Abstract
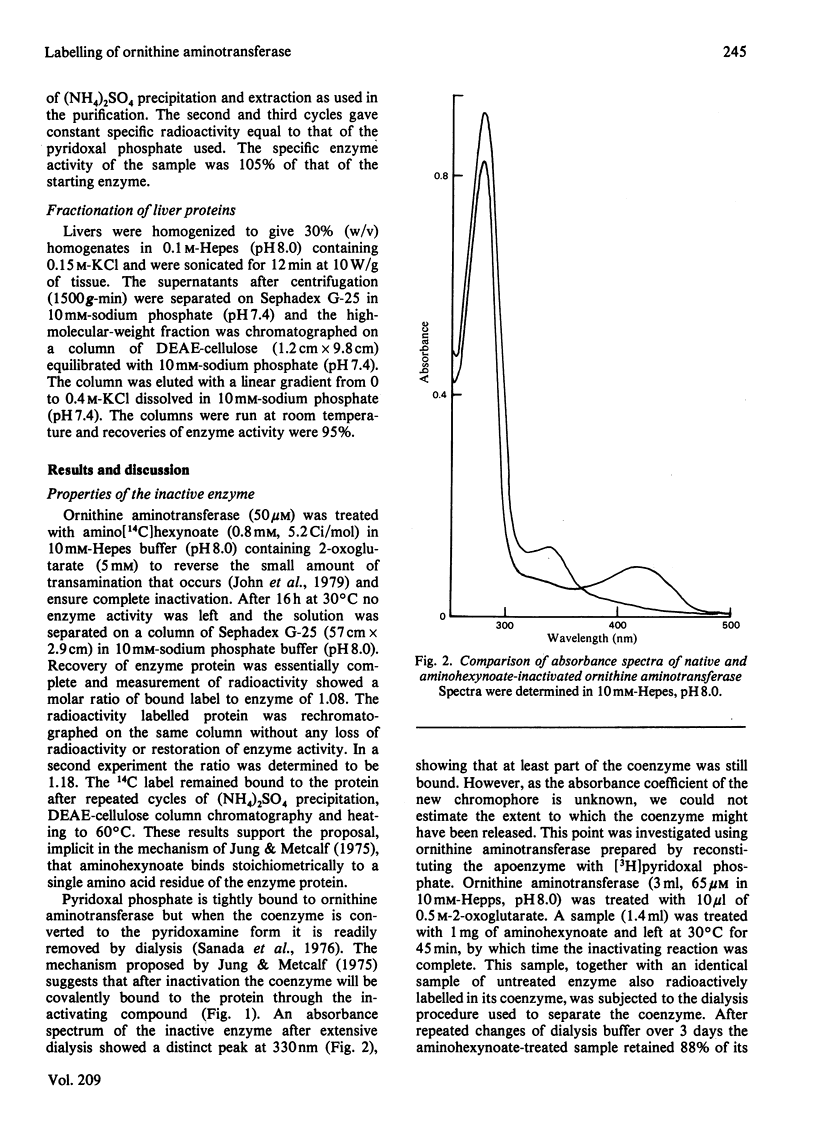
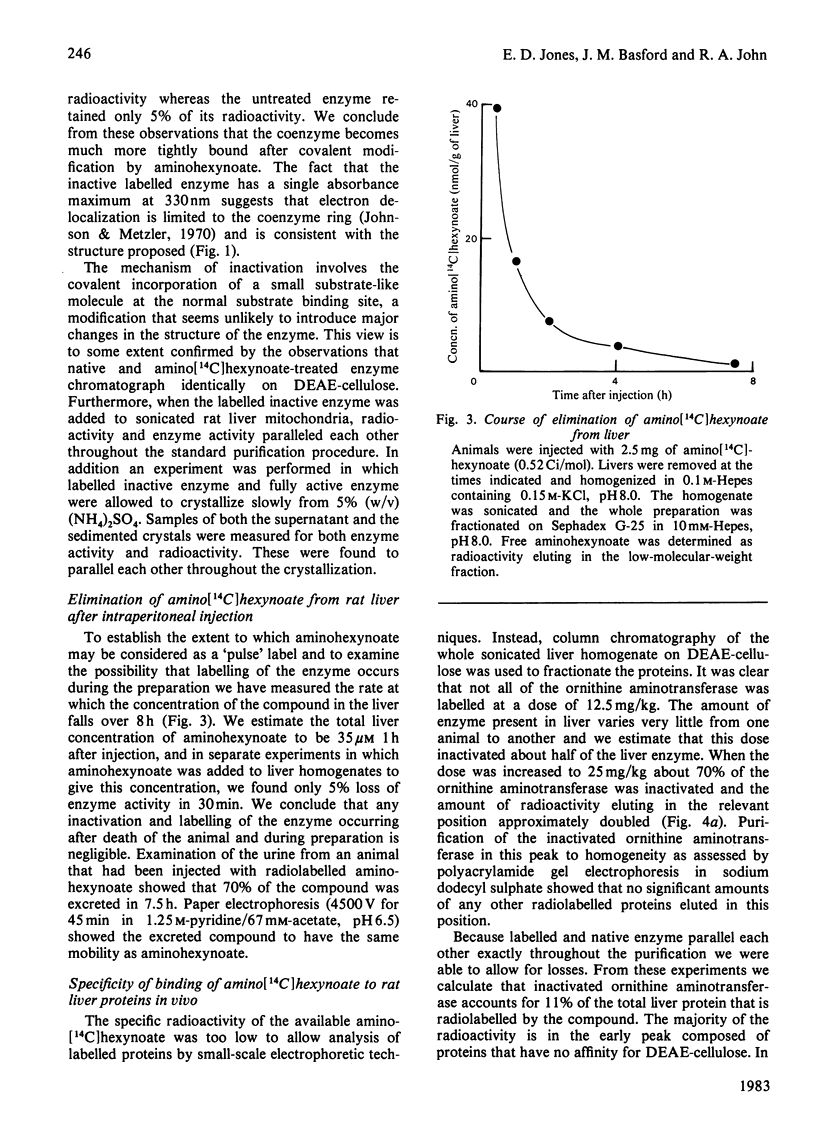
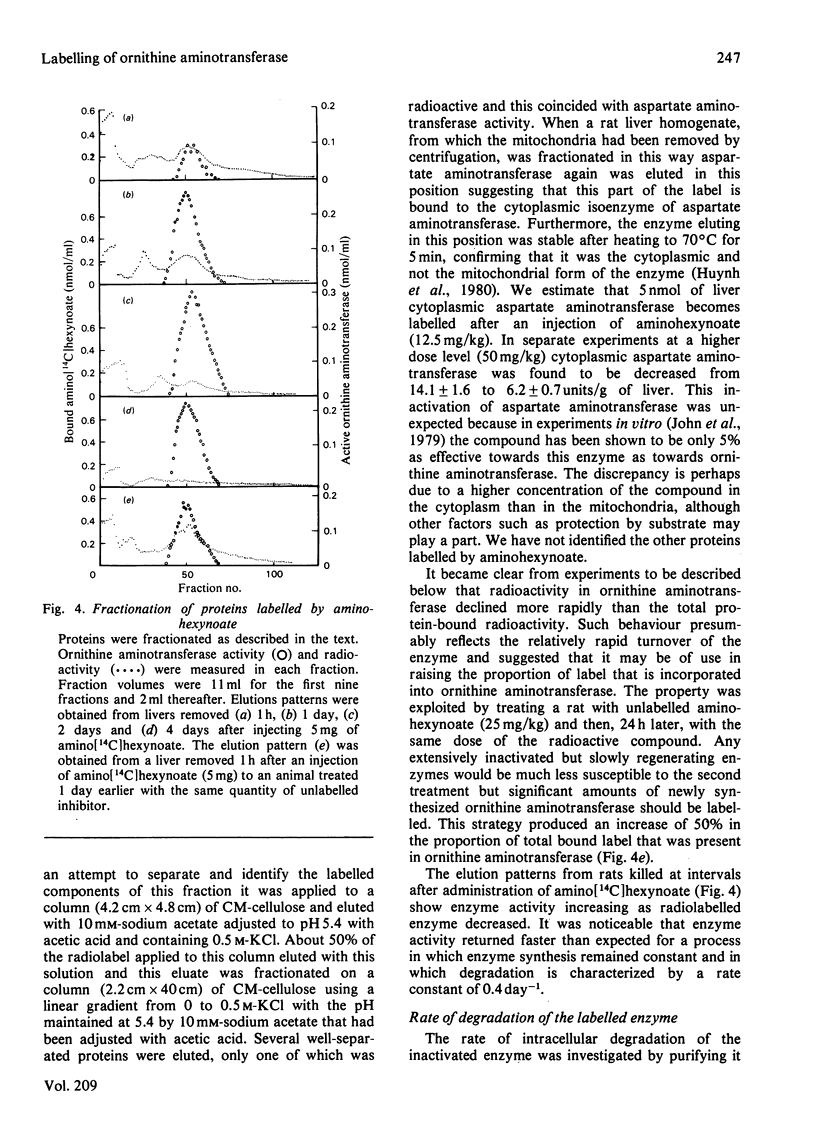
Ornithine aminotransferase is shown to bind 1 mol of amino[14C]hexynoate per mol of coenzyme in the 'suicide' inactivation process. At the same time the coenzyme pyridoxal phosphate becomes irreversibly bound to the enzyme protein. Apart from the inactivation, the labelled enzyme is indistinguishable from native ornithine aminotransferase by several separation techniques. Because the rate of degradation of the labelled enzyme is the same as that of the normal enzyme it is concluded that loss of coenzyme does not initiate turnover. Free aminohexynoate is rapidly eliminated from the liver, and 70% of the compound is excreted unchanged in 7.5 h. Inactivated ornithine aminotransferase accounts for 11% of the total labelled liver protein and significant amounts of label are found in aspartate aminotransferase which is also extensively inactivated. The rate of return of enzyme activity is determined and found to be more rapid than expected for a process in which the enzyme is synthesized at a constant rate and degraded in a single, first-order process.
Full text
PDF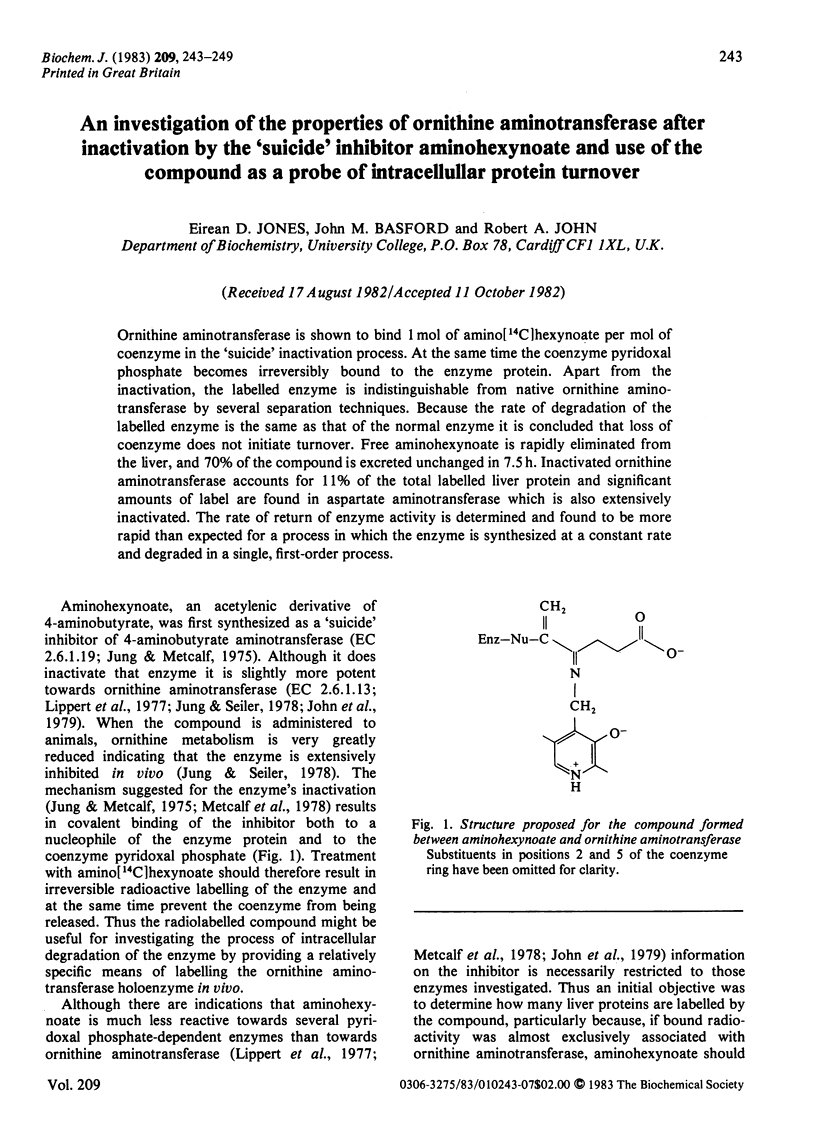


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlin C. M., Schimke R. T. Influence of turnover rates on the responses of enzymes to cortisone. Mol Pharmacol. 1965 Sep;1(2):149–156. [PubMed] [Google Scholar]
- Chee P. Y., Swick R. W. Effect of dietary protein and tryptophan and the turnover of rat liver ornithine aminotransferase. J Biol Chem. 1976 Feb 25;251(4):1029–1034. [PubMed] [Google Scholar]
- Huynh Q. K., Sakakibara R., Watanabe T., Wada H. Glutamic oxaloacetic transaminase isozymes from rat liver. Purification and physicochemical characterization. J Biochem. 1980 Jul;88(1):231–239. [PubMed] [Google Scholar]
- John R. A., Jones E. D., Fowler L. J. Enzyme-induced inactivation of transminases by acetylenic and vinyl analogues of 4-aminobutyrate. Biochem J. 1979 Feb 1;177(2):721–728. doi: 10.1042/bj1770721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung M. J., Metcalf B. W. Catalytic inhibition of gamma-aminobutyric acid - alpha-ketoglutarate transaminase of bacterial origin by 4-aminohex-5-ynoic acid, a substrate analog. Biochem Biophys Res Commun. 1975 Nov 3;67(1):301–306. doi: 10.1016/0006-291x(75)90316-2. [DOI] [PubMed] [Google Scholar]
- Jung M. J., Seiler N. Enzyme-activated irreversible inhibitors of L-ornithine:2-oxoacid aminotransferase. Demonstration of mechanistic features of the inhibition of ornithine aminotransferase by 4-aminohex-5-ynoic acid and gabaculine and correlation with in vivo activity. J Biol Chem. 1978 Oct 25;253(20):7431–7439. [PubMed] [Google Scholar]
- KARMEN A. A note on the spectrometric assay of glutamic-oxalacetic transaminase in human blood serum. J Clin Invest. 1955 Jan;34(1):131–133. [PubMed] [Google Scholar]
- Lippert B., Metcalf B. W., Jung M. J., Casara P. 4-amino-hex-5-enoic acid, a selective catalytic inhibitor of 4-aminobutyric-acid aminotransferase in mammalian brain. Eur J Biochem. 1977 Apr 15;74(3):441–445. doi: 10.1111/j.1432-1033.1977.tb11410.x. [DOI] [PubMed] [Google Scholar]
- Litwack G., Rosenfield S. Coenzyme dissociation, a possible determinant of short half-life of inducible enzymes in mammalian liver. Biochem Biophys Res Commun. 1973 May 1;52(1):181–188. doi: 10.1016/0006-291x(73)90971-6. [DOI] [PubMed] [Google Scholar]
- Martinez-Carrion M., Turano C., Chiancone E., Bossa F., Giartosio A., Riva F., Fasella P. Isolation and characterization of multiple forms of glutamate-asparate aminotransferase from pig heart. J Biol Chem. 1967 May 25;242(10):2397–2409. [PubMed] [Google Scholar]
- Peraino C., Bunville L. G., Tahmisian T. N. Chemical, physical, and morphological properties of ornithine Aminotransferase from rat liver. J Biol Chem. 1969 May 10;244(9):2241–2249. [PubMed] [Google Scholar]
- SAKAMI W., LAFAYE J. M. The metabolism of acetone in the intact rat. J Biol Chem. 1951 Nov;193(1):199–203. [PubMed] [Google Scholar]
- SEGAL H. L., KIM Y. S. GLUCOCORTICOID STIMULATION OF THE BIOSYNTHESIS OF GLUTAMIC-ALANINE TRANSAMINASE. Proc Natl Acad Sci U S A. 1963 Nov;50:912–918. doi: 10.1073/pnas.50.5.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRECKER H. J. PURIFICATION AND PROPERTIES OF RAT LIVER ORNITHINE DELTA-TRANSAMINASE. J Biol Chem. 1965 Mar;240:1225–1230. [PubMed] [Google Scholar]
- Swick R. W., Rexroth A. K., Stange J. L. The metabolism of mitochondrial proteins. 3. The dynamic state of rat liver mitochondria. J Biol Chem. 1968 Jul 10;243(13):3581–3587. [PubMed] [Google Scholar]
- Williams J. A., Bridge G., Fowler L. J., John R. A. The reaction of ornithine aminotransferase with ornithine. Biochem J. 1982 Jan 1;201(1):221–225. doi: 10.1042/bj2010221. [DOI] [PMC free article] [PubMed] [Google Scholar]


