Abstract
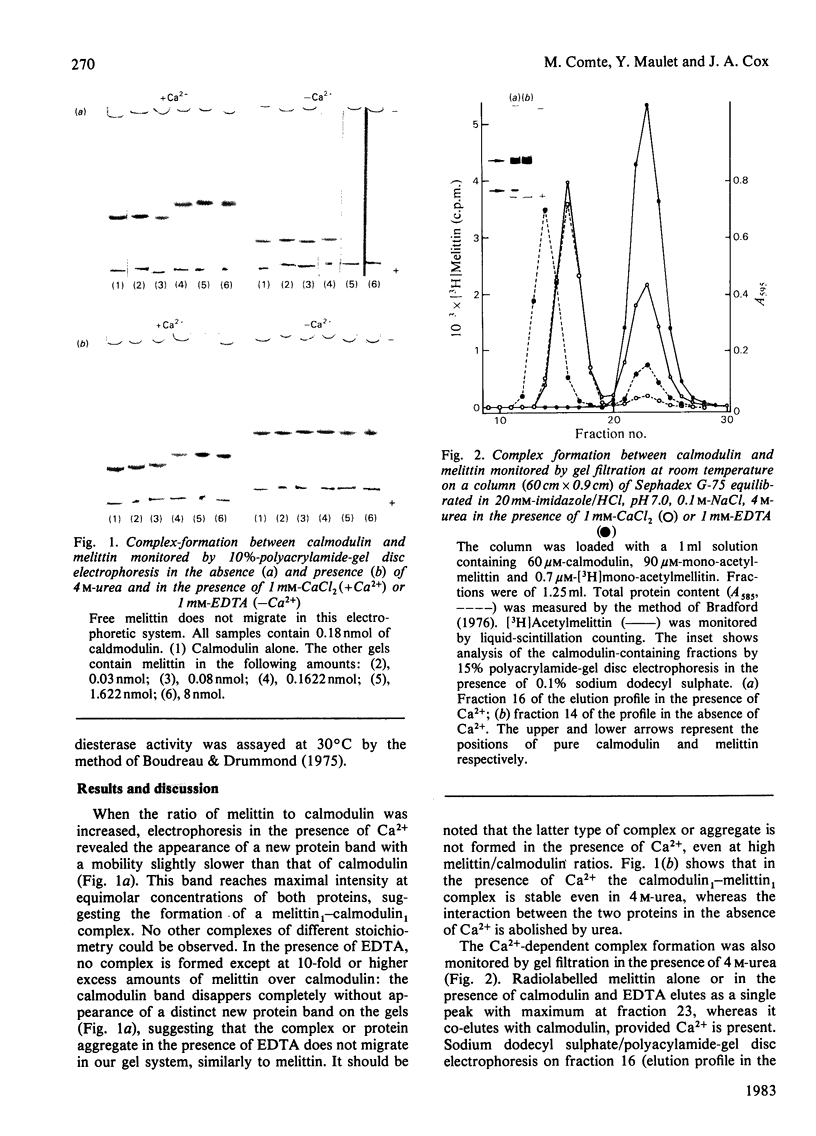
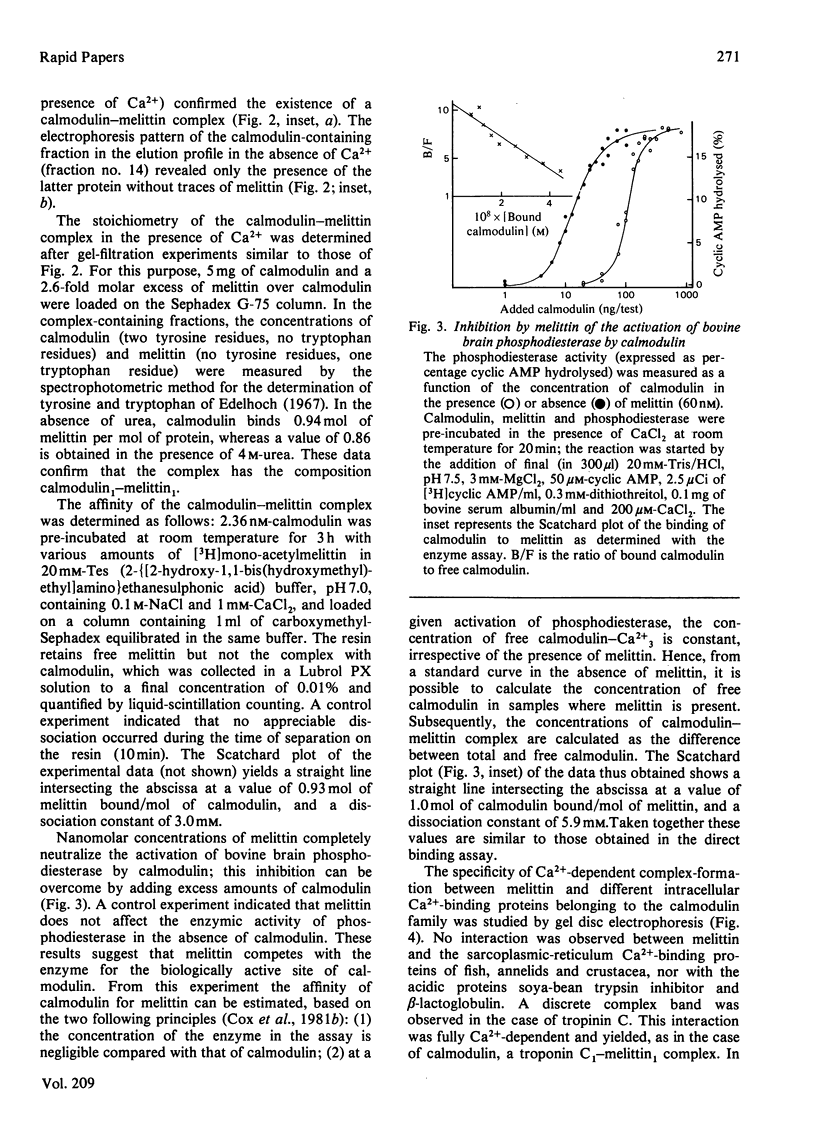
The amphiphatic polypeptide melittin migrates as an equimolar complex with bovine brain calmodulin when monitored by gel disc electrophoresis or gel filtration in the presence of Ca2+, even in 4M-urea. The complex disassociates in the presence of EDTA and urea. The affinity is of the same order as that of calmodulin for its target enzymes, and more than 1000-fold higher than that of calmodulin for basic peptide hormones or hydrophobic drugs. The activation of brain phosphodiesterase by calmodulin is inhibited by melittin. The kinetics of inhibition suggest competition between the enzyme and melittin for calmodulin. The calmodulin-melittin interaction may constitute a model for that existing between calmodulin and its target enzymes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. G., Kenrick J. R. Melittin : an inhibitor of chloroplast photochemical reactions. Biochem Biophys Res Commun. 1980 Dec 16;97(3):1082–1090. doi: 10.1016/0006-291x(80)91486-2. [DOI] [PubMed] [Google Scholar]
- Blumenthal D. K., Stull J. T. Effects of pH, ionic strength, and temperature on activation by calmodulin an catalytic activity of myosin light chain kinase. Biochemistry. 1982 May 11;21(10):2386–2391. doi: 10.1021/bi00539a017. [DOI] [PubMed] [Google Scholar]
- Boone L. R., Skalka A. Two species of full-length cDNA are synthesized in high yield by melittin-treated avian retrovirus particles. Proc Natl Acad Sci U S A. 1980 Feb;77(2):847–851. doi: 10.1073/pnas.77.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau R. J., Drummond G. I. A modified assay of 3':5'-cyclic-AMP phosphodiesterase. Anal Biochem. 1975 Feb;63(2):388–399. doi: 10.1016/0003-2697(75)90361-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown L. R., Lauterwein J., Wüthrich K. High-resolution 1H-NMR studies of self-aggregation of melittin in aqueous solution. Biochim Biophys Acta. 1980 Apr 25;622(2):231–244. doi: 10.1016/0005-2795(80)90034-3. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of calcium ions, calmodulin and troponin in the regulation of phosphorylase kinase from rabbit skeletal muscle. Eur J Biochem. 1980 Oct;111(2):563–574. doi: 10.1111/j.1432-1033.1980.tb04972.x. [DOI] [PubMed] [Google Scholar]
- Cox J. A., Comte M., Stein E. A. Activation of human erythrocyte Ca2+-dependent Mg2+-activated ATPase by calmodulin and calcium: quantitative analysis. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4265–4269. doi: 10.1073/pnas.79.14.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. A., Comte M., Stein E. A. Calmodulin-free skeletal-muscle troponin C prepared in the absence of urea. Biochem J. 1981 Apr 1;195(1):205–211. doi: 10.1042/bj1950205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. A., Malnoë A., Stein E. A. Regulation of brain cyclic nucleotide phosphodiesterase by calmodulin. A quantitative analysis. J Biol Chem. 1981 Apr 10;256(7):3218–3222. [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Perry S. V. The binding of calmodulin to myelin basic protein and histone H2B. Biochem J. 1980 Aug 1;189(2):227–240. doi: 10.1042/bj1890227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head J. F., Perry S. V. The interaction of the calcium-binding protein (troponin C) with bivalent cations and the inhibitory protein (troponin I). Biochem J. 1974 Feb;137(2):145–154. doi: 10.1042/bj1370145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head J. F., Weeks R. A., Perry S. V. Affinity-chromatographic isolation and some properties of troponin C from different muscle types. Biochem J. 1977 Mar 1;161(3):465–471. doi: 10.1042/bj1610465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh N., Raynor R. L., Wise B. C., Schatzman R. C., Turner R. S., Helfman D. M., Fain J. N., Kuo J. F. Inhibition by melittin of phospholipid-sensitive and calmodulin-sensitive Ca2+-dependent protein kinases. Biochem J. 1982 Jan 15;202(1):217–224. doi: 10.1042/bj2020217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lad P. L., Shier W. T. Effect of melittin-induced membrane alterations on rat heart adenylate cyclase activity. Arch Biochem Biophys. 1980 Oct 15;204(2):418–424. doi: 10.1016/0003-9861(80)90052-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R. Binding of simple peptides, hormones, and neurotransmitters by calmodulin. Biochemistry. 1982 Jul 6;21(14):3480–3486. doi: 10.1021/bi00257a035. [DOI] [PubMed] [Google Scholar]
- Malencik D. A., Anderson S. R., Bohnert J. L., Shalitin Y. Functional interactions between smooth muscle myosin light chain kinase and calmodulin. Biochemistry. 1982 Aug 17;21(17):4031–4039. doi: 10.1021/bi00260a019. [DOI] [PubMed] [Google Scholar]
- Malnoë A., Cox J. A., Stein E. A. Ca2+-dependent regulation of calmodulin binding and adenylate cyclase activation in bovine cerebellar membranes. Biochim Biophys Acta. 1982 Jan 12;714(1):84–92. doi: 10.1016/0304-4165(82)90129-5. [DOI] [PubMed] [Google Scholar]
- Marcum J. M., Dedman J. R., Brinkley B. R., Means A. R. Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3771–3775. doi: 10.1073/pnas.75.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshak D. R., Watterson D. M., Van Eldik L. J. Calcium-dependent interaction of S100b, troponin C, and calmodulin with an immobilized phenothiazine. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6793–6797. doi: 10.1073/pnas.78.11.6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulet Y., Mathey-Prevot B., Kaiser G., Rüegg U. T., Fulpius B. W. Purification and chemical characterization of melittin and acetylated derivatives. Biochim Biophys Acta. 1980 Oct 21;625(2):274–280. doi: 10.1016/0005-2795(80)90291-3. [DOI] [PubMed] [Google Scholar]
- Sellinger-Barnette M., Weiss B. Interaction of beta-endorphin and other opioid peptides with calmodulin. Mol Pharmacol. 1982 Jan;21(1):86–91. [PubMed] [Google Scholar]
- Terwilliger T. C., Eisenberg D. The structure of melittin. II. Interpretation of the structure. J Biol Chem. 1982 Jun 10;257(11):6016–6022. [PubMed] [Google Scholar]
- Vesely D. L. Bee venom enhances guanylate cyclase activity. Science. 1981 Jul 17;213(4505):359–360. doi: 10.1126/science.6113689. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]