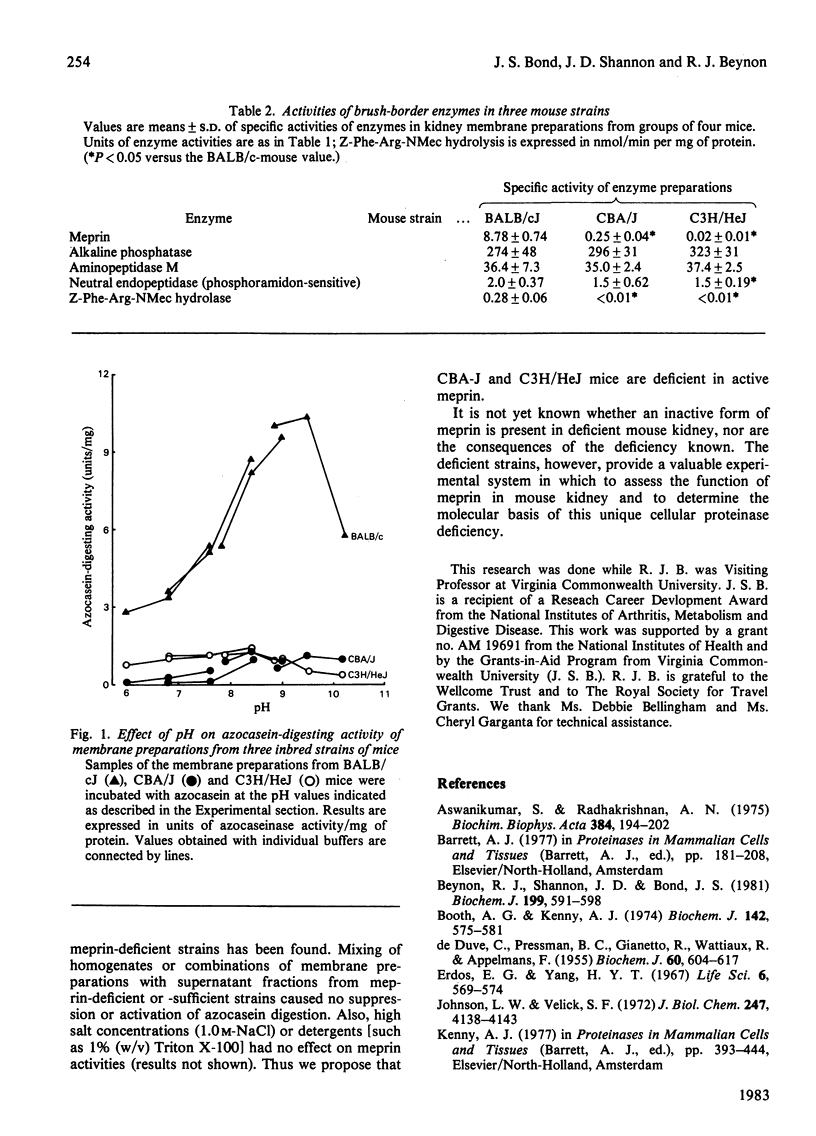
Abstract
Preparations of microvilli from kidneys of BALB/c mice contain an alkaline metallo-endopeptidase, meprin (metallo-endopeptidase from renal tissue). Certain genealogically related inbred mice are markedly deficient in meprin activity. The meprin-deficient strains (CBA/J and C3H/HeJ) exhibit normal levels of other brush-border enzymes: alkaline phosphatase, aminopeptidase M and another proteinase, a phosphoramidon-sensitive neutral endopeptidase. Meprin deficiency cannot be attributed to a shift in pH optimum and is unlikely to be due to the presence of endogenous inhibitors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aswanikumar S., Radhakrishnan A. N. Purification and properties of a peptidase acting on a synthetic substrate for collagenase from monkey kidney. Biochim Biophys Acta. 1975 Mar 28;384(1):194–202. doi: 10.1016/0005-2744(75)90108-4. [DOI] [PubMed] [Google Scholar]
- Beynon R. J., Shannon J. D., Bond J. S. Purification and characterization of a metallo-endoproteinase from mouse kidney. Biochem J. 1981 Dec 1;199(3):591–598. doi: 10.1042/bj1990591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth A. G., Kenny A. J. A rapid method for the preparation of microvilli from rabbit kidney. Biochem J. 1974 Sep;142(3):575–581. doi: 10.1042/bj1420575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdos E. G., Yang H. Y. An enzyme in microsomal fraction of kidney that inactivates bradykinin. Life Sci. 1967 Mar 15;6(6):569–574. doi: 10.1016/0024-3205(67)90090-2. [DOI] [PubMed] [Google Scholar]
- Johnson L. W., Velick S. F. The synthesis and degradation of fructose diphosphate-aldolase and glyceraldehyde 3-phosphate dehydrogenase in rabbit liver. J Biol Chem. 1972 Jul 10;247(13):4138–4143. [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The molecular weight and properties of a neutral metallo-endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):489–495. doi: 10.1042/bj1370489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr M. A., Kenny A. J. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem J. 1974 Mar;137(3):477–488. doi: 10.1042/bj1370477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Place G. A., Beynon R. J. The effect of ionic environment on pig heart mitochondrial malate dehydrogenase. Int J Biochem. 1982;14(4):305–309. doi: 10.1016/0020-711x(82)90091-x. [DOI] [PubMed] [Google Scholar]
- Sogawa K., Ichihara Y., Takahashi K. Comparison of some characteristics of membrane-bound neutral proteinase activity in the microsomal fractions of rat kidney and small intestine. J Biochem. 1981 Nov;90(5):1243–1248. doi: 10.1093/oxfordjournals.jbchem.a133588. [DOI] [PubMed] [Google Scholar]
- Varandani P. T., Shroyer L. A. A rat kidney neutral peptidase that degrades B chain of insulin, glucagon, and ACTH: purification by affinity chromatography and some properties. Arch Biochem Biophys. 1977 May;181(1):82–93. doi: 10.1016/0003-9861(77)90486-6. [DOI] [PubMed] [Google Scholar]



