Abstract
Purpose
To quantify tracheal collapsibility using low-dose four-dimensional (4D) CT and to compare visual and quantitative 4D CT–based assessments with assessments from paired inspiratory-expiratory CT, bronchoscopy, and spirometry.
Materials and Methods
The authors retrospectively analyzed 4D CT examinations (January 2016–December 2022) during shallow respiration in 52 patients (mean age, 66 years ± 12 [SD]; 27 female, 25 male), including 32 patients with chronic obstructive pulmonary disease (mean forced expiratory volume in 1 second percentage predicted [FEV1%], 50% ± 27), with suspected tracheal collapse. Paired CT data were available for 27 patients and bronchoscopy data for 46 patients. Images were reviewed by two radiologists in consensus, classifying patients into three groups: 50% or greater tracheal collapsibility, less than 50% collapsibility, or fixed stenosis. Changes in minimal tracheal lumen area, tracheal volume, and lung volume from inspiration to expiration were quantified using YACTA software. Tracheal collapsibility between groups was compared employing one-way analysis of variance (ANOVA). For related samples within one group, ANOVA with repeated measures was used. Spearman rank order correlation coefficient was calculated for collapsibility versus pulmonary function tests.
Results
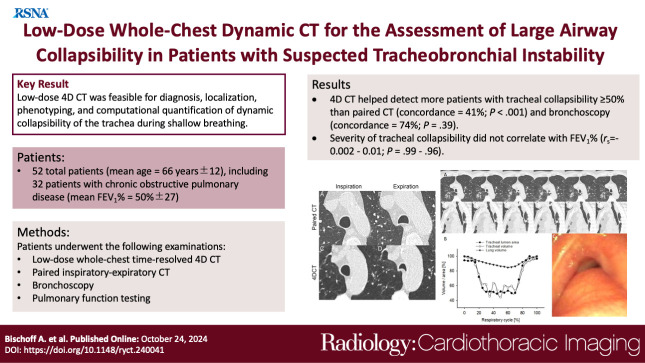
At 4D CT, 25 of 52 (48%) patients had tracheal collapsibility of 50% or greater, 20 of 52 (38%) less than 50%, and seven of 52 (13%) had fixed stenosis. Visual assessment of 4D CT detected more patients with collapsibility of 50% or greater than paired CT, and concordance was 41% (P < .001). 4D CT helped identify more patients with tracheal collapsibility of 50% or greater than did bronchoscopy, and concordance was 74% (P = .39). Mean collapsibility of tracheal lumen area and volume at 4D CT were higher for 50% or greater visually assessed collapsibility (area: 53% ± 9 and lumen: 52% ± 10) compared with the less than 50% group (27% ± 9 and 26% ± 6, respectively) (P < .001), whereas both tracheal area and volume were stable for the fixed stenosis group (area: 16% ± 12 and lumen: 21% ± 11). Collapsibility of tracheal lumen area and volume did not correlate with FEV1% (rs = −0.002 to 0.01, P = .99–.96).
Conclusion
The study demonstrated that 4D CT is feasible and potentially more sensitive than paired CT for central airway collapse. Expectedly, FEV1% was not correlated with severity of tracheal collapsibility.
Keywords: CT-Quantitative, Tracheobronchial Tree, Chronic Obstructive Pulmonary Disease, Imaging Postprocessing, Thorax
Supplemental material is available for this article.
© RSNA, 2024
Keywords: CT-Quantitative, Tracheobronchial Tree, Chronic Obstructive Pulmonary Disease, Imaging Postprocessing, Thorax
Summary
Low-dose four-dimensional CT was feasible for diagnosis, localization, phenotyping, and computational quantification of dynamic collapsibility of the trachea during shallow breathing; concordance with bronchoscopy was moderate and with paired CT was low.
Key Points
■ Visual assessment of four-dimensional (4D) CT detected more patients with tracheal collapsibility of 50% or greater than paired inspiratory-expiratory CT, and concordance was 41% (P < .001).
■ Visual assessment of 4D CT identified more patients with tracheal collapsibility of 50% or greater than bronchoscopy, and concordance was 74% (P = .39).
■ Severity of tracheal collapsibility in shallow breathing at rest assessed using computer-aided quantification did not correlate with forced expiratory volume in 1 second percentage predicted (rs = −0.002 to 0.01, P = .99–.96), as expected.
Introduction
Dynamic instability of the central airways can cause severe airflow limitation. In addition to congenital causes, acquired diseases like smoking-related chronic obstructive pulmonary disease (COPD), infections, or postinterventional weakness of the tracheobronchial cartilage can be associated with airway collapsibility (1–5). Tracheal collapse can be differentiated into tracheobronchomalacia (TBM) and excessive dynamic airway collapse (EDAC), which both can be found in COPD (6). While TBM is caused by tracheal cartilage destruction or softening, EDAC is characterized by an inward bulging of the posterior membranous part of the trachea only (7,8). Central airway collapsibility is often not detected with spirometry measurements, and its contribution to morbidity in COPD needs to be evaluated further given that current standards of care may fail to recognize the diagnosis as a phenotype of COPD (2,9,10).
Central airway collapse is typically diagnosed using flexible bronchoscopy (11), which is highly user-dependent, nonquantitative, and limited to the segmental level or by proximal stenosis. Further, both the characterization as well as the grading of airway collapse are not standardized (12–14). Established imaging techniques such as paired inspiratory-expiratory CT during breath holding or dynamic single-section two-dimensional CT (2D CT or cine CT) showed an equal diagnostic sensitivity to bronchoscopy, but due to improper timing and patient coordination, as well as unintentional Valsalva maneuvers during scanning, focal tracheal collapse may be underestimated or missed using both (2,5,9,15–18). A four-dimensional (4D) CT technique could be introduced that employs full coverage of the central airways and even of the whole chest in adults and allows objective quantification of respiratory dynamics along the respiratory cycle. At 4D CT, it is not required to prespecify the level of suspected collapse as in 2D CT. Further, respiratory z-axis displacement of the site of collapse during respiration does not impair diagnostic sensitivity. As an advantage over bronchoscopy, instabilities down to the level of first subsegmental airways, which are nonperpendicular to the transversal plane, can be also evaluated using 4D CT (18,19).
The aim of the present study was to quantify tracheal collapsibility using low-dose chest 4D CT. We assessed the concordance of visual and quantitative assessment of tracheal collapsibility at 4D CT with paired inspiratory-expiratory CT and flexible bronchoscopy assessments, as well as the correlation of quantitative tracheal collapsibility with clinical dyspnea assessment tests and pulmonary function tests.
Materials and Methods
Study Patients and Data Collection
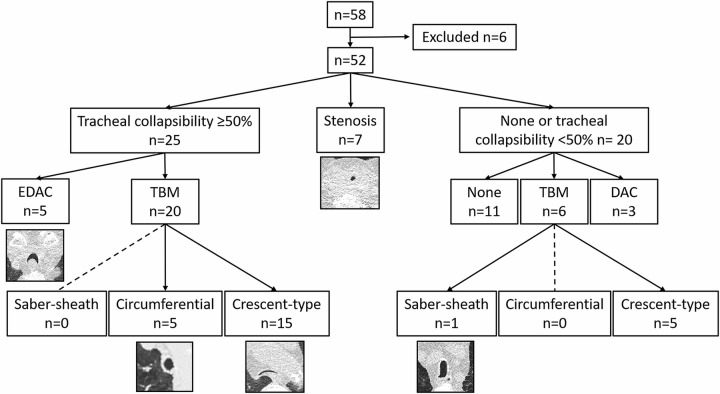
Institutional ethics committee approval (no. S-646–2016) was obtained for this retrospective study, and the requirement for written informed consent was waived. Low-dose 4D CT of the chest was performed in 58 patients according to clinical standards of care at our center (Thoraxklinik at Heidelberg University Hospital) from January 2012 to December 2016 for suspected large airway collapse. Clinical suspicion of airway instability was based on clinical assessment (anamneses, clinical examination, standard pulmonary function tests) and in patients with uncontrolled dyspnea despite optimal therapy according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommendations (20). Further factors were findings at previous breath-hold CT, as well as at bronchoscopy in earlier visits. Four patients were excluded from analysis because of tracheal deformation due to rare conditions (eg, scoliosis, amyloidosis [n = 3]) or insufficient diagnostic quality (n = 1). Two patients with a combination of TBM and fixed stenosis were also excluded to avoid possible bias (Fig 1). Clinical dyspnea assessment tests (modified British Medical Research Council dyspnea scale, 6-minute walking distance test) were performed in 29 patients within the first visit at our center. In 36 patients, a low-dose CT scan with quantitative analyses including emphysema index was available. A total of 27 patients underwent paired inspiratory-expiratory CT as per clinical standard in COPD, which was used for further quantitative analyses and evaluated for concordance with 4D CT. A total of 50 patients underwent full-body plethysmography according to American Thoracic Society and European Respiratory Society standards, with reference values according to the Global Lung Function Initiative (21,22).
Figure 1:
Study flowchart. Patients were grouped as having fixed stenosis or tracheal collapsibility, the latter with a threshold of 50% or greater reduction of minimal lumen area. The most frequent diagnosis was tracheobronchomalacia (TBM), and its most frequent subtype was crescent-type collapse. (E)DAC = (excessive) dynamic airway collapse.
Bronchoscopy
Bronchoscopy was performed in 46 patients either within the same visit as 4D CT or in a previous visit. The procedure was performed either under local anesthesia only or in combination with sedation. For rigid bronchoscopy, general anesthesia was preferred (23,24). During the examination, coughing or Valsalva maneuvers were initiated to investigate abnormal airway wall movement if necessary and tolerable for the patient. Three operators (D.G., R.E., and F.T.) with equivocal experience (>10 years) in interventional pneumology performed bronchoscopy and subjectively rated tracheal collapsibility. Bronchoscopy and 4D CT were both independently indicated in cases of suspicion for central airway instability. Bronchoscopy was typically performed before 4D CT. Six patients who underwent bronchoscopy in an earlier visit were referred to 4D CT because of suspicious findings. However, patients with negative bronchoscopy findings were also referred to 4D CT if there was no sufficient explanation for symptoms or if suspicion of airway instability could not be ruled out. Generally, the individual operator independently rated a clinically significant collapse of the trachea if the subjective reduction in the lumen was greater than 50%. Similarly, fixed stenosis was rated. These findings were used for concordance testing between bronchoscopy and CT techniques.
Low-dose 4D CT of the Whole Chest
Acquisition and reconstruction.— Low-dose noncontrast 4D CT of the whole chest (Somatom Definition AS64; Siemens Healthineers) was performed with 80-kV tube voltage, 10-mAs reference, 0.09 pitch, and 340-msec rotation time (18). All patients were instructed over 5 minutes to perform regular shallow breathing before being transferred in a head-first supine position onto the CT table. Nasal oxygen supplementation was provided if needed. A belt system was applied around the chest wall for recording respiratory movements for retrospective gating. Total scan duration was approximately 90 seconds. The mean dose length product was 154 mGy · cm ± 125 (SD), with a resulting effective dose of 2.7 mSv ± 2.4. The median breathing rate was 20 breaths/min. A total of 21 separate three-dimensional datasets were computed per patient by dividing the respiratory cycle into 5%-wide temporal steps. Together, this results in an average temporal increment of 16 msec and overlap of 324 msec. Images were iteratively reconstructed with 1.5-mm and 1.0-mm section thickness (SAFIRE, strength 3; Siemens Healthineers) in a medium-soft (I40f) and sharp lung kernel (I70f) with two fields of view, one focused on the trachea (200 mm) to enhance resolution and one encompassing the whole thorax (300 mm).
Visual evaluation of tracheal collapsibility.— Tracheal collapsibility was graded visually with a 4D viewing tool (commercially available software SyngoVia, version VB70A; Siemens Healthineers) by two radiologists (A.B. with 3 years and M.O.W. with 14 years of experience in chest radiology) in consensus according to three categories: (a) 50% or greater or (b) less than 50% tracheal collapsibility and (c) fixed tracheal stenosis (6,8,12,25). A clinically significant tracheal collapse was defined by a threshold of 50% or greater reduction of the tracheal lumen area from the maximum at inspiration to the minimum at expiration (6,8,26). The phenotype of tracheal collapsibility was defined as either TBM with differentiation into its three subtypes (circumferential, crescent, or saber-sheath type) or as excessive or dynamic airway collapse (EDAC or DAC) (Fig 1) (6). Of note, 4D CT was reviewed blinded to bronchoscopy results.
Quantification of tracheal collapsibility.— All 21 datasets per patient (ie, 1092 in total) were subjected to automatic segmentation and quantification by scientific software YACTA (software “Yet Another CT Analyzer,” version 2.8.5.5; programming by O.W.) (27–30). Automatic segmentation was reviewed and corrected manually where necessary by two radiologists (A.B. and M.O.W.) in consensus. Minimal transverse tracheal lumen area perpendicular to a centerline, tracheal volume, and lung volume were measured and were normalized to maximal values per patient for comparison (18). Accordingly, the datasets of the available standard breath hold and paired inspiratory-expiratory CT were analyzed. The software used the medium-soft kernel I40f images for quantification of tracheal lumen area and tracheal volume at 200-mm field of view and of lung volume at 300-mm field of view.
Statistical Analysis
Statistical analysis was performed with SigmaPlot (version 14.0; Systat Software). Data are presented as means ± SDs unless specified otherwise. Comparisons of tracheal collapsibility between groups along the 21 steps of the respiratory cycle were made employing one-way analysis of variance (ANOVA). For related samples within one patient group, ANOVA with repeated measures was used. In both, adjustment for multiple comparisons by Bonferroni method was applied. Pairwise correlation of airway instability and pulmonary function tests were assessed using Spearman rank order correlation coefficient rs. McNemar test was used to assess concordance of 4D CT measurements with bronchoscopy and paired inspiratory-expiratory CT. The patients with missing bronchoscopy, quantitative CT, or pulmonary function testing data were excluded for these specific analyses, and the sample size was reduced in these investigations as mentioned in the results. A P value less than .05 was considered to indicate a statistically significant difference, including corrections for multiple comparisons using the Bonferroni method, as appropriate.
Results
Patient Characteristics
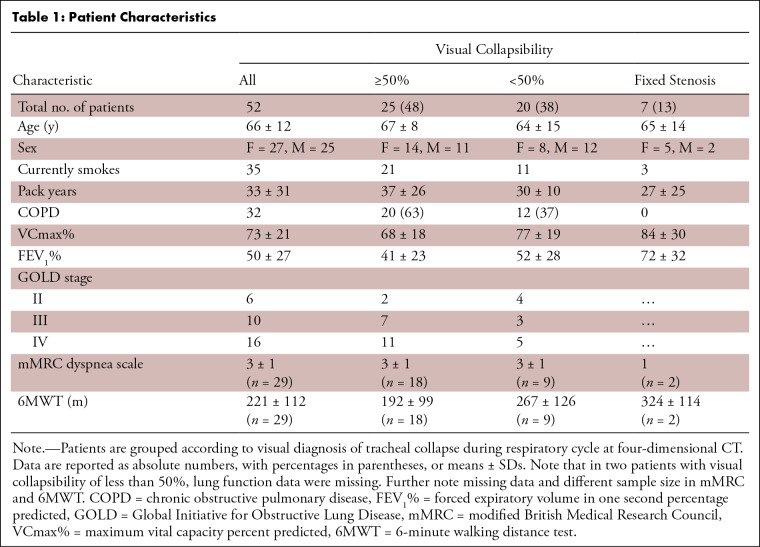
The final cohort included 52 patients (mean age, 66 years ± 12; 27 female and 25 male) with suspected tracheal collapse. Paired CT data were available for 27 patients and bronchoscopy data for 46 patients. A total of 32 patients were diagnosed with COPD (mean forced expiratory volume in 1 second percentage predicted [FEV1%], 50% ± 27) (Fig 1, Table 1). Other underlying diagnoses were asthma, history of long-term intubation, surgery for goiter, or other tracheal trauma.
Table 1:
Patient Characteristics
Visual Assessment of Extent and Type of Tracheal Collapsibility
Tracheal collapsibility of 50% or greater was found in 25 of 52 (48%), less than 50% in 20 (38%), and fixed stenosis in seven patients (13%). For tracheal collapsibility of 50% or greater, TBM was the major morphologic finding (20 of 25), with crescent-type TBM being the most frequent subtype. Findings in 11 patients of the less than 50% group were classified as normal without any signs of changes in tracheal shape or lumen area or with nonpathologic changes. Some patients with collapsibility of less than 50% also showed morphologic changes of tracheal shape, of which six could be classified as TBM and three as DAC. Location of maximum collapse was mainly found in the distal trachea below the level of the aortic arch until the level of the carina (n = 24).
Quantification of Tracheal Collapsibility
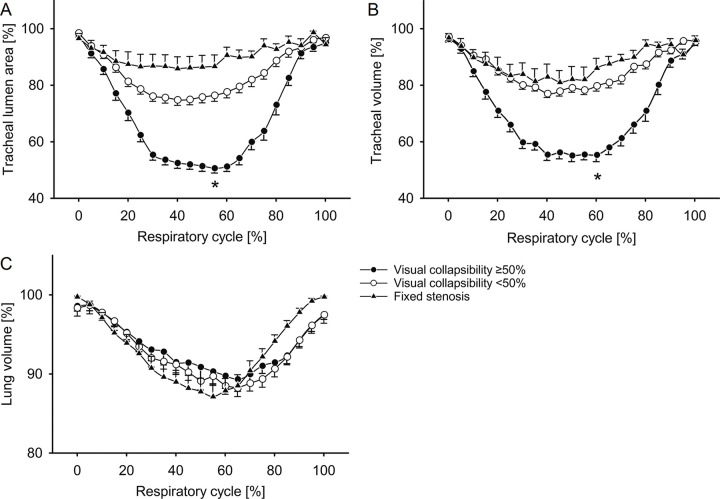
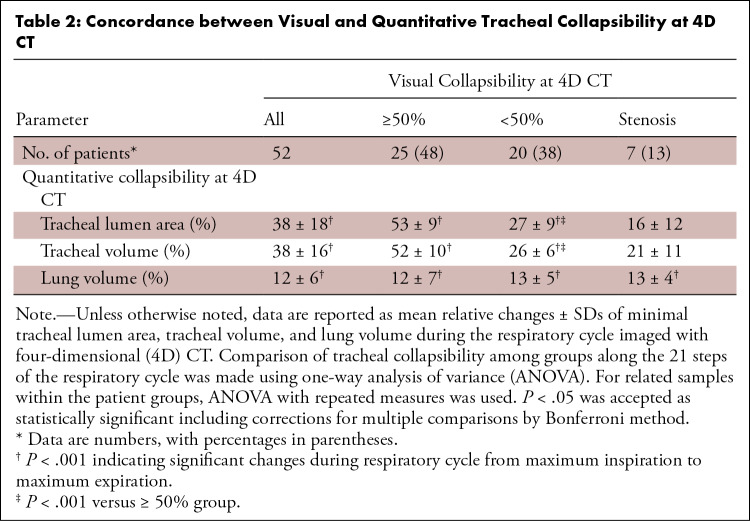
Along the respiratory cycle, significant changes in tracheal lumen area, tracheal volume, and lung volume were detected in all three groups (Fig 2) (P < .001). The tracheal collapse occurred mainly in early expiration. The reduction in tracheal volume was kept relatively constant until the beginning of inspiration, whereas the lung volume continuously decreased during expiration until inspiration was exerted. The visual classification in tracheal collapsibility of 50% or greater was consistent with quantitative measurements in 18 of 25 (72%) patients, using change of tracheal lumen area, and in 14 of 25 (56%) patients, using change of tracheal volume. In patients with a mismatch of 50% or greater collapsibility, the distance to the 50% threshold was less than 5% in three patients for tracheal lumen area and in seven patients for tracheal volume. In the remaining patients, the differences ranged from 5% to 15%. Tracheal lumen area was reduced on average by 53% ± 9 in the group with visual collapsibility of 50% or greater and by 27% ± 9 in the group with visual collapsibility less than 50% (Table 2). Importantly, using tracheal volume and a threshold of 50% did not yield different results as in tracheal lumen area.
Figure 2:
Quantification of (A) minimal tracheal lumen area, (B) tracheal volume, and (C) lung volume during the respiratory cycle by using four-dimensional (4D) CT. Patients were grouped according to visual degree of collapsibility. Relative changes during free shallow breathing are shown with the respiratory cycle separated into 21 discrete time points equal to 5%-wide steps at 4D CT. Symbols represent group means, and whiskers indicate standard error of the mean; statistical significance is given for maximum inspiration versus end expiration, with * indicating P < .001. Comparisons of tracheal collapsibility between groups along the 21 steps of the respiratory cycle were made by one-way analysis of variance (ANOVA). For related samples within the patient groups, ANOVA with repeated measures was used. P < .05 was accepted as statistically significant, including corrections for multiple comparisons by Bonferroni method.
Table 2:
Concordance between Visual and Quantitative Tracheal Collapsibility at 4D CT
Concordance of Tracheal Collapsibility at 4D CT with Paired Inspiratory-Expiratory CT
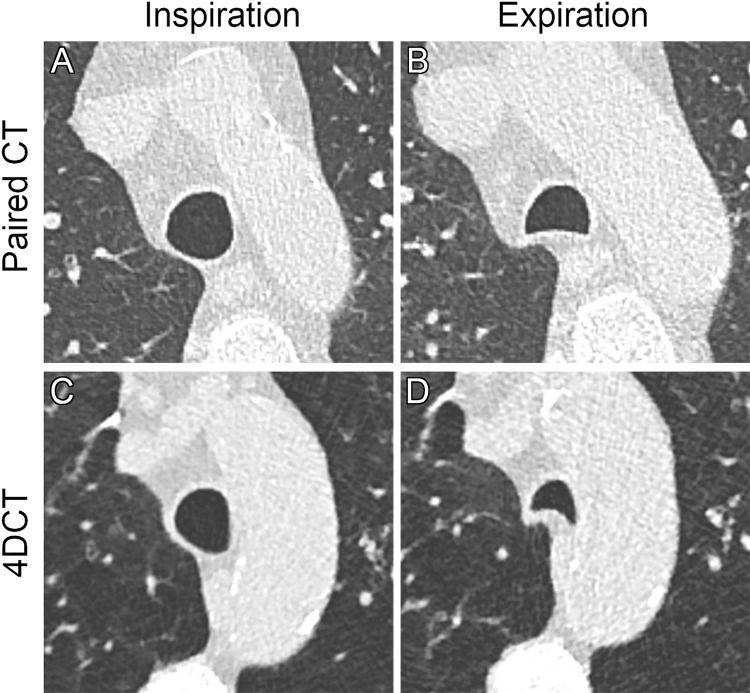
Data from paired inspiratory-expiratory CT were available for 27 patients. Eighteen of 27 (66%) patients were classified with clinically significant tracheal collapsibility (≥50%) visually at 4D CT compared with only four of 27 (15%) patients at paired inspiratory-expiratory CT (P < .001). Only one patient was additionally identified to have clinically significant collapse at paired inspiratory-expiratory CT not found at 4D CT. Overall, visual assessment of tracheal collapsibility at paired inspiratory-expiratory CT was in agreement with visual assessment at 4D CT in 11 of 27 (41%) patients and with computer-aided quantification at 4D CT in 15 of 27 (55%) patients (Fig 3, Table 3).
Figure 3:
Low concordance of paired inspiratory-expiratory CT with four-dimensional (4D) CT. The top row represents paired inspiratory-expiratory CT (noncontrast, lung window) in (A) inspiratory and (B) expiratory breath hold in axial plane in a 77-year-old male patient. The bottom row shows the same patient under shallow breathing at 4D CT (noncontrast, lung window) in (C) maximum inspiration and (D) end expiration in axial plane. Note that the tracheal collapsibility was less than 50% at paired inspiratory-expiratory CT but 50% or greater at 4D CT. The level of strongest collapse varied slightly between paired CT and 4D CT.
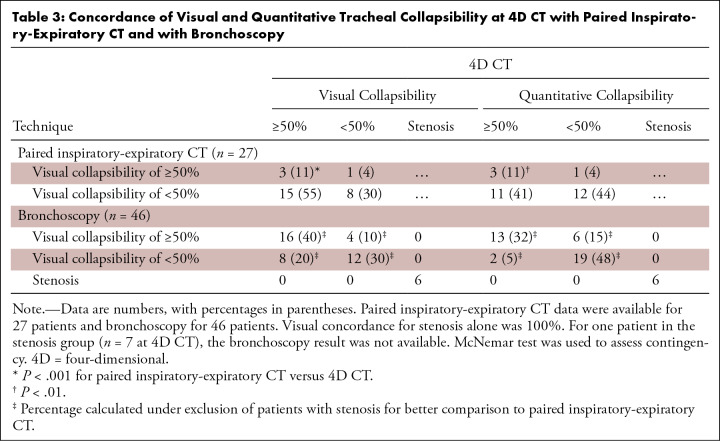
Table 3:
Concordance of Visual and Quantitative Tracheal Collapsibility at 4D CT with Paired Inspiratory-Expiratory CT and with Bronchoscopy
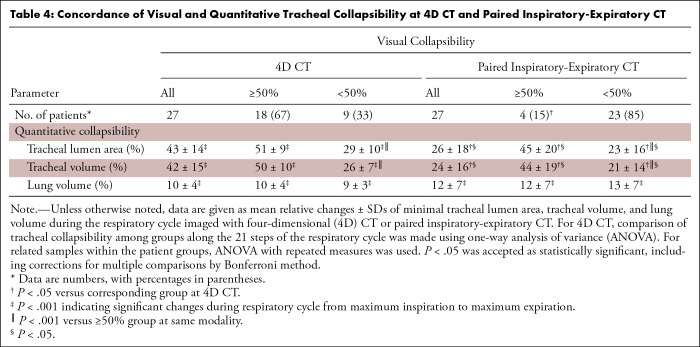
Quantification of paired inspiratory-expiratory CT found that three of 27 (11%) patients had 50% or greater collapsibility of the tracheal lumen area and one of 27 (4%) patients of the tracheal volume. Overall concordance of visual assessment and computer-aided quantification at paired inspiratory-expiratory CT was 22 of 27 (81%) patients. In patients with a mismatch of 50% or greater collapsibility, the distance to the 50% threshold was less than 5% in one patient for tracheal lumen area and in three patients for tracheal volume. On average, computer-aided quantification of paired inspiratory-expiratory CT revealed changes in tracheal lumen area of 26% ± 18 and tracheal volume of 24% ± 16 (Table 4). Tracheal lumen area was reduced on average by 45% ± 20 in the group with visual collapsibility of 50% or greater and by 23% ± 16 in the group with visual collapsibility less than 50%. Using tracheal volume did not yield different results as for tracheal lumen area (Table 4). Thus, the mean changes of tracheal lumen area and tracheal volume on paired inspiratory-expiratory CT did not reach the 50% or greater threshold, in contrast to 4D CT quantification.
Table 4:
Concordance of Visual and Quantitative Tracheal Collapsibility at 4D CT and Paired Inspiratory-Expiratory CT
Concordance with Bronchoscopy
In patients with fixed stenosis, the concordance of bronchoscopy and 4D CT was 100%. There was overall agreement of tracheal collapsibility of 50% or greater, less than 50%, and fixed stenosis in 34 of 46 (74%) patients. Excluding the patients diagnosed with fixed stenosis, bronchoscopy and 4D CT agreed for the diagnosis of 50% or greater tracheal collapse in 16 of 40 (40%) patients and for less than 50% in 12 of 40 (30%) patients, with an overall agreement in 28 of 40 (70%) patients. In four patients, tracheal collapsibility was classified as less severe at 4D CT than at bronchoscopy and vice versa in eight patients (Fig 3). Overall, concordance of visual assessment at paired inspiratory-expiratory CT and bronchoscopy was found in 13 of 27 (48%) patients. In both visual 4D CT evaluation and bronchoscopy, TBM was more often reported than EDAC (26 and 23 patients with TBM and eight and nine patients with EDAC, respectively). However, the definition of EDAC was often used interchangeably with TBM diagnosis at bronchoscopy, although they represent different physiologic and morphologic forms of collapse. Representative examples for each phenotype of tracheal collapsibility and stenosis are given with Figures 4–6, as well as with Figures S1 and S2 and Movies 1–10.
Figure 4:
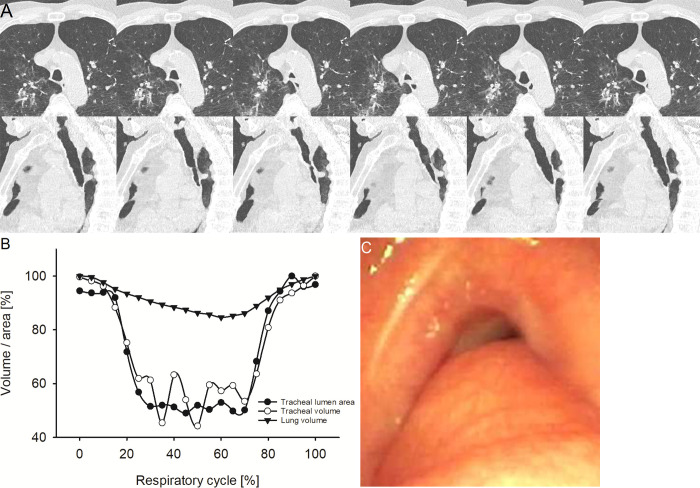
Representative example of circumferential-type tracheobronchomalacia (TBM). (A) Image from four-dimensional (4D) CT (noncontrast, lung window) in a 72-year-old male patient with circumferential-type TBM at the level of maximum observed tracheal collapsibility at representative selected time points of the respiratory cycle. The upper row shows axial view, the lower row sagittal view. The axial view demonstrates the circular collapse of the trachea because of cartilaginous malacia. (B) Graph shows the changes of minimal tracheal lumen area, tracheal volume, and lung volume in the same patient over the respiratory cycle with 21 time points in 5%-wide steps at 4D CT. (C) Image from correlated bronchoscopy.
Figure 6:
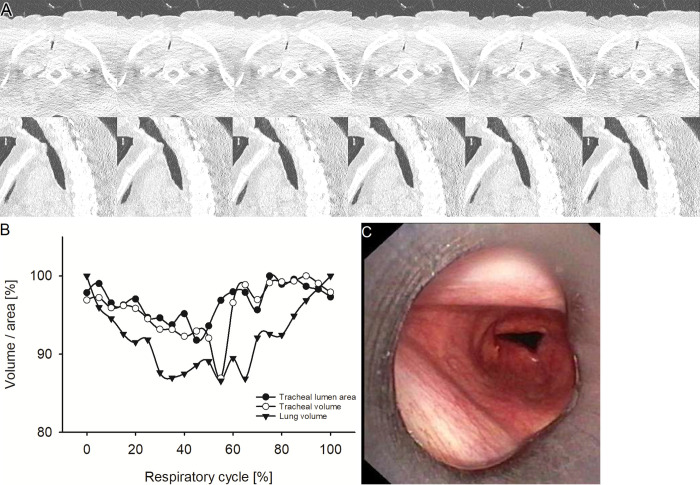
Representative example of fixed tracheal stenosis. (A) Images from four-dimensional (4D) CT (noncontrast, lung window) in a 53-year-old female patient with fixed tracheal stenosis at the level of maximum observed tracheal narrowing at representative selected time points of the respiratory cycle. The upper row shows axial view, the lower row sagittal view. No dynamic changes were observed. (B) Graph shows the changes of minimal tracheal lumen area, tracheal volume, and lung volume of the same patient over the respiratory cycle with 21 time points in 5%-wide steps at 4D CT. (C) Image from correlated bronchoscopy.
Movie 1:
4DCT (noncontrast, lung window) in axial orientation of a 72-year-old male patient with circumferential type TBM (same patient as in Fig 4) at the level of maximum observed tracheal collapsibility.
Movie 2:
4DCT (noncontrast, lung window) in sagittal orientation of a 72-year-old male patient with circumferential type TBM (same patient as in Fig 4).
Movie 3:
4DCT (noncontrast, lung window) in axial orientation of a 62-year-old male patient with crescent type TBM (same patient as in Fig S1) at the level of maximum observed tracheal collapsibility.
Movie 4:
4DCT (noncontrast, lung window) in sagittal orientation of a 62-year-old male patient with crescent type TBM (same patient as in Fig S1).
Movie 5:
4DCT (noncontrast, lung window) in axial orientation of a 76-year-old male patient with saber-sheath TBM (same patient as in Fig S2) at the level of maximum observed tracheal collapsibility.
Movie 6:
4DCT (noncontrast, lung window) in coronal orientation of a 76-year-old male patient with saber-sheath TBM (same patient as in Fig S2).
Movie 7:
4DCT (noncontrast, lung window) in axial orientation of a 51-year-old female patient with EDAC (same patient as in Fig 5) at the level of maximum observed tracheal collapsibility.
Figure 5:
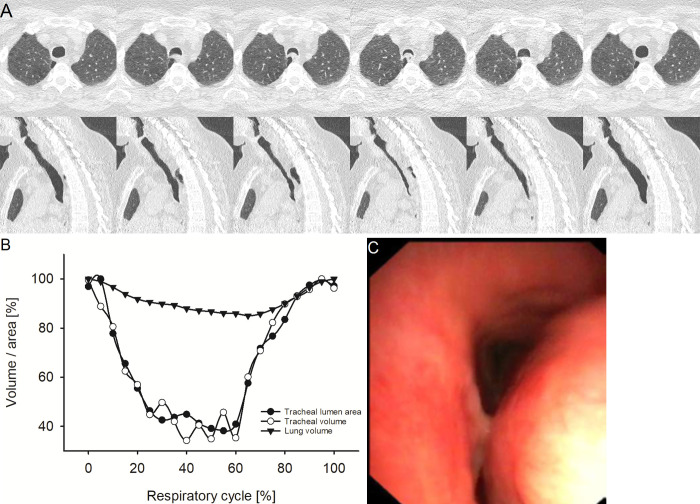
Representative example of excessive dynamic airway collapse (EDAC). (A) Images from four-dimensional (4D) CT (noncontrast, lung window) in a 51-year-old female patient with EDAC at the level of maximum observed tracheal collapsibility at representative selected time points of the respiratory cycle. The upper row shows axial view, the lower row sagittal view. The axial view demonstrates the inward bowing of the posterior membrane while the cartilage retains its normal shape. (B) Graph shows the changes of minimal tracheal lumen area, tracheal volume, and lung volume of the same patient over the respiratory cycle with 21 time points in 5%-wide steps at 4D CT. (C) Image from correlated bronchoscopy.
Movie 8:
4DCT (noncontrast, lung window) in sagittal orientation of a 51-year-old female patient with EDAC (same patient as in Fig 5).
Movie 9:
4DCT (noncontrast, lung window) in axial orientation of a 53-year-old female patient with fixed tracheal stenosis (same patient as in Fig 6) at the level of maximum observed tracheal narrowing.
Movie 10:
4DCT (noncontrast, lung window) in sagittal orientation of a 53-year-old female patient with fixed tracheal stenosis (same patient as in Fig 6).
Correlation of Tracheal Collapsibility with Clinical Data and Pulmonary Function Tests
A diagnosis of COPD was documented in 12 of 20 patients (60%) with a visual collapse less than 50% and 20 of 25 patients (80%) with a visual collapse of 50% or greater (Table 1). Patients with visual collapsibility of 50% or greater and less than 50% showed no evidence of a difference in FEV1% (37% ± 26 in 50% or greater vs 30% ± 10 in less than 50%, P = .44). Clinical dyspnea assessment tests (modified British Medical Research Council dyspnea scale, 6-minute walking distance test) did not correlate with tracheal collapsibility (Table S1). In patients with COPD, the quantitative emphysema index correlated inversely with FEV1% (rs = −0.50, P = .007). However, there was no correlation between the emphysema index and changes in tracheal lumen area or tracheal volume (rs = 0.17 and 0.15, P = .38 and .44, respectively).
Changes in tracheal lumen area and tracheal volume correlated inversely with FEV1% when including all patients regardless of visual airway collapsibility (rs = −0.44 and −0.43, respectively; each, P = .002) (Table S1). This result is mainly driven by the group with visual collapsibility less than 50% (rs = −0.46 and −0.30, P = .06 and .23, respectively). In patients with visual tracheal collapsibility of 50% or greater, quantitative changes of tracheal lumen area and tracheal volume did not correlate with spirometry parameters such as FEV1%, maximum vital capacity percentage predicted, or obstruction-related spirometric indexes such as maximum expiratory flow at 25% and 50% of vital capacity (P = .65–.99). The change in lung volume at 4D CT showed moderate to excellent correlations with FEV1% in all tracheal collapsibility groups (rs = 0.56–0.91, P = .021 to <.001; Table S1).
Discussion
We demonstrate the feasibility of retrospectively gated low-dose whole-chest 4D CT for the assessment of tracheal collapsibility. In a retrospective study setting, we found a moderate concordance of 74% for visual categorization with bronchoscopy and a good concordance with objective software-based quantification. In comparison to paired inspiratory-expiratory CT, 4D CT helped detect significantly more patients with a tracheal collapse based on visual (P < .001) and quantitative assessment (P = .009), and concordance was 41% and 55%, respectively. Moreover, change in tracheal area and volume in the group of visual collapsibility of 50% or greater expectedly did not correlate with spirometry (rs = −0.002 to 0.01, P = .99–.96 [Table S1]).
The type and severity of tracheal collapse was categorized in accordance with previous studies (6,8,26). Stern et al (31) described a wide range of dynamic changes of the trachea using cine CT imaging that would lead to a possible overdiagnosis of TBM in healthy or asymptomatic patients. Further, Boiselle et al (9,15) recommended a threshold of greater than 80% reduction of tracheal lumen area based on paired inspiratory and dynamic expiratory CT imaging findings in a cohort of healthy volunteers. A higher threshold of 50% reduction of the minimal tracheal lumen or more restrictive criteria were proposed to avoid false-positive findings, that is, clinically irrelevant results.
The concordance between visual and quantitative assessment in the change of tracheal lumen area and tracheal volume was good in classifying patients with tracheal collapsibility less than 50%. However, visual assessment classified more patients with a collapse of 50% or greater than quantitative assessment did. Individual differences in measurements or overinterpretation of a human reader versus quantification of the computer software could be an explanation. Also, the software quantifies true axial lumen and planes perpendicular to the tracheal axis, thus correcting for axis deviations similar to centerline analyses known from cardiovascular imaging. In addition, the quantitative changes of tracheal volume did not yield significantly different results from those of tracheal lumen area. However, we suggest assessing correlation between the changes in airway volume and clinical parameters in further studies, as airflow resistance as defined by the Hagen-Poiseuille equation may better be represented by changes in tracheal volume (ie, three dimensions) than changes only in minimal luminal area (ie, two dimensions). On the other hand, a high-grade stenosis of a short segment might be underrepresented by focusing on tracheal volume alone.
The initiation of the reduction of tracheal lumen area and volume were found mainly in early expiration, with a fast reduction over a short time followed by a relatively constant state until the beginning of inspiration. Sera et al (32) described a similar observation in their investigation of compliance of small airways in excised rat lungs. The authors summarize that small airways collapse at a specific pressure and compliance is dependent on the surrounding tissue. With respect to our cohort, the finding could implicate the same behavior for large airways, as this feature was found more prominent in patients with visual collapsibility of 50% or greater. However, these changes were also found in patients with visual collapsibility of less than 50% and irrespective of a diagnosis of COPD. This feature of early and sustained collapse from the beginning of expiration could be interpreted as meaning that a simple expiratory scan could be similarly suited to help detect airway collapse even in patients with not ideal expiration maneuvers. However, visual and quantitative assessment of paired inspiratory-expiratory CT scans identified far fewer patients with 50% or greater tracheal collapsibility compared with 4D CT in our study.
The quantitative changes in lung volume at paired CT were equal to those at 4D CT, indicating that the level of expiration was similar in both groups. Nevertheless, visually and using computer-aided quantification, 4D CT could help classify more patients with tracheal collapsibility using a threshold of 50% or greater. Hence, 4D CT in shallow breathing could be interpretated as at least not inferior to standard breath-hold expiratory CT. Further, our results emphasize findings in the literature suggesting that tracheal collapsibility at paired inspiratory-expiratory CT do not necessarily predict the severeness of tracheal collapsibility at dynamic imaging. As previous studies using 2D CT suggested, a dynamic imaging technique could be superior over static breath-hold CT for patients with clinically suspected tracheal collapsibility, especially in COPD (17,33). In light of the findings of the present study, 4D CT could be a suitable, potentially more sensitive technique than paired inspiratory-expiratory CT for the diagnosis of large airway collapsibility.
In the present study, there was no significant correlation of either clinical dyspnea assessment tests or spirometry with quantitative tracheal collapsibility in the group of patients with visual collapsibility of 50% or greater, which mainly consisted of patients with GOLD III and IV stage COPD with multiple comorbidities. The missing correlation of clinical parameters with instability of the large airways supports findings from other studies using different imaging techniques (9,34). Thus, our data encourage the notion that spirometry alone is not sufficient to detect large airway collapsibility in patients with COPD. Moreover, the missing correlation of FEV1% with clinically significant collapsibility may not represent the actual clinical impact in patients with severe COPD. Importantly, FEV1% relates to a forced expiration maneuver and might not represent the airway changes detected at 4D CT under shallow breathing (33). Investigation of changes of more peripheral airways, as well as parenchyma quantification, could be helpful in future studies to assess pathophysiologic significance of dynamic airway collapse in obstructive airway diseases. The significant correlation of the change in tracheal lumen area and tracheal volume with FEV1% in the overall study cohort should not be overemphasized. On the one hand, this could implicate a possible trend for a larger cohort. On the other hand, the presence of tracheal collapsibility should not be considered equal to clinical indications or symptoms of lung disease. The change in lung volume at 4D CT showed moderate to excellent correlations with FEV1% in all groups, which indicates that overall, dynamic and fixed stenoses of the trachea will lead to a reduction in forced volume.
4D CT was performed during shallow tidal breathing, resulting in small differences of quantitative assessed lung volume. O’Donnell et al (33) and Boiselle et al (35) investigated TBM by performing 2D CT during repeated forced coughing maneuvers, as these stress the weakness of the tracheobronchial tree and may reveal instability otherwise compensated for in shallow breathing. At present, retrospectively gated 4D CT does not yield sufficient temporal resolution for forced maneuvers, and no direct comparison of 4D CT in shallow breathing with forced 2D CT has been performed yet to address these questions (18). In contrast to the slow-spiral acquisition in the present study, 4D CT can be performed using large-area-detector CT without z-axis movement for the assessment of instabilities of the trachea and main bronchi during continuous acquisition of a full respiratory cycle (36–38). As a disadvantage, this technique is limited to investigation of the central airways in adults and excludes the assessment of lung volumes. Ley-Zaporozhan et al (39) used 4D CT of the whole chest with a technique using slow pitch and retrospective gating similar to our study for the investigation of changes of lung parenchyma density and emphysema parameters. The authors divided the respiratory cycle into 10%-wide steps with reduced radiation dose exposure and a smaller number of images as resulting advantages. A disadvantage for our focus on airway dynamics, though, would be possible information loss and missed maximum collapse because of reduced temporal resolution. Mean radiation dose at 4D CT in our study was 2.7 mSv ± 2.4, which is even lower compared with paired inspiratory-expiratory CT, which yields usually up to 5 mSv (3,18). Nevertheless, radiation dose is a relevant factor and stresses a careful indication for whole-chest 4D CT. As an alternative without radiation dose, time-resolved MRI could be considered, but this is currently not well-established and is limited to the central airways (40,41).
We acknowledge that our study had limitations. First, the included patient cohort was relatively small and heterogeneous. Under the retrospective setting, the available data were incomplete, thereby limiting statistical analyses. Second, in our monocentric retrospective study setting, the 4D CT technique showed excellent technical performance, but transferability to other centers remains to be shown. Third, 4D CT is of limited temporal resolution and therefore not appropriate for being combined with forced maneuvers (ie, cough, forced exhalation). Fourth, correlation of type and severity of tracheal collapse with bronchoscopy was limited under retrospective investigation because of the variety of description and lack of a standardized reporting system for bronchoscopy. Fifth, the 4D tool used is actually part of the heart function diagnostics in SyngoVia and is not particularly developed for airway imaging. A clinically available alternative is the 4D-Viewer by Osirix MD. To date, there are no available tools specifically designed for airway visualization with 4D CT, as this is a new indication.
In conclusion, our study demonstrates that low-dose whole-chest 4D CT is feasible for the diagnosis, localization, phenotyping, and computational quantification of dynamic collapsibility of the trachea during shallow breathing, showing moderate concordance with bronchoscopy. In patients with clinical suspicion of clinically significant tracheal collapsibility, 4D CT helped detect clinically significant tracheal collapsibility of 50% or greater more accurately than paired inspiratory-expiratory CT on visual assessment and with computer-aided quantification. As a technique in shallow breathing, it can be more easily tolerated than examinations with forced maneuvers, especially in patients with comorbidities. Objective quantification showed good concordance with visual grading but did not correlate with spirometry for a collapsibility of 50% or greater. With computer-aided quantification, 4D CT may aid in identifying patients with clinically significant tracheal collapse as a possible cause for persisting symptoms of tracheal collapse despite optimal conservative therapy. Further, it may be used for precise monitoring of these pathologies and as a possible tool for planning of regional interventions such as stent placement or development of emerging therapeutic options.
Supported by grants from the German Federal Ministry of Education and Research (BMBF) (82DZL004A1). Funders had no involvement in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the article for publication.
Disclosures of conflicts of interest: A.B. No relevant relationships. O.W. Airway analysis technology is licensed to Imbio. A.D. No relevant relationships. R.R. No relevant relationships. H.U.K. Support for the present manuscript from the German Center of Lung Research, paid to author’s institution. D.G. Grant from Olympus for bronchoscopy courses; lecture fees from Pulmonx, Olympus, AstraZeneca, Chiesi, Berlin Chemie, MSD, and Boehringer Ingelheim. R.E. No relevant relationships. F.T. No relevant relationships. C.P.H. Consultation or other fees from Schering-Plough (2009, 2010), Pfizer (2008–2014), Basilea (2008, 2009, 2010), Boehringer Ingelheim (2010–2024), Novartis (2010, 2012, 2014), Roche (2010), Astellas (2011, 2012), Gilead (2011–2015), MSD (2011–2013), Lilly (2011), Intermune (2013–2014), and Fresenius (2013–2014); research funding from Siemens (2012–2014), Pfizer (2012–2014), MeVis (2012, 2013), Boehringer Ingelheim (2014), and Exscientia (2023–2024); lecture fees from Gilead (2008–2014), Essex (2008, 2009, 2010), Schering-Plough (2008, 2009, 2010), AstraZeneca (2008–2014, 2022), Lilly (2008, 2009, 2012), Roche (2008, 2009), MSD (2009–2014), Pfizer (2010–2014, 2023–2024), Bracco (2010, 2011), MEDA Pharma (2011), Intermune (2011–2014), Chiesi (2012), Siemens (2012), Covidien (2012), Pierre Fabre (2012), Boehringer Ingelheim (2012–2024), Grifols (2012), Novartis (2013–2016), Basilea (2015, 2016), and Bayer (2016); patent: “Method and Device for Representing the Microstructure of the Lungs,” IPC8 class: AA61B5055FI, PAN: 20080208038, co-inventor; committee membership on the Chest Working Group of the German Roentgen Society; consultant of ECIL-3, ECCMID, and EORTC/MSG; founding member of the working team in infections in immunocompromised hosts of the German Society of Hematology/Oncology; faculty member of the European Society of Thoracic Radiology (ESTI), European Respiratory Society (ERS), and European Imaging Biomarkers Alliance (EIBALL); editor of Medizinische Klinik, Intensivmedizin und Notfallmedizin (Springer); stock or stock options in GSK. F.J.F.H. No relevant relationships. M.H. No relevant relationships. F.F. Support for the present manuscript from the University of Lübeck; grants or contracts from the University of Lübeck; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Technische Hochscule Lübeck; support for attending meetings and/or travel from the University of Lübeck; receipt of equipment, material, drugs, medical writing, gifts, or other services from the University of Lübeck. M.O.W. Study grant from Vertex Pharmaceuticals, paid to author’s institution; consulting fees from Vertex Pharmaceuticals, paid to author’s institution; leadership or fiduciary role in ESTI, IWPFI, and DRG (unpaid).
Abbreviations:
- ANOVA
- analysis of variance
- COPD
- chronic obstructive pulmonary disease
- EDAC
- excessive dynamic airway collapse
- FEV1%
- forced expiratory volume in 1 second percentage predicted
- 4D
- four-dimensional
- GOLD
- Global Initiative for Chronic Obstructive Lung Disease
- MEF
- maximum expiratory flow
- TBM
- tracheobronchomalacia
- 2D
- two-dimensional
References
- 1. Aquino SL , Shepard JA , Ginns LC , et al . Acquired tracheomalacia: detection by expiratory CT scan . J Comput Assist Tomogr 2001. ; 25 ( 3 ): 394 – 399 . [DOI] [PubMed] [Google Scholar]
- 2. Boiselle PM , Litmanovich DE , Michaud G , et al . Dynamic expiratory tracheal collapse in morbidly obese COPD patients . COPD 2013. ; 10 ( 5 ): 604 – 610 . [DOI] [PubMed] [Google Scholar]
- 3. Kauczor HU , Wielpütz MO , Owsijewitsch M , Ley-Zaporozhan J . Computed tomographic imaging of the airways in COPD and asthma . J Thorac Imaging 2011. ; 26 ( 4 ): 290 – 300 . [DOI] [PubMed] [Google Scholar]
- 4. Leong P , Tran A , Rangaswamy J , et al . Expiratory central airway collapse in stable COPD and during exacerbations . Respir Res 2017. ; 18 ( 1 ): 163 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Raoof S , Shah M , Braman S , et al . Lung imaging in COPD part 2: emerging concepts . Chest 2023. ; 164 ( 2 ): 339 – 354 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murgu SD , Colt HG . Tracheobronchomalacia and excessive dynamic airway collapse . Respirology 2006. ; 11 ( 4 ): 388 – 406 . [DOI] [PubMed] [Google Scholar]
- 7. Kalra A , Abouzgheib W , Gajera M , Palaniswamy C , Puri N , Dellinger RP . Excessive dynamic airway collapse for the internist: new nomenclature or different entity? Postgrad Med J 2011. ; 87 ( 1029 ): 482 – 486 . [DOI] [PubMed] [Google Scholar]
- 8. Leong P , Bardin PG , Lau KK . What’s in a name? Expiratory tracheal narrowing in adults explained . Clin Radiol 2013. ; 68 ( 12 ): 1268 – 1275 . [DOI] [PubMed] [Google Scholar]
- 9. Boiselle PM , Michaud G , Roberts DH , et al . Dynamic expiratory tracheal collapse in COPD: correlation with clinical and physiologic parameters . Chest 2012. ; 142 ( 6 ): 1539 – 1544 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lynch DA , Austin JH , Hogg JC , et al . CT-definable subtypes of chronic obstructive pulmonary disease: a statement of the Fleischner Society . Radiology 2015. ; 277 ( 1 ): 192 – 205 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heidinger BH , Occhipinti M , Eisenberg RL , Bankier AA . Imaging of large airways disorders . AJR Am J Roentgenol 2015. ; 205 ( 1 ): 41 – 56 . [DOI] [PubMed] [Google Scholar]
- 12. Boiselle PM , Ernst A . State-of-the-art imaging of the central airways . Respiration 2003. ; 70 ( 4 ): 383 – 394 . [DOI] [PubMed] [Google Scholar]
- 13. Hysinger EB , Hart CK , Burg G , De Alarcon A , Benscoter D . Differences in flexible and rigid bronchoscopy for assessment of tracheomalacia . Laryngoscope 2021. ; 131 ( 1 ): 201 – 204 . [DOI] [PubMed] [Google Scholar]
- 14. Kugler C , Stanzel F . Tracheomalacia . Thorac Surg Clin 2014. ; 24 ( 1 ): 51 – 58 . [DOI] [PubMed] [Google Scholar]
- 15. Boiselle PM , O’Donnell CR , Bankier AA , et al . Tracheal collapsibility in healthy volunteers during forced expiration: assessment with multidetector CT . Radiology 2009. ; 252 ( 1 ): 255 – 262 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ciet P , Boiselle PM , Michaud G , O’Donnell C , Litmanovich DE . Optimal imaging protocol for measuring dynamic expiratory collapse of the central airways . Clin Radiol 2016. ; 71 ( 1 ): e49 – e55 . [DOI] [PubMed] [Google Scholar]
- 17. Heussel CP , Hafner B , Lill J , Schreiber W , Thelen M , Kauczor HU . Paired inspiratory/expiratory spiral CT and continuous respiration cine CT in the diagnosis of tracheal instability . Eur Radiol 2001. ; 11 ( 6 ): 982 – 989 . [DOI] [PubMed] [Google Scholar]
- 18. Wielpütz MO , Eberhardt R , Puderbach M , Weinheimer O , Kauczor HU , Heussel CP . Simultaneous assessment of airway instability and respiratory dynamics with low-dose 4D-CT in chronic obstructive pulmonary disease: a technical note . Respiration 2014. ; 87 ( 4 ): 294 – 300 . [DOI] [PubMed] [Google Scholar]
- 19. Iwasawa T , Yoshiike Y , Saito K , Kagei S , Gotoh T , Matsubara S . Paradoxical motion of the hemidiaphragm in patients with emphysema . J Thorac Imaging 2000. ; 15 ( 3 ): 191 – 195 . [DOI] [PubMed] [Google Scholar]
- 20. Singh D , Agusti A , Anzueto A , et al . Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019 . Eur Respir J 2019. ; 53 ( 5 ): 1900164 . [DOI] [PubMed] [Google Scholar]
- 21. Miller MR , Hankinson J , Brusasco V , et al . Standardisation of spirometry . Eur Respir J 2005. ; 26 ( 2 ): 319 – 338 . [DOI] [PubMed] [Google Scholar]
- 22. Quanjer PH , Stanojevic S , Cole TJ , et al . Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations . Eur Respir J 2012. ; 40 ( 6 ): 1324 – 1343 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eberhardt R , Holland A , Petermann C , Bornitz F , Gesierich W . Interventional bronchoscopy-an overview [in German] . Pneumologe (Berl) 2021. ; 18 ( 6 ): 405 – 418 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wälscher J , Gompelmann D . Bronchoscopy [in German] . Dtsch Med Wochenschr 2016. ; 141 ( 17 ): 1236 – 1238 . [DOI] [PubMed] [Google Scholar]
- 25. Shepard JO , Flores EJ , Abbott GF . Imaging of the trachea . Ann Cardiothorac Surg 2018. ; 7 ( 2 ): 197 – 209 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sverzellati N , Rastelli A , Chetta A , et al . Airway malacia in chronic obstructive pulmonary disease: prevalence, morphology and relationship with emphysema, bronchiectasis and bronchial wall thickening . Eur Radiol 2009. ; 19 ( 7 ): 1669 – 1678 . [DOI] [PubMed] [Google Scholar]
- 27. Jobst BJ , Weinheimer O , Buschulte T , et al . Longitudinal airway remodeling in active and past smokers in a lung cancer screening population . Eur Radiol 2019. ; 29 ( 6 ): 2968 – 2980 . [DOI] [PubMed] [Google Scholar]
- 28. Konietzke P , Weinheimer O , Wielpütz MO , et al . Quantitative CT detects changes in airway dimensions and air-trapping after bronchial thermoplasty for severe asthma . Eur J Radiol 2018. ; 107 : 33 – 38 . [DOI] [PubMed] [Google Scholar]
- 29. Weinheimer O , Achenbach T , Düber C . Fully automated extraction of airways from CT scans based on self-adapting region growing . Second International Workshop on Pulmonary Image Analysis, part of MICCAI ; London , 2009. . https://www.lungworkshop.org/2009/proc2009/315.pdf . [Google Scholar]
- 30. Wielpütz MO , Eichinger M , Weinheimer O , et al . Automatic airway analysis on multidetector computed tomography in cystic fibrosis: correlation with pulmonary function testing . J Thorac Imaging 2013. ; 28 ( 2 ): 104 – 113 . [DOI] [PubMed] [Google Scholar]
- 31. Stern EJ , Graham CM , Webb WR , Gamsu G . Normal trachea during forced expiration: dynamic CT measurements . Radiology 1993. ; 187 ( 1 ): 27 – 31 . [DOI] [PubMed] [Google Scholar]
- 32. Sera T , Fujioka H , Yokota H , et al . Localized compliance of small airways in excised rat lungs using microfocal X-ray computed tomography . J Appl Physiol 2004. ; 96 ( 5 ): 1665 – 1673 . [DOI] [PubMed] [Google Scholar]
- 33. O’Donnell CR , Bankier AA , O’Donnell DH , Loring SH , Boiselle PM . Static end-expiratory and dynamic forced expiratory tracheal collapse in COPD . Clin Radiol 2014. ; 69 ( 4 ): 357 – 362 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yamashiro T , Moriya H , Tsubakimoto M , Matsuoka S , Murayama S . Continuous quantitative measurement of the proximal airway dimensions and lung density on four-dimensional dynamic-ventilation CT in smokers . Int J Chron Obstruct Pulmon Dis 2016. ; 11 : 755 – 764 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boiselle PM , Lee KS , Lin S , Raptopoulos V . Cine CT during coughing for assessment of tracheomalacia: preliminary experience with 64-MDCT . AJR Am J Roentgenol 2006. ; 187 ( 2 ): W175 – W177 . [DOI] [PubMed] [Google Scholar]
- 36. Lee EY , Zucker EJ , Restrepo R , Daltro P , Boiselle PM . Advanced large airway CT imaging in children: evolution from axial to 4-D assessment . Pediatr Radiol 2013. ; 43 ( 3 ): 285 – 297 . [DOI] [PubMed] [Google Scholar]
- 37. Uddin AK , Mansfield DR , Farmer MW , Lau KK . Primary tracheobronchial amyloidosis associated with tracheobronchomegaly evaluated by novel four-dimensional functional CT . Respirol Case Rep 2015. ; 3 ( 4 ): 151 – 154 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wagnetz U , Roberts HC , Chung T , Patsios D , Chapman KR , Paul NS . Dynamic airway evaluation with volume CT: initial experience . Can Assoc Radiol J 2010. ; 61 ( 2 ): 90 – 97 . [DOI] [PubMed] [Google Scholar]
- 39. Ley-Zaporozhan J , Ley S , Mews J , Weinheimer O , Kandel S , Rogalla P . Changes of emphysema parameters over the respiratory cycle during free breathing: preliminary results using respiratory gated 4D-CT . COPD 2017. ; 14 ( 6 ): 597 – 602 . [DOI] [PubMed] [Google Scholar]
- 40. Ciet P , Boiselle PM , Heidinger B , et al . Cine MRI of tracheal dynamics in healthy volunteers and patients with tracheobronchomalacia . AJR Am J Roentgenol 2017. ; 209 ( 4 ): 757 – 761 . [DOI] [PubMed] [Google Scholar]
- 41. Heussel CP , Ley S , Biedermann A , et al . Respiratory lumenal change of the pharynx and trachea in normal subjects and COPD patients: assessment by cine-MRI . Eur Radiol 2004. ; 14 ( 12 ): 2188 – 2197 . [DOI] [PubMed] [Google Scholar]