Graphical Abstract
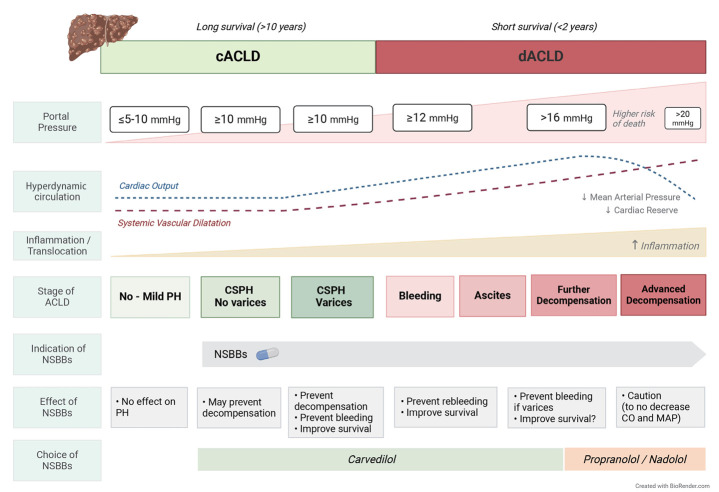
Advanced chronic liver disease (ACLD) progresses over time, from a compensated stage (cACLD) to development of decompensation (dACLD), markedly declining life expectancy [1,2]. Portal hypertension (PH), usually estimated by the hepatic-venous pressure gradient (HVPG), is the main determinant leading to decompensation [2-4]. Variceal bleeding, overt ascites (or pleural effusion), and/or overt encephalopathy define decompensation [2]. An HVPG ≥10 mmHg defines clinically significant PH (CSPH), the main substage of cACLD, since varices and decompensating events develop above this threshold [2]. The presence of varices identifies a substage of cACLD with CSPH, since patients with varices have an increased risk of decompensation [5,6].
Increased hepatic vascular resistance is the primary factor leading to PH in early cACLD, and is related to liver fibrosis with architectural distortion, endothelial dysfunction, and vascular occlusion [7,8]. At this stage, mild increases in portal pressure activate vasodilatory and angiogenic signals, developing portosystemic collaterals and progressive splanchnic vasodilatation. The ensuing increase in portal blood flow leads to hyperdynamic circulation, exacerbating PH [6-8]. The persistence of etiological/co-etiological factors, such as obesity, diabetes or alcohol consumption, by facilitating systemic delivery of PAMPs and DAMPs, as well as bacterial translocation induced by PH, may favor the release of pro-inflammatory cytokines [9]. This may further increase intrahepatic vascular resistance and exacerbate splanchnic vasodilatation and hyperdynamic circulation, worsening PH and eventually leading to decompensation [8-10]. Cardiac output progressively increases until the advanced stages of dACLD, when cardiac compensatory reserve may be reduced, mainly in stressful situations such as severe bacterial infections or acute-on-chronicl iver failure, which may negatively impact survival [11,12].
Among patients with cACLD, hyperdynamic circulation is more developed in those with CSPH than in those with mild PH (HVPG between 5 and 10 mmHg) [6], and among patients with CSPH is more accentuated in those with varices [6,12]. Non-selective beta-blockers (NSBBs) decrease PH by β1-adrenergic blockade (reducing heart rate and cardiac output) and by β2-adrenergic blockade, causing splanchnic vasoconstriction due to unopposed adrenergic tone [13-15]. NSBBs have a portal-pressure-decreasing effect once CSPH has developed, but have a minimal effect in patients with mild PH, when hyperdynamic circulation is poorly developed [6]. The HVPG-lowering effect of NSBBs is also smaller in decompensated vs. compensated patients [11]. This may be related to vascular dysfunction in dACLD, with hypo-contractility induced by dysregulation of vasoactive proteins [16]. Altogether, indicates that patients with cACLD and CSPH may benefit the most from NSBBs.
Preventing complications of PH is the goal of therapy in cACLD. This is particularly relevant in patients with CSPH and mainly in those with varices, due to their higher risk of decompensation [2]. Strong evidence supports the efficacy of NSBBs to prevent bleeding in cirrhosis with high-risk varices [13-18]. Furthermore, the PREDESCI study demonstrated that NSBBs can also prevent decompensation in cACLD with CSPH, with a 50% risk reduction. This was mainly achieved by preventing ascites, the most frequent and severe decompensation in cACLD [19]. Subsequent studies reinforce the value of NSBBs to prevent decompensation [20,21]. At present, CSPH can be confirmed non-invasively, mainly relying on liver stiffness measurement (LSM) by transient elastography [18,22]. LSM ≤15 KPa plus platelets ≥150x109/L rule-out CSPH, and LSM of ≥25 KPa rule it in quite accurately [18,22]. Detecting varices by endoscopy or collateral circulation by imaging also identifies patients with CSPH [2]. In the PREDESCI study, the benefit of NSBBs in cACLD was consistent in patients either with or without small varices, but was more apparent with small varices, probably due to the higher risk of decompensation [19].
In patients with high-risk varices, both NSBBs and endoscopic variceal ligation (EVL) have similar efficacy to prevent a first bleeding in RCTs [13,14,17]. A recent individual patient data (IPD) meta-analysis (MA) of RCTs comparing NSBBs vs. EVL for primary prophylaxis, stratified risk according to cirrhosis decompensation [23]. This IPD-MA, by optimizing the assessment of cirrhosis as a multistate disease and that of outcomes as time-dependent events, demonstrated a significant reduction of mortality risk by half in cACLD favoring NSBBs over EVL [23]. This was mainly due to a decreased risk of ascites, while the risk of bleeding was similar. The benefit did not improve by adding EVL to NSBBs [23]. These results strongly support for the preference of NSBBs over EVL in cACLD with high-risk varices, because NSBBs further reduce the risk of ascites and improve survival, in addition to a similar bleeding risk.
Carvedilol is the preferred NSBB in cACLD. It has anti-αadrenergic activity and also enhances intrahepatic NO release, inducing a decrease in intra-hepatic vascular-resistance [24], a key factor leading to inducing PH in cACLD [6,8]. Carvedilol has a greater portal-pressure-decreasing effect than classical-NSBBs, such as propranolol or nadolol, and may achieve a hemodynamic response in previous non-responders to classical-NSBBs [24,25]. Furthermore, carvedilol has additional antioxidant, anti-inflammatory, and antifibrotic effects [26,27]. A recent IPD-MA has investigated the efficacy of carvedilol in cACLD with CSPH, including RCTs comparing carvedilol with a control group receiving no active therapy (in patients with small varices or without varices) or EVL (if high-risk varices) [28]. This IPD-MA has demonstrated that carvedilol can effectively prevent decompensation and significantly improves survival in cACLD with CSPH [28]. This supports the strategy of screening patients with cACLD for CSPH to start therapy with carvedilol, as suggested in the last Baveno meeting.
The goal of treatment in dACLD is to prevent death. This implies preventing further decompensation, which is closely related to death. Whether NSBBs may be effective in dACLD without varices has not been clarified. In patients with dACLD and high-risk varices, NSBBs are no better than EVL to prevent first bleeding, according to a previously commented IPD-MA [23]. After variceal bleeding, current guidelines advise combining NSBBs and EVL to prevent rebleeding [2,3]. According to another IPD-MA, adding NSBBs to EVL significantly decreases rebleeding risk and improves survival compared with EVL monotherapy, particularly in Child-Pugh B/C [29]. In this IPD-MA, NSBBs monotherapy performed as well as combined therapy [29], suggesting that NSBBs are the cornerstone of treatment in patients with previous bleeding. The benefit of NSBBs is particularly relevant in patients with a marked decrease in HVPG [30,31], and guiding therapy based on HVPG response has been suggested to potentially improve efficacy [32]. Nevertheless, the limited availability and invasiveness limit such strategy [33]. Identifying non-responders using non-invasive tools is an unmet clinical need, since this is a promising strategy to guide therapy, particularly in the high-risk setting of dACLD [18,22].
In patients with advanced ascites, NSBBs should be dose-reduced or discontinued if persistently low arterial pressure (systolic <90 mmHg), or if renal impairment [2]. This is also the case in patients with intercurrent conditions determining hemodynamic instability, such as bleeding, SBP or other severe infections [13,14]. After recovery, NSBBs should be re-started at lower doses and under close monitoring. Carvedilol, given its non-selective vasodilatory effect, may increase sodium and water retention in patients with advanced ascites, when classical NSBBs may be preferable [24]. Patients with dACLD and contraindication/intolerance to NSBBs should be considered for trans-jugular intrahepatic porto-systemic shunt [34-36], particularly those with uncontrolled ascites or recurrent decompensation.
Abbreviations
- CI
confidence interval
- CSPH
clinically significant portal hypertension
- FU
follow-up
- HVPG
hepatic venous pressure gradient
- INR
international normalized ratio
- MELD
model for end stage liver disease
- NSBBs
non-selective β-blockers
- OLT
orthotopic liver transplantation
- PH
portal hypertension
- RCT
randomized controlled trial
- SD
standard deviation
- SHR
subdistribution hazard ratio
Footnotes
Authors’ contribution
A.B. manuscript writing and approval.
C.V. concept of the work, manuscript writing and approval.
Conflicts of Interest
The authors have no conflicts to disclose.
REFERENCES
- 1.D’Amico G, Morabito A, D’Amico M, Pasta L, Malizia G, Rebora P, et al. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68:563–576. doi: 10.1016/j.jhep.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 2.de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C, Baveno VII Faculty Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76:959–974. doi: 10.1016/j.jhep.2021.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaplan DE, Ripoll C, Thiele M, Fortune BE, Simonetto DA, Garcia-Tsao G, et al. AASLD Practice Guidance on risk stratification and management of portal hypertension and varices in cirrhosis. Hepatology. 2024;79:1180–1211. doi: 10.1097/HEP.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–335. doi: 10.1002/hep.28906. [DOI] [PubMed] [Google Scholar]
- 5.D’Amico G, Pasta L, Morabito A, D’Amico M, Caltagirone M, Malizia G, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39:1180–1193. doi: 10.1111/apt.12721. [DOI] [PubMed] [Google Scholar]
- 6.Villanueva C, Albillos A, Genescà J, Abraldes JG, Calleja JL, Aracil C, et al. Development of hyperdynamic circulation and response to β-blockers in compensated cirrhosis with portal hypertension. Hepatology. 2016;63:197–206. doi: 10.1002/hep.28264. [DOI] [PubMed] [Google Scholar]
- 7.European Association for the Study of the Liver EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Felli E, Nulan Y, Selicean S, Wang C, Gracia-Sancho J, Bosch J. Emerging therapeutic targets for portal hypertension. Curr Hepatol Rep. 2023;22:51–66. doi: 10.1007/s11901-023-00598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albillos A, Martin-Mateos R, Van der Merwe S, Wiest R, Jalan R, Álvarez-Mon M. Cirrhosis-associated immune dysfunction. Nat Rev Gastroenterol Hepatol. 2022;19:112–134. doi: 10.1038/s41575-021-00520-7. [DOI] [PubMed] [Google Scholar]
- 10.Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272–1284. doi: 10.1016/j.jhep.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Alvarado-Tapias E, Ardevol A, Garcia-Guix M, Montañés R, Pavel O, Cuyas B, et al. Short-term hemodynamic effects of β-blockers influence survival of patients with decompensated cirrhosis. J Hepatol. 2020;73:829–841. doi: 10.1016/j.jhep.2020.03.048. [DOI] [PubMed] [Google Scholar]
- 12.Turco L, Garcia-Tsao G, Magnani I, Bianchini M, Costetti M, Caporali C, et al. Cardiopulmonary hemodynamics and C-reactive protein as prognostic indicators in compensated and decompensated cirrhosis. J Hepatol. 2018;68:949–958. doi: 10.1016/j.jhep.2017.12.027. [DOI] [PubMed] [Google Scholar]
- 13.Rabiee A, Garcia-Tsao G, Tapper EB. Nonselective beta-blockers in portal hypertension: Why, when, and how? Clin Liver Dis (Hoboken) 2022;19:118–123. doi: 10.1002/cld.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigues SG, Mendoza YP, Bosch J. Beta-blockers in cirrhosis: Evidence-based indications and limitations. JHEP Rep. 2019;2:100063. doi: 10.1016/j.jhepr.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Albillos A, Krag A. Beta-blockers in the era of precision medicine in patients with cirrhosis. J Hepatol. 2023;78:866–872. doi: 10.1016/j.jhep.2022.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Trebicka J, von Heydebrand M, Lehmann J, Tofteng F, Busk T, Jensen HL, et al. Assessment of response to beta-blockers by expression of βArr2 and RhoA/ROCK2 in antrum mucosa in cirrhotic patients. J Hepatol. 2016;64:1265–1273. doi: 10.1016/j.jhep.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 17.Sharma M, Singh S, Desai V, Shah VH, Kamath PS, Murad MH, et al. Comparison of therapies for primary prevention of esophageal variceal bleeding: A systematic review and network meta-analysis. Hepatology. 2019;69:1657–1675. doi: 10.1002/hep.30220. [DOI] [PubMed] [Google Scholar]
- 18.Abraldes JG, Caraceni P, Ghabril M, Garcia-Tsao G. Update in the treatment of the complications of cirrhosis. Clin Gastroenterol Hepatol. 2023;21:2100–2109. doi: 10.1016/j.cgh.2023.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Villanueva C, Albillos A, Genescà J, Garcia-Pagan JC, Calleja JL, Aracil C, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393:1597–1608. doi: 10.1016/S0140-6736(18)31875-0. [DOI] [PubMed] [Google Scholar]
- 20.Rowe IA, Villanueva C, Shearer JE, Torres F, Albillos A, Genescà J, et al. Quantifying the benefit of nonselective beta-blockers in the prevention of hepatic decompensation: A Bayesian reanalysis of the PREDESCI trial. Hepatology. 2023;78:530–539. doi: 10.1097/HEP.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 21.Serper M, Kaplan DE, Taddei TH, Tapper EB, Cohen JB, Mahmud N. Nonselective beta blockers, hepatic decompensation, and mortality in cirrhosis: A national cohort study. Hepatology. 2023;77:489–500. doi: 10.1002/hep.32737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong YJ, Zhaojin C, Tosetti G, Degasperi E, Sharma S, Agarwal S, et al. Baveno-VII criteria to predict decompensation and initiate non-selective beta-blocker in compensated advanced chronic liver disease patients. Clin Mol Hepatol. 2023;29:135–145. doi: 10.3350/cmh.2022.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Villanueva C, Sapena V, Lo GH, Seo YS, Shah HA, Singh V, et al. Improving primary prophylaxis of variceal bleeding by adapting therapy to the clinical stage of cirrhosis. A competing-risk meta-analysis of individual participant data. Aliment Pharmacol Ther. 2024;59:306–321. doi: 10.1111/apt.17824. [DOI] [PubMed] [Google Scholar]
- 24.Villanueva C, Tripathi D. Carvedilol, probably the β-blocker of choice for everyone with cirrhosis and portal hypertension: But not so fast! Liver Int. 2023;43:1154–1156. doi: 10.1111/liv.15582. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie SL, Hanrahan TP, Rockey DC, Majumdar A, Hayes PC. Review article: controversies surrounding the use of carvedilol and other beta blockers in the management of portal hypertension and cirrhosis. Aliment Pharmacol Ther. 2023;57:454–463. doi: 10.1111/apt.17380. [DOI] [PubMed] [Google Scholar]
- 26.McDowell HR, Chuah CS, Tripathi D, Stanley AJ, Forrest EH, Hayes PC. Carvedilol is associated with improved survival in patients with cirrhosis: a long-term follow-up study. Aliment Pharmacol Ther. 2021;53:531–539. doi: 10.1111/apt.16189. [DOI] [PubMed] [Google Scholar]
- 27.Turco L, Reiberger T, Vitale G, La Mura V. Carvedilol as the new non-selective beta-blocker of choice in patients with cirrhosis and portal hypertension. Liver Int. 2023;43:1183–1194. doi: 10.1111/liv.15559. [DOI] [PubMed] [Google Scholar]
- 28.Villanueva C, Torres F, Shah HA, Tripathi D, Sarin SK, Brujats A, et al. Carvedilol improves risk of decompensation and survival in compensated cirrhosis. A competing-risk meta-analysis of individual patient data. J Hepatol. 2021;75:S378–S380. [Google Scholar]
- 29.Albillos A, Zamora J, Martínez J, Arroyo D, Ahmad I, De-la-Peña J, et al. Stratifying risk in the prevention of recurrent variceal hemorrhage: Results of an individual patient meta-analysis. Hepatology. 2017;66:1219–1231. doi: 10.1002/hep.29267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandorfer M, Hernández-Gea V, Reiberger T, García-Pagán JC. Hepatic venous pressure gradient response in non-selective beta-blocker treatment—Is it worth measuring? Curr Hepatology Rep. 2019;18:174–186. [Google Scholar]
- 31.Turco L, Villanueva C, La Mura V, García-Pagán JC, Reiberger T, Genescà J, et al. Lowering portal pressure improves outcomes of patients with cirrhosis, with or without ascites: A meta-analysis. Clin Gastroenterol Hepatol. 2020;18:313–327.e6. doi: 10.1016/j.cgh.2019.05.050. [DOI] [PubMed] [Google Scholar]
- 32.Villanueva C, Graupera I, Aracil C, Alvarado E, Miñana J, Puente Á, et al. A randomized trial to assess whether portal pressure guided therapy to prevent variceal rebleeding improves survival in cirrhosis. Hepatology. 2017;65:1693–1707. doi: 10.1002/hep.29056. [DOI] [PubMed] [Google Scholar]
- 33.La Mura V, Garcia-Guix M, Berzigotti A, Abraldes JG, García-Pagán JC, Villanueva C, et al. A prognostic strategy based on stage of cirrhosis and HVPG to improve risk stratification after variceal bleeding. Hepatology. 2020;72:1353–1365. doi: 10.1002/hep.31125. [DOI] [PubMed] [Google Scholar]
- 34.Lee EW, Eghtesad B, Garcia-Tsao G, Haskal ZJ, Hernandez-Gea V, Jalaeian H, et al. AASLD Practice Guidance on the use of TIPS, variceal embolization, and retrograde transvenous obliteration in the management of variceal hemorrhage. Hepatology. 2024;79:224–250. doi: 10.1097/HEP.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 35.Lee HL, Lee SW. The role of transjugular intrahepatic portosystemic shunt in patients with portal hypertension: Advantages and pitfalls. Clin Mol Hepatol. 2022;28:121–134. doi: 10.3350/cmh.2021.0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Larrue H, D’Amico G, Olivas P, Lv Y, Bucsics T, Rudler M, et al. TIPS prevents further decompensation and improves survival in patients with cirrhosis and portal hypertension in an individual patient data meta-analysis. J Hepatol. 2023;79:692–703. doi: 10.1016/j.jhep.2023.04.028. [DOI] [PubMed] [Google Scholar]