the Biomedical Research Ethics Committee of Huaxi Hospital and adhered to the guidelines of the Declaration of Helsinki (2013 edition). Each participant understood the experiment and signed the written consent. Before the scan, all participants provided written informed consent to participate in this study.
Image acquisition
All subjects underwent MRS scans using the Siemens Tim Trio 3.0 T scanner from Siemens Healthcare in Erlangen, Germany, which was outfitted with eight-channel phased array head coils. During scanning, we eliminated noise by using muted earplugs and reduced head motion by using sponge cushions. We used a magnetization-prepared rapid gradient echo in the first high-resolution T1-weighted sagittal imaging sequence, with the following settings: repetition time/echo time = 2250/2.6 ms, flip angle = 9°, slice thickness=1 mm, field of view = 256 × 256 mm2, matrix size = 256 × 256, and a total of 192 continuous sagittal slices. In the process of MRS localization, we generated three-dimensional perspectives of the volumes of interest (VOIs) by reconstructing axial and coronal images.
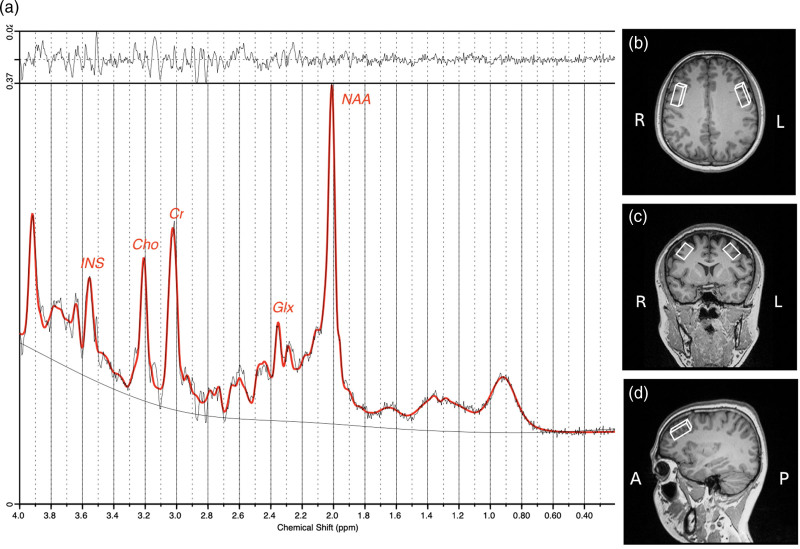
The single-voxel proton MRS acquisition was performed using a point-resolved spectroscopy sequence (PRESS), with a repetition time/echo time of 2000/30 ms, a bandwidth of 1200 Hz, and 128 averages. Yue Q, an expert radiologist, positioned VOIs in the regions of the bilateral DLPFCs containing the most gray matter and the least white matter. Each VOI had a fixed size of 40 × 15 × 20 mm3 (anterior-posterior × right-left × head-foot), and the locations of the VOI is shown in Fig. 1. The presaturation bands are placed around VOIs to prevent outside signals from interfering with the VOIs. There were two types of spectral data acquired, one with and one without water suppression. Before the implementation of the PRESS module, water suppression was achieved using three-chemical shift-selective pulses. The internal reference used to quantify absolute metabolites and correct for eddy currents was the unsuppressed tissue water signal. By implementing fastmap shimming, we were able to reduce the line width and improve the signal-to-noise ratio (SNR). The overall duration of the scan was around 16 min. All subjects were MRI negative. For any uncertainties, more sequences (such as T2 and T2_FLAIR) were performed to confirm that all participants had normal MRI findings.
Fig. 1.
(a) Metabolite concentration (Ins, myoinositol; Cho, choline-containing compounds; Cr, creatine and phosphocreatine; Glx, glutamate and glutamine; NAA, N-acetyl aspartate) estimated by LCmodel. (b), (c) and (d), respectively, correspond to the axial, coronal, and sagittal views of reference images, and white boxes represent the locations of the volumes of interest (VOIs) in the bilateral dorsolateral prefrontal cortices (DLPFCs).
Magnetic resonance spectroscopy data processing
The raw MRS spectral data was analyzed with a linear combination model (LCModel, Version 6.3-1H, Provencher SW, http://s-provencher.com/lcmodel.shtml), which enables the automatic determination of metabolite concentrations in living organisms. Metabolites typically consist of N-acetyl aspartate (NAA), choline-containing compounds (Cho), creatine and phosphocreatine (Cr), myoinositol, and glutamate and glutamine (Glu and Gln, Glx). The unit of final metabolite concentrations was mmol/kg wet weight. Statistical analysis was conducted on only the data that met the following criteria: SNR ≥ 10, full width at half maximum ≤ 0.08 ppm, and Cramer–Rao lower bounds ≤ 15%.
To eliminate the partial volume effect of cerebrospinal fluid (CSF) in the VOIs, the T1-weighted images of each subject were automatically segmented into gray matter, white matter, and CSF using Advanced Normalization Tools (ANTs, http://www.picsl.upenn.edu/ANTS/). Finally, we calculated the proportion of CSF in the VOIs by covering each voxel in the segmented T1-weighted images according to its location. The formula was utilized to calculate ultimate metabolite levels adjusted for CSF volume [17], with Craw representing the initial estimate of the metabolite concentration, while Vtotal and VCSF represent the overall volume and the volume of CSF in the whole voxel, respectively.
Statistical analysis
SPSS 26 was utilized for all statistical analyses. All histograms and graphs were generated with GraphPad Prism 7.0 (GraphPad Software, La Jolla, California, USA). The normal distribution of the data was assessed with the Shapiro–Wilk test. In terms of clinical data comparisons, differences in age and education level among the four groups were assessed by one-way analysis of variance. The disease duration and frequency between EWM and epilepsy or MWoA were compared by independent sample t-test. The VAS and HIT-90 scores between EWM and MWoA were assessed by independent sample t-test. The rest were tested by a chi-square test. For MRS data comparisons, we performed a multivariate analysis of variance (MANOVA) for four groups (MWoA, epilepsy, EWM, healthy controls), two locations (left and right DLPFCs), and five metabolites (myoinositol, NAA, Cho, Cr, Glx). Multiple comparisons were calculated using Bonferroni correction. Finally, Spearman or Pearson correlations were performed between metabolites and main clinical data of epilepsy, EWM, and MWoA, including the disease attack frequency, disease duration, VAS score, and HIT-90 score. The magnitude of the correlation was determined by the absolute value of r, with ranges indicating the strength of the correlation: 0.00–0.10 for negligible correlation, 0.10–0.39 for weak correlation, 0.40–0.69 for moderate correlation, 0.70–0.89 for strong correlation, 0.90–1.00 for very strong correlation) [20]. A significance level of less than 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
Of the 84 participants, one healthy control was excluded because of imperfect data, and eight participants’ spectra (including five EWMs, one epilepsy, one MWoA, and one healthy control) were excluded due to poor spectral qualities. Finally, 19 patients with EWM (age 18–57 years, mean 32.26 years), 19 patients with epilepsy (age 18–51 years, mean 28.74 years), 19 patients with MWoA (age 22–48 years, mean 30.00 years), and 18 healthy controls (age 24–60 years, mean 28.94 years) were included in the further statistical analyses. The detailed demographic and clinical characteristics are summarized in Table 1. The four groups did not differ in terms of age (P = 0.649) or education level (P = 0.098). The use of analgetic drugs in MWoA was significantly higher than that in EWM (P = 0.022). EWM and MWoA did not differ in the disease duration, frequency, VAS, or HIT-90 scores of migraine (all P > 0.05). EWM and epilepsy also had no differences in the disease duration, frequency, or antiepileptic drugs (AEDs) use of epilepsy (all P > 0.05).
Table 1.
Clinical and demographic data
| Parameters | HCs | EP | EWM | MWoA | t/F/x2 | P |
|---|---|---|---|---|---|---|
| Age (years) | 28.94 ± 10.94 | 28.74 ± 7.55 | 32.26 ± 11.53 | 30.00 ± 7.01 | 0.552 | 0.649 |
| Education level (years) | 16.00 ± 3.66 | 12.88 ± 3.96 | 12.95 ± 3.70 | 15.95 ± 3.12 | 8.480 | 0.098 |
| Epilepsy aspects | ||||||
| Seizure type (N) | ||||||
| Focal onset | / | 11 | 10 | / | / | |
| Generalized onset | / | 8 | 9 | / | / | |
| Disease duration (years) | / | 9.31 ± 5.96 | 11.47 ± 8.50 | / | 0.947 | 0.350 |
| Frequency (times/year) | / | 51.44 ± 93.44 | 90.16 ± 33.31 | / | 0.450 | 0.655 |
| AEDs (treated/untreated) | 17/2 | 16/3 | / | 0.230 | 1.000 | |
| Migraine aspects | ||||||
| Disease duration (years) | / | / | 7.47 ± 5.03 | 5.50 ± 3.46 | 1.410 | 0.168 |
| Frequency (times/year) | / | / | 47.84 ± 65.48 | 46.79 ± 86.91 | 0.042 | 0.967 |
| Analgetic drugs (treated/untreated) | / | / | 7/12 | 14/5 | 5.216 | 0.022 |
| Migraine side | ||||||
| Bilateral | / | / | 12 | 8 | / | / |
| Unilateral (left/right) | / | / | 2/5 | 6/5 | 1.169 | 0.367 |
| VAS score | / | / | 5.68 ± 1.06 | 6.32 ± 1.06 | −1.842 | 0.074 |
| HIT-90 score | / | / | 16.53 ± 18.00 | 16.42 ± 21.98 | 0.016 | 0.987 |
Values are presented as the mean ± SD or N. Bold font indicates P < 0.05.
AEDs, antiepileptic drugs; EP, epilepsy; EWM, EP comorbid with MWoA; HCs, healthy controls; HIT-90, Headache Impact Test-90; MWoA, migraine without aura; VAS, Visual Analog Scale.
Magnetic resonance spectroscopy results
The MRS results were shown in Table 2 and Fig. 2. The results of the multivariate test in MANOVA showed that metabolites were statistically significant in both group (P = 0.000) and location (P = 0.046), and there was no interaction between them (P = 0.574). The results of univariate test in MANOVA showed that the NAA level of bilateral DLPFCs were asymmetric, with the left DLPFC higher than the right (7.41 ± 1.15 vs. 7.07 ± 0.79; P = 0.031). There were also significant differences in myoinositol, NAA, and Cho among the four groups (P = 0.028, 0.021, and 0.005). Further Bonferroni correction revealed that myoinositol (4.34 ± 0.71 vs. 4.96 ± 0.71; P = 0.038) and NAA levels (7.02 ± 0.76 vs. 7.66 ± 1.40; P = 0.030) in EWM were significantly lower than that in healthy controls, while Cho level in MWoA were significantly higher than that in the other three groups (MWoA vs. epilepsy, EWM and healthy controls; 1.59 ± 0.59 vs. 1.37 ± 0.19, 1.33 ± 0.22, and 1.36 ± 0.21; P = 0.047, 0.007, and 0.036).
Table 2.
Metabolite concentrations (mmol/kg wet weight) of groups and statistical results of MANOVA analysis
| Metabolites | Multivariate test | |||||||
|---|---|---|---|---|---|---|---|---|
| Ins | NAA | Cho | Cr | Glx | F | P | ||
| Location | Left DLPFC | 4.79 ± 1.18 | 7.41 ± 1.15 | 1.44 ± 0.41 | 5.54 ± 0.94 | 9.81 ± 1.77 | 2.322 | 0.046 |
| Right DLPFC | 4.63 ± 0.69 | 7.07 ± 0.79 | 1.38 ± 0.29 | 5.51 ± 0.58 | 9.73 ± 1.85 | |||
| F | 1.030 | 4.723 | 1.191 | 0.056 | 0.083 | |||
| P | 0.312 | 0.031 | 0.277 | 0.813 | 0.774 | |||
| Group | MWoA | 4.88 ± 1.47 | 7.23 ± 0.82 | 1.59 ± 0.59 | 5.45 ± 1.08 | 9.22 ± 1.91 | 4.676 | 0.000 |
| EP | 4.66 ± 0.65 | 7.07 ± 0.79 | 1.37 ± 0.19b | 5.66 ± 0.66 | 10.08 ± 1.96 | |||
| EWM | 4.34 ± 0.71a | 7.02 ± 0.76a | 1.33 ± 0.22b | 5.30 ± 0.45 | 9.58 ± 1.61 | |||
| HCs | 4.96 ± 0.71 | 7.66 ± 1.40 | 1.36 ± 0.21b | 5.72 ± 0.75 | 10.23 ± 1.57 | |||
| F | 3.108 | 3.361 | 4.441 | 2.275 | 2.524 | |||
| P | 0.028 | 0.021 | 0.005 | 0.083 | 0.060 | |||
| Location × Group | F | 0.126 | 1.259 | 0.186 | 0.116 | 0.481 | 0.891 | 0.574 |
| P | 0.945 | 0.291 | 0.905 | 0.950 | 0.696 | |||
Values are presented as the mean ± SD. Bold font indicates P < 0.05. Bonferroni correction: a indicates P less than 0.05 when compared with HCs; b indicates P less than 0.05 when compared with MWoA.
Cho, choline-containing compounds; Cr, creatine and phosphocreatine; DLPFC, dorsolateral prefrontal cortex; EP, epilepsy; EWM, EP comorbid with MWoA; Glx, glutamate and glutamine (Glx, Glu and Gln); HCs, healthy controls; Ins, myoinositol; MWoA, migraine without aura; NAA, N-acetyl aspartate.
Fig. 2.
Metabolic differences in locations [left and right dorsolateral prefrontal cortices (DLPFCs)] and groups (EP, MWoA, EWM, and HCs). (a) Metabolic differences between the left and right DLPFCs. The comparison revealed that NAA was asymmetrical, with a higher level in the left DLPFC than in the right (P = 0.031). (b) Metabolic differences among the four groups. The multiple comparison revealed that both NAA and Ins were significantly reduced in EWM when compared with HCs (P = 0.030 and 0.038). In addition, MWoA had a higher Cho level than that of HCs, EP and EWM (P = 0.036, 0.047, and 0.007). The upper edge and whisker of the rectangle represent the mean and range, respectively. Cho, choline-containing compounds; Cr, creatine and phosphocreatine; EP, epilepsy; EWM, EP comorbid with MWoA; Glx, glutamate and glutamine (Glx, Glu and Gln); HCs, healthy controls; Ins, myoinositol; L, the left DLPFC; MWoA, migraine without aura; NAA, N-acetyl aspartate; R, the right DLPFC.. P < 0.05 indicates statistical significance.
Correlation analysis
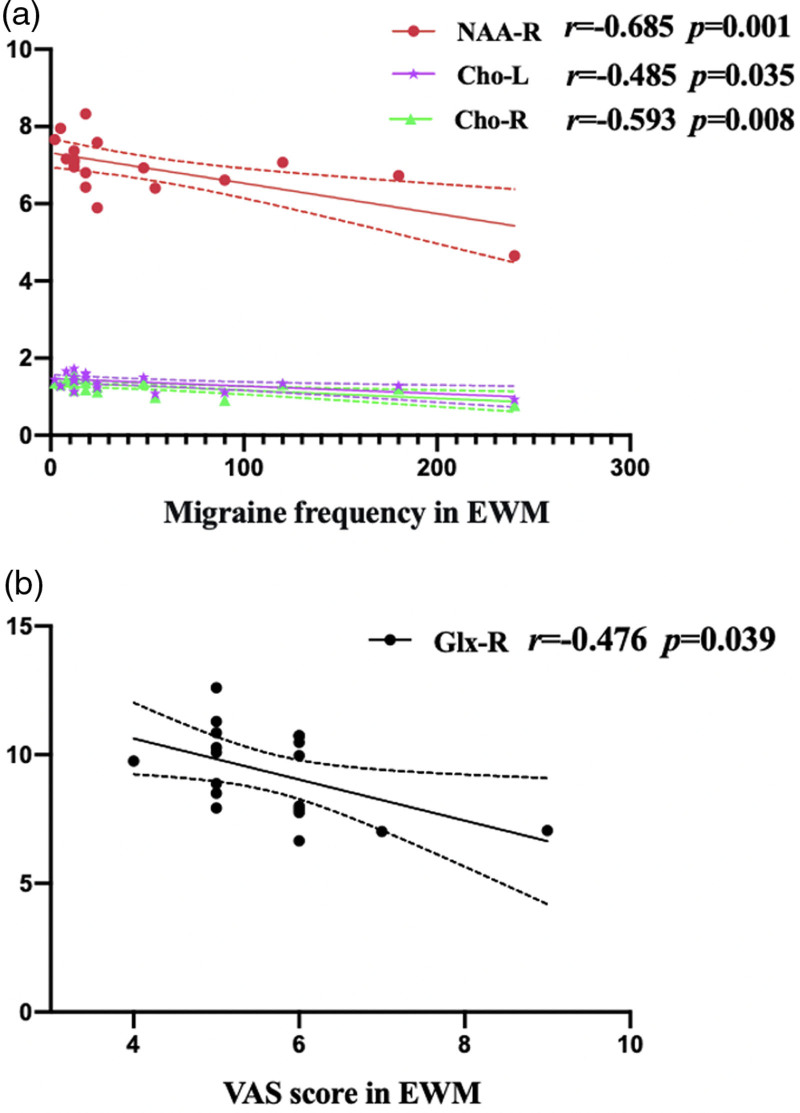
The results of correlation analyses were shown in Fig. 3. Both NAA (r = −0.685, P = 0.001) of the right DLPFC and Cho of the bilateral DLPFCs (left, r = −0.485, P = 0.035; right, r = −0.593, P = 0.008) in EWM showed a moderately negative correlation with migraine frequency. In addition, the Glx of the right DLPFC in EWM was moderately negatively related to migraine severity (evaluated by VAS score) (r = −0.476, P = 0.039). There were no additional connections discovered correlations between metabolites and the primary clinical data in the four groups (all P > 0.05).
Fig. 3.
(a) Correlations between metabolite concentrations and migraine frequency in EWM. (b) Correlations between metabolite concentrations and VAS score for migraine severity in EWM. Both NAA of the right DLPFC (r = −0.685, P = 0.001) and Cho of the bilateral DLPFCs (left, r = −0.485, P = 0.035; right, r = −0.593, P = 0.008) in EWM showed a negative correlation with migraine frequency. In addition, the Glx of the right DLPFC in EWM was negatively related to the VAS score for migraine severity (r = −0.476, P = 0.039). The solid line represents the linear regression line; the dotted line represents the 95% confidence interval of the linear fit. Cho, choline-containing compounds; DLPFC, dorsolateral prefrontal cortex; EWM, epilepsy comorbid with migraine without aura; Glx, glutamate and glutamine (Glx, Glu and Gln); L, left dorsolateral prefrontal cortex; NAA, N-acetyl aspartate; R, right dorsolateral prefrontal cortex; VAS, Visual Analog Scale.
Discussion
NAA is mainly synthesized and stored in neuronal mitochondria and is present in high concentrations in neurons. It is a marker of neuronal structure and function [21]. Our study found that the NAA level between bilateral DLPFCs was asymmetric (left > right), which was similar to previous studies [16,17]. Studies have shown that biochemical asymmetry is closely related to anatomical asymmetry between the cerebral hemispheres [22,23]. For example, in a study by Jayasundar and his colleagues [22], it was discovered that metabolites showed asymmetry between the cerebral hemispheres of right-handed individuals, with a higher NAA/Cr ratio observed in the left frontal lobe compared to the right. Frontal anatomical asymmetry was also reported to be associated with handedness [24]. Notably, in this study, all subjects were right-handed. Therefore, the observed NAA asymmetry may reflect the difference in the number of neurons caused by anatomical asymmetry between the dominant and nondominant hemispheres, that is, the neuronal number in the dominant left hemisphere of right-handed individuals was greater.
Our study found that NAA level in MWoA, epilepsy, and EWM was gradually decreased when compared to healthy controls, but was only significant in EWM. This suggested that when epilepsy and MWoA are comorbid (EWM), there may be a ‘synergistic effect’ on NAA reduction. Previous studies revealed that NAA was obviously lower in patients with epilepsy [16,17] or MWoA [25,26] than that in healthy controls, which was inconsistent with our findings. This may be caused by differences in subject factors, scanning time, statistical methods, and so on. The obviously reduced NAA in EWM may be the combined results of the following two aspects. First, both epilepsy [21,27] and migraine [28] attacks can result in neuronal mitochondrial dysfunction. NAA is mainly synthesized by neuronal mitochondria, and abnormal energy metabolism caused by neuronal mitochondrial dysfunction can lead to reduced NAA level [27]. Furthermore, extended and recurrent epilepsy [29] and migraine [25,26] attacks may also lead to progressive neuronal damage and loss, demonstrated by a gradual reduction in NAA levels, a marker for neurons. Our study also found a negative correlation between NAA of the right DLPFC and migraine frequency in EWM. This result indicated that more frequent migraine attacks can cause greater damage to neuronal integrity or function in the right DLPFC of EWM. A study by Schmitz et al. also revealed that migraine attack frequency affected neuronal structure and density [30].
Observed changes in myoinositol were similar to those in NAA: Compared with healthy controls, myoinositol was gradually reduced in MWoA, epilepsy, and EWM, but only obviously in EWM. This indicated that neither epilepsy nor MWoA leads to a significant reduction in myoinositol. However, when they are comorbid, there may be also a ‘synergistic effect’ that causes myoinositol to decrease substantially. As an organic osmolyte, myoinositol plays an important role in cellular osmoregulation [31]. Myoinositol stability may represent the stability of cell osmotic pressure [31]. Both epilepsy and migraine episodes can alter neural electrophysiology, accompanied by osmotic imbalance. An animal study revealed that hypoosmolality caused by osmotic pressure imbalance may be compensated for by rapidly decreasing myoinositol, which can slowly recover after osmolarity normalization [32]. The slightly reduced myoinositol level observed in MWoA and epilepsy may reflect the process of returning to normal osmolarity after the disease attack, while the significantly reduced myoinositol level found in EWM may indicate the presence of more serious and uncompensated cell osmolarity disorders. In addition, prolonged exposure of cells to stressful environments like serious osmotic pressure imbalance was more likely to cause the injury of molecules such as DNA, resulting in cellular dysfunction and even potential apoptosis [33]. Myoinositol is found predominantly in glial cells and is regarded as a biomarker for the number and function of glial cells [34,35]. Significantly reduced myoinositol was also reported to be associated with decreased glial cell density and dysfunction [34,35]. Therefore, we speculate that glial cell loss and dysfunction may also occur in EWM.
The Cho primarily provides information regarding cell membrane integrity, density, and turnover (phospholipid synthesis and degradation), which occurs at a higher level in glial cells than in neurons [36,37]. We found that the Cho level in MWoA was significantly higher than that in the other three groups. The study by Dehghan et al. found that MWoA had a higher Cho/Cr ratio in the occipital lobe than healthy controls [26]. The elevation in Cho level was generally indicative of cellular membrane injury and glial proliferation [36,37]. Therefore, the higher Cho level observed in MWoA may reflect the membrane integrity breakdown and reactive glial proliferation caused by recurrent migraine attacks. We did not find a significant Cho change in epilepsy or EWM, which may be due to the therapeutic effects of AEDs. Comparisons of clinical data in this study showed no difference in the use of AEDs between epilepsy and EWM, and more than 80% of epilepsy and EWM were on AEDs. Studies show that AEDs can prevent seizures and produce some substances that protect neurons and axons from damage [38]. In addition, some AEDs can also prevent migraine attacks to some extent [39]. We also found that Cho level in the bilateral DLPFCs of EWM was negatively correlated with migraine attack frequency, suggesting that frequent migraine attacks have a potential effect on impaired maintenance of membrane turnover, glial dysfunction, and reduced cell density in EWM.
Our study found that migraine intensity (assessed by VAS score) was negatively correlated with Glx in the right DLPFC of EWM. Ashina et al.’s study [40] showed that the pain intensity of migraine was associated with increased emotional stress and depression. In addition, decreased Glx was also reported to be related to emotional factors [41,42]. For example, a study by Luykx et al. showed that Glx in the anterior cingulate cortex was reduced in patients with major depression [42]. The DLPFC plays a crucial role in processing emotions such as anxiety or depression [13], with research indicating that depression-related brain dysfunction primarily affects the right hemisphere [43]. Therefore, we speculated that as migraine intensity increases, the decline in Glx level in the right DLPFC of EWM may be related to depression. However, further research is needed to confirm whether EWM patients are prone to depression as pain intensity increases.
In conclusion, our study confirms that the DLPFC is an important brain region in both epilepsy and MWoA. Neither epilepsy nor MWoA leads to a significant reduction in myoinositol and NAA, however, when they are comorbid (EWM), there may be a ‘synergistic’ effect leading NAA and myoinositol to decline obviously. Since NAA and myoinositol are respective markers for neurons and glial cells, these results suggest that more serious injuries of both neurons and glial cells may be involved in the biochemical mechanisms of EWM in female patients. Further research with larger sample sizes is necessary to confirm these findings and the interaction mechanism between epilepsy and MWoA needs to be further explored.
There are some limitations in this study. First, the sample size of this study was small, which may reduce the statistical power. This result would be more convincing if a study with a larger sample could confirm our findings. Second, due to difficulties in patient inclusion, epilepsy seizure types were mixed in epilepsy and EWM patients, so the results may be controversial. However, we tried to keep the epileptic seizure types as balanced as possible between epilepsy and EWM patients, believing that this effect would be canceled out. Third, although all patients stopped taking drugs 72 h before the scan, we cannot guarantee whether long-term drug use has any effect on brain metabolism. However, AEDs have the potential to increase NAA levels and shield neurons and axons from damage [38]. Therefore, we believe that the reduction in NAA and myoinositol of EWM in this study was not caused by AEDs.
Acknowledgements
We wish to thank members of the Epilepsy Center at West China Hospital of Sichuan University for their assistance and thank all the subjects who participated in this study.
We thank the support of foundations named the National Natural Science Foundation of China (Grant no. 82471961; 82271961); the Sichuan Provincial Foundation of Science and Technology (Grant no. 2019YFS0428); and West China Hospital of Sichuan University-University of Electronic Science and Technology Talent Cultivation Fund for Medical-Industrial Integration (Grant no. ZYGX2022YGRH019).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Liping Wang, Huaxia Pua, and Jingyuan Zhou contributed equally to the writing of this article.
References
- 1.Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019; 393:689–701. [DOI] [PubMed] [Google Scholar]
- 2.Keezer MR, Sisodiya SM, Sander JW. Comorbidities of epilepsy: current concepts and future perspectives. Lancet Neurol. 2016; 15:106–115. [DOI] [PubMed] [Google Scholar]
- 3.Fanella M, Fattouch J, Casciato S, Lapenta L, Morano A, Egeo G, et al. Ictal epileptic headache as ‘subtle’ symptom in generalized idiopathic epilepsy. Epilepsia. 2012; 53:e67–e70. [DOI] [PubMed] [Google Scholar]
- 4.Garg D, Tripathi M. Borderlands of migraine and epilepsy. Neurol India. 2021; 69:S91–S97. [DOI] [PubMed] [Google Scholar]
- 5.Keezer MR, Bauer PR, Ferrari MD, Sander JW. The comorbid relationship between migraine and epilepsy: a systematic review and meta-analysis. Eur J Neurol. 2015; 22:1038–1047. [DOI] [PubMed] [Google Scholar]
- 6.Kanner AM. Management of psychiatric and neurological comorbidities in epilepsy. Nat Rev Neurol. 2016; 12:106–116. [DOI] [PubMed] [Google Scholar]
- 7.Nye BL, Thadani VM. Migraine and epilepsy: review of the literature. Headache. 2015; 55:359–380. [DOI] [PubMed] [Google Scholar]
- 8.Begasse De Dhaem O, Aldana SI, Kanner AM, Sperling M, French J, Nadkarni SS, et al. Association between migraine comorbidity and psychiatric symptoms among people with newly diagnosed focal epilepsy. J Neuropsychiatry Clin Neurosci. 2022; 34:182–187. [DOI] [PubMed] [Google Scholar]
- 9.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 10.Huang Q, Lv X, He Y, Wei X, Ma M, Liao Y, et al. Structural differences in interictal migraine attack after epilepsy: a diffusion tensor imaging analysis. Epilepsy Behav. 2017; 77:8–12. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Cai XT, Zu MD, Zhang J, Deng ZR, Wang Y. Decreased resting-state functional connectivity of periaqueductal gray in temporal lobe epilepsy comorbid with migraine. Front Neurol. 2021; 12:636202–636214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Z, Wan X, Zhao X, Rong Y, Wu Y, Cao Z, et al. Brain neurometabolites differences in individuals with subjective cognitive decline plus: a quantitative single- and multi-voxel proton magnetic resonance spectroscopy study. Quant Imaging Med Surg. 2021; 11:4074–4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farchione TR, Moore GJ, Rosenberg DR. Proton magnetic resonance spectroscopic imaging in pediatric major depression. Biol Psychiatry. 2002; 52:86–92. [DOI] [PubMed] [Google Scholar]
- 14.Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003; 126:1079–1091. [DOI] [PubMed] [Google Scholar]
- 15.Qin L, Jiang W, Zheng J, Zhou X, Zhang Z, Liu J. Alterations functional connectivity in temporal lobe epilepsy and their relationships with cognitive function: a longitudinal resting-state fMRI study. Front Neurol. 2020; 11:625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Wu X, Su X, Sun H, Tan Q, Zhang S, et al. Metabolic alterations of the dorsolateral prefrontal cortex in sleep-related hypermotor epilepsy: a proton magnetic resonance spectroscopy study. J Neurosci Res. 2021; 99:2657–2668. [DOI] [PubMed] [Google Scholar]
- 17.Tan Q, Sun H, Wang W, Wu X, Hao N, Su X, et al. Quantitative MR spectroscopy reveals metabolic changes in the dorsolateral prefrontal cortex of patients with temporal lobe epilepsy. Eur Radiol. 2018; 28:4496–4503. [DOI] [PubMed] [Google Scholar]
- 18.Stewart WF, Wood C, Reed ML, Roy J, Lipton RB; AMPP Advisory Group. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008; 28:1170–1178. [DOI] [PubMed] [Google Scholar]
- 19.Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017; 58:522–530. [DOI] [PubMed] [Google Scholar]
- 20.Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018; 126:1763–1768. [DOI] [PubMed] [Google Scholar]
- 21.Signoretti S, Marmarou A, Tavazzi B, Lazzarino G, Beaumont A, Vagnozzi R. N-Acetylaspartate reduction as a measure of injury severity and mitochondrial dysfunction following diffuse traumatic brain injury. J Neurotrauma. 2001; 18:977–991. [DOI] [PubMed] [Google Scholar]
- 22.Jayasundar R. Human brain: biochemical lateralization in normal subjects. Neurol India. 2002; 50:267–271. [PubMed] [Google Scholar]
- 23.Kristofiková Z, Rícný J, Ort M, Rípová D. Aging and lateralization of the rat brain on a biochemical level. Neurochem Res. 2010; 35:1138–1146. [DOI] [PubMed] [Google Scholar]
- 24.Amunts K, Jäncke L, Mohlberg H, Steinmetz H, Zilles K. Interhemispheric asymmetry of the human motor cortex related to handedness and gender. Neuropsychologia. 2000; 38:304–312. [DOI] [PubMed] [Google Scholar]
- 25.Sarchielli P, Tarducci R, Presciutti O, Gobbi G, Pelliccioli GP, Stipa G, et al. Functional 1H-MRS findings in migraine patients with and without aura assessed interictally. Neuroimage. 2005; 24:1025–1031. [DOI] [PubMed] [Google Scholar]
- 26.Dehghan A, Saatchian E, Sobhani M, Montazerabadi A. Neurochemical metabolite alterations of the occipital lobe in migraine without aura by proton magnetic resonance spectroscopy. Neuroradiol J. 2020; 33:410–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowley S, Patel M. Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic Biol Med. 2013; 62:121–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filippi V, Steiger R, Beliveau V, Frank F, Kaltseis K, Gizewski ER, Broessner G. Investigating the migraine cycle over 21 consecutive days using proton magnetic resonance spectroscopy and resting-state fMRI: a pilot study. Brain Sci. 2022; 12:646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun H, Li X, Guo Q, Liu S. Research progress on oxidative stress regulating different types of neuronal death caused by epileptic seizures. Neurol Sci. 2022; 43:6279–6298. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz N, Admiraal-Behloul F, Arkink EB, Kruit MC, Schoonman GG, Ferrari MD, van Buchem MA. Attack frequency and disease duration as indicators for brain damage in migraine. Headache. 2008; 48:1044–1055. [DOI] [PubMed] [Google Scholar]
- 31.Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem. 2002; 82:736–754. [DOI] [PubMed] [Google Scholar]
- 32.Brand A, Leibfritz D, Richter-Landsberg C. Oxidative stress-induced metabolic alterations in rat brain astrocytes studied by multinuclear NMR spectroscopy. J Neurosci Res. 1999; 58:576–585. [PubMed] [Google Scholar]
- 33.Bradley AI, Marsh NM, Borror HR, Mostoller KE, Gama AI, Gardner RG. Acute ethanol stress induces sumoylation of conserved chromatin structural proteins in Saccharomyces cerevisiae. Mol Biol Cell. 2021; 32:1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byun MS, Choi JS, Yoo SY, Kang DH, Choi CH, Jang DP, et al. Depressive symptoms and brain metabolite alterations in subjects at ultra-high risk for psychosis: a preliminary study. Psychiatry Investig. 2009; 6:264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheikhi Koohsar J, Faeghi F, Rafaiee R, Niroumand Sarvandani M, Masjoodi S, Kalalian Moghaddam H. Metabolite alternations in the dopamine circuit associated with methamphetamine-related psychotic symptoms: a proton magnetic resonance spectroscopy study. Iran J Psychiatry. 2022; 17:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000; 13:129–153. [DOI] [PubMed] [Google Scholar]
- 37.Fernández-García P, Malet-Engra G, Torres M, Hanson D, Rosselló CA, Román R, et al. Evolving diagnostic and treatment strategies for pediatric CNS tumors: the impact of lipid metabolism. Biomedicines. 2023; 11:1365–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romoli M, Mazzocchetti P, D’Alonzo R, Siliquini S, Rinaldi VE, Verrotti A, et al. Valproic acid and epilepsy: from molecular mechanisms to clinical evidences. Curr Neuropharmacol. 2019; 17:926–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ingram EE, Bocklud BE, Corley SC, Granier MA, Neuchat EE, Ahmadzadeh S, et al. Non-CGRP antagonist/non-triptan options for migraine disease treatment: clinical considerations. Curr Pain Headache Rep. 2023; 27:497–502. [DOI] [PubMed] [Google Scholar]
- 40.Ashina M, Cohen JM, Gandhi SK, Du E. Reduction in the severity and duration of headache following fremanezumab treatment in patients with episodic and chronic migraine. Headache. 2021; 61:916–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widerström-Noga E, Pattany PM, Cruz-Almeida Y, Felix ER, Perez S, Cardenas DD, Martinez-Arizala A. Metabolite concentrations in the anterior cingulate cortex predict high neuropathic pain impact after spinal cord injury. Pain. 2013; 154:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luykx JJ, Laban KG, van den Heuvel MP, Boks MP, Mandl RC, Kahn RS, et al. Region and state specific glutamate downregulation in major depressive disorder: a meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev. 2012; 36:198–205. [DOI] [PubMed] [Google Scholar]
- 43.Hecht D. Depression and the hyperactive right-hemisphere. Neurosci Res. 2010; 68:77–87. [DOI] [PubMed] [Google Scholar]