Abstract
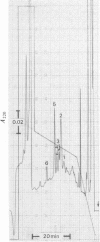
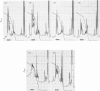
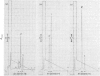
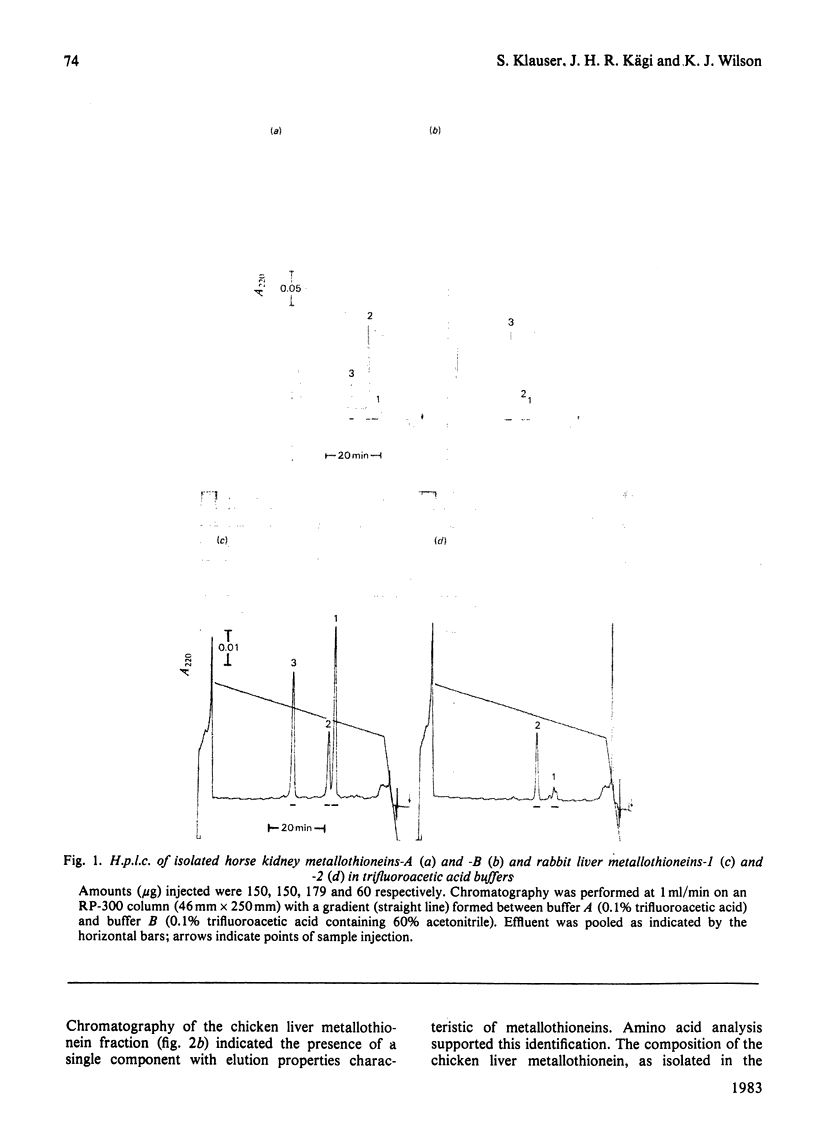
Reverse-phase high-pressure ('performance') liquid chromatography was used to characterize the isometallothioneins in preparations isolated from tissues of a variety of animals by 'conventional' chromatographic methods. The resolution was such that isoproteins differing by a single serine leads to leucine difference in 61 residues could be easily separated. Yields from the reverse-phase support were typically 60-70% for the isoproteins. Comparisons of isometallothionein patterns after Cd2+-induction in rabbits indicated that total metallothionein concentrations were about 4-fold higher in liver than kidney extracts from the same animal. In the extracts a minimum of four and six isometallothionein peaks were detected in kidney and liver respectively. Under acidic conditions, where the metals are removed from the protein, the chromatographic properties, i.e. hydrophobicities, of the isoproteins from kidney were identical with those of four of those found in liver. Although the same peaks appeared in tissue extracts from individual animals, concentration differences were apparent. Remarkably, no differences were observed between the isoprotein patterns of liver or kidney as a consequence of either Cd2+- or Zn2+-induction. Chromatography of the metal-containing forms at neutral pH in Tris buffer indicated that the relative ratios of the complexed metal ions in the isoproteins were found to be effectively identical, not only before and after chromatography, but also within the separated forms from a single tissue source.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen R. D., Winter W. P., Maher J. J., Bernstein I. A. Turnover of metallothioneins in rat liver. Biochem J. 1978 Jul 15;174(1):327–338. doi: 10.1042/bj1740327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler R. H., Kägi J. H. Human hepatic metallothioneins. FEBS Lett. 1974 Feb 15;39(2):229–234. doi: 10.1016/0014-5793(74)80057-8. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem. 1981 Jun 10;256(11):5712–5716. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Glanville N., Durnam D. M., Palmiter R. D. Structure of mouse metallothionein-I gene and its mRNA. Nature. 1981 Jul 16;292(5820):267–269. doi: 10.1038/292267a0. [DOI] [PubMed] [Google Scholar]
- Kissling M. M., Kägi H. R. Primary structure of human hepatic metallothionein. FEBS Lett. 1977 Oct 15;82(2):247–250. doi: 10.1016/0014-5793(77)80594-2. [DOI] [PubMed] [Google Scholar]
- Kägi J. H., Himmelhoch S. R., Whanger P. D., Bethune J. L., Vallee B. L. Equine hepatic and renal metallothioneins. Purification, molecular weight, amino acid composition, and metal content. J Biol Chem. 1974 Jun 10;249(11):3537–3542. [PubMed] [Google Scholar]
- Otvos J. D., Armitage I. M. Structure of the metal clusters in rabbit liver metallothionein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7094–7098. doi: 10.1073/pnas.77.12.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overnell J., Coombs T. L. Purification and properties of plaice metallothionein, a cadmium-binding protein from the liver of the plaice (Pleuronectes platessa). Biochem J. 1979 Nov 1;183(2):277–283. doi: 10.1042/bj1830277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piotrowski J. K., Bolanowska W., Sapota A. Evaluation of metallothionein content in animal tissues. Acta Biochim Pol. 1973;20(3):207–215. [PubMed] [Google Scholar]
- Suzuki K. T., Yamamura M. Changes of metal contents and isometallothionein levels in rat tissues after cadmium loading. Biochem Pharmacol. 1980 Sep 15;29(18):2407–2412. doi: 10.1016/0006-2952(80)90342-1. [DOI] [PubMed] [Google Scholar]
- Vasák M., Galdes A., Hill H. A., Kägi J. H., Bremner I., Young B. W. Investigation of the structure of metallothioneins by proton nuclear magnetic resonance spectroscopy. Biochemistry. 1980 Feb 5;19(3):416–425. doi: 10.1021/bi00544a003. [DOI] [PubMed] [Google Scholar]
- Vasák M., Kägi J. H. Metal thiolate clusters in cobalt(II)-metallothionein. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6709–6713. doi: 10.1073/pnas.78.11.6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K. J., Honegger A., Hughes G. J. Comparison of buffers and detection systems for high-pressure liquid chromatography of peptide mixtures. Biochem J. 1981 Oct 1;199(1):43–51. doi: 10.1042/bj1990043. [DOI] [PMC free article] [PubMed] [Google Scholar]