Abstract
Background
There is conflicting evidence regarding the effect of residual transmitral mean pressure gradient (TMPG) after mitral transcatheter edge-to-edge repair (M-TEER). Different TMPG cutoffs have been employed in prior studies with varying results.
Objectives
The purpose of this study was to examine the association between residual TMPG and M-TEER outcomes.
Methods
Consecutive patients undergoing M-TEER at our institution between 2014 and 2022 were included and divided based on quartiles of predischarge TMPG. Outcomes were assessed using Kaplan-Meier analysis and Cox proportional hazard models. We performed subgroup analyses according to mitral regurgitation (MR) mechanism. The primary outcome was all-cause mortality or heart failure hospitalization.
Results
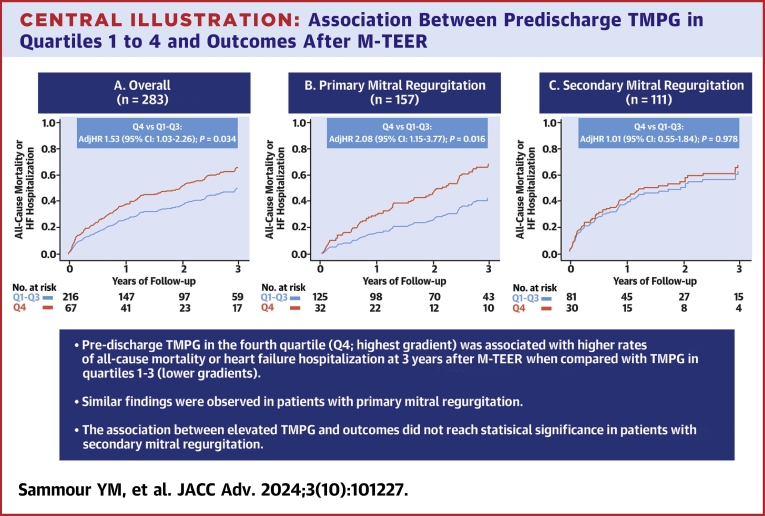
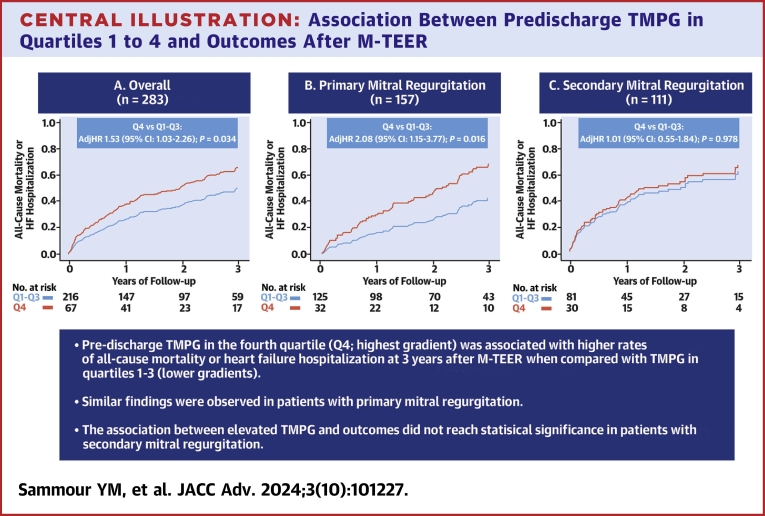
We included 283 patients (age 76.7 ± 10.8 years, 42.8% women, 78.4% Caucasian, and baseline TMPG 2.4 ± 1.3 mm Hg). Higher baseline TMPG was a predictor of increased TMPG after M-TEER (coefficient 0.60 [95% CI: 0.40-0.70]; P < 0.001). In comparison with predischarge TMPG quartiles 1 to 3, those in quartile 4 (7.0 ± 1.1 mm Hg) had an increased risk of 3-year all-cause mortality or heart failure hospitalization (adjHR: 1.53 [95% CI: 1.03-2.26]; P = 0.034), as well as all-cause mortality alone (adjusted HR [adjHR]: 1.68 [95% CI: 1.09-2.60]; P = 0.020). Among patients with primary MR, similar findings were seen for the composite endpoint (adjHR: 2.08 [95% CI: 1.15-3.77]; P = 0.016), and all-cause mortality (adjHR: 2.70 [95% CI: 1.40-5.19]; P = 0.003). However, this association did not reach statistical significance in secondary MR.
Conclusions
In this single-center study, higher residual TMPG after M-TEER was associated with worse outcomes at intermediate- to long-term follow-up. The effect was mainly driven by increased mortality especially in patients with primary MR. Operators should strive to lower residual TMPG before the conclusion of the procedure.
Key words: mean gradient, MitraClip, mitral repair, MVG, outcomes, TEER, TMPG
Central Illustration
Mitral transcatheter edge-to-edge repair (M-TEER) has become a safe and effective option for management of patients with primary mitral regurgitation (MR) at high to prohibitive surgical risk, and those with secondary MR who remain symptomatic despite optimal guideline-directed medical therapy.1,2 The guidelines recommend careful assessment of several parameters after M-TEER including mitral valve area, residual transmitral mean pressure gradient (TMPG), peak velocity, and Doppler velocity index to evaluate for functional failure of the implanted device.3 There is conflicting evidence regarding the impact of residual TMPG on outcomes after M-TEER which may also vary based on the mechanism of MR, including primary versus secondary.3 Furthermore, there is no consensus regarding what TMPG cutoff predicts poor outcomes with studies reporting different cutoffs including 4.4 mm Hg, 5 mm Hg, or quartiles.4, 5, 6, 7 Given this uncertainty in relationship between residual TMPG cutoff and outcome, we sought to assess outcomes of M-TEER based on quartiles of TMPG obtained prior to discharge. Additionally, we evaluated the relationship between TMPG and outcomes stratified by mechanism, primary, and secondary MR.
Methods
Patient selection and data collection
We included all consecutive patients with moderate-to-severe or severe MR who underwent successful M-TEER using the MitraClip device (Abbott Vascular) at Houston Methodist Hospital between March 2014 and March 2022. Candidates with symptomatic primary MR at increased surgical risk or secondary MR on guideline-directed medical therapy were selected for the procedure if they had suitable anatomy for M-TEER after careful evaluation by a multidisciplinary heart team. We excluded patients who had aborted M-TEER procedures (N = 19), and those with missing data on TMPG at discharge (N = 3).
The study was conducted with proper ethical oversight and was approved by the Institutional Review Board at Houston Methodist Hospital. Informed consent was waived given the retrospective nature of the study. Baseline characteristics, procedural details, and outcomes were extracted from our prospectively collected institutional registry. Missing data were collected by review of the electronic medical records. All supporting data are available within the manuscript and online supplementary files.
Echocardiographic assessment
All included patients in this study underwent intraprocedural guidance using transesophageal echocardiograms (TEEs). Baseline and predischarge transthoracic echocardiograms (TTEs) were performed by experienced sonographers and interpreted by level-3 echocardiographers. The severity of MR was graded according to the American Society of Echocardiography guidelines including mild, moderate, moderate-to-severe, and severe.8 MR was classified according to mechanism into: 1) primary or degenerative MR if the primary pathology was related to the valve leaflet or chordae; 2) secondary or functional MR if the mechanism of the regurgitation was related to left ventricular dysfunction, or mitral annulus dilation including secondary to atrial fibrillation; or 3) mixed MR if both mechanisms were observed. Postprocedural TTEs obtained prior to hospital discharge were used to calculate mean TMPG using continuous Doppler waveform tracing of the mitral diastolic inflow as described in the guidelines.9 In patients with atrial fibrillation, the average measurement over 3 to 5 consecutive beats was reported.
Study outcomes
The study outcomes included a composite endpoint of all-cause mortality or heart failure hospitalization, as well as its individual endpoints at 3 years of follow-up. Additionally, we performed subgroup analyses based on the mechanism of MR including primary and secondary after excluding patients with mixed etiology. We conducted a secondary analysis to compare outcomes according to different hemodynamic profiles of residual TMPG and MR after M-TEER. We divided the study cohort to 4 groups including TMPG quartiles 1 to 3 with less than moderate MR (≤1+), TMPG quartiles 1 to 3 with moderate or greater MR (≥2+), TMPG quartile 4 with MR ≤1+, and lastly those with TMPG quartile 4 and MR ≥2+.
Statistical analysis
Patients were stratified based on quartiles of TMPG on predischarge TTE. Categorical variables were described as counts and proportions and compared using chi-square test. Continuous variables were expressed as mean ± SD and compared by analysis of variance test, or as median (IQR) and compared using the Kruskal-Wallis test. A multivariable linear regression model was performed to identify the predictors of elevated TMPG after M-TEER. Variables with P value <0.20 in the univariable analysis were considered eligible to be entered into the multivariable model. The unadjusted and adjusted coefficients and 95% CIs were reported. Next, we performed survival analysis to compare time to endpoints using the log-rank test and Kaplan-Meier curves were constructed. To adjust for between-group differences including quartiles 1 to 3 versus quartile 4, we performed Cox proportional hazards regression analysis. Similarly, variables with P value <0.20 in the univariable analysis were considered eligible to be entered into the multivariable model. These variables included: 1) age, atrial fibrillation/flutter, baseline hemoglobin, baseline creatinine, left ventricular ejection fraction, baseline tricuspid regurgitation, and residual MR at discharge in the overall cohort; 2) sex, race, atrial fibrillation/flutter, baseline hemoglobin, baseline creatinine, left ventricular ejection fraction, and baseline mitral valve area in patients with primary MR; and 3) age, baseline hemoglobin, baseline tricuspid regurgitation, mitral annular calcification severity, and baseline mitral valve area in those with secondary MR (Supplemental Tables 1 to 3). The associations were expressed as HRs with respective 95% CI. Adjusted survival curves were performed for graphical representation of these comparisons. A 2-tailed P value of 0.05 was used for significance testing. Statistical analysis was conducted using SPSS, version 26.0 (SPSS Inc, Chicago, Illinois, USA).
Results
Baseline characteristics
We included 283 patients who met the study inclusion criteria. Overall, the mean age was 76.7 ± 10.8 years, 42.8% were women, 78.4% were Caucasians, and the mean Society of Thoracic Surgeons risk score for mitral valve repair was 5.4% ± 5.6%. Patients with TMPG in the fourth quartile had higher baseline TMPG 3.2 ± 1.5 mm Hg with lower mitral valve area 4.9 ± 1.4 cm2 in comparison with lower quartiles (Table 1). The median follow-up time was 23.6 months (IQR: 11.3-40.5).
Table 1.
Baseline Demographic, Clinical, and Echocardiographic Characteristics According to Quartiles of Residual Post-TEER TMPG at Discharge
|
P Value |
|||||||
|---|---|---|---|---|---|---|---|
| Overall (N = 283) | Quartile 1 (n = 65) | Quartile 2 (n = 67) | Quartile 3 (n = 84) | Quartile 4 (n = 67) | Overall | Q4 vs Q1-3 | |
| Demographics and clinical characteristics | |||||||
| Age (y) | 76.7 ± 10.8 | 79.6 ± 10.1 | 77.1 ± 10.7 | 76.6 ± 10.8 | 76.7 ± 10.8 | 0.023 | 0.010 |
| Women (%) | 121 (42.8%) | 22 (33.8%) | 22 (32.8%) | 42 (50.0%) | 35 (52.2%) | 0.028 | 0.073 |
| Caucasian (%) | 222 (78.4%) | 40 (75.4%) | 55 (82.1%) | 67 (79.8%) | 51 (76.1%) | 0.756 | 0.596 |
| BMI (kg/m2) | 26.2 ± 6.0 | 24.3 ± 4.7 | 25.7 ± 5.3 | 26.8 ± 6.1 | 27.6 ± 7.1 | 0.010 | 0.023 |
| STS risk MV repair | 5.4 ± 5.6 | 5.1 ± 4.1 | 5.5 ± 4.6 | 4.9 ± 4.5 | 5.9 ± 7.6 | 0.683 | 0.427 |
| STS risk MV replacement | 7.4 ± 6.6 | 6.9 ± 4.8 | 7.4 ± 5.9 | 6.7 ± 5.1 | 8.4 ± 9.9 | 0.512 | 0.288 |
| Prior surgical ring (%) | 25 (8.8%) | 2 (3.1%) | 2 (3.0%) | 12 (14.3%) | 9 (13.4%) | 0.016 | 0.129 |
| Hypertension (%) | 198 (70.0%) | 46 (70.8%) | 48 (71.6%) | 58 (69.0%) | 46 (68.7%) | 0.978 | 0.789 |
| Diabetes (%) | 75 (26.5%) | 12 (18.5%) | 13 (19.4%) | 26 (31.0%) | 24 (35.8%) | 0.052 | 0.048 |
| Coronary artery disease (%) | 104 (36.7%) | 28 (43.1%) | 23 (34.3%) | 28 (33.3%) | 25 (37.3%) | 0.633 | 0.913 |
| Prior PCI (%) | 51 (18.1%) | 17 (26.6%) | 13 (19.4%) | 10 (11.9%) | 11 (16.4%) | 0.140 | 0.596 |
| Prior CABG (%) | 61 (21.6%) | 13 (20.0%) | 11 (16.4%) | 21 (25.0%) | 16 (23.9%) | 0.584 | 0.204 |
| Prior myocardial infarction (%) | 67 (23.7%) | 20 (30.85) | 16 (23.9%) | 19 (22.6%) | 12 (17.9%) | 0.377 | 0.685 |
| Prior stroke (%) | 37 (13.1%) | 7 (10.8%) | 11 (16.4%) | 8 (9.5%) | 11 (16.4%) | 0.466 | 0.353 |
| Atrial fibrillation/flutter (%) | 165 (58.3%) | 37 (59.9%) | 48 (71.6%) | 51 (60.7%) | 29 (43.3%) | 0.010 | 0.004 |
| Prior pacemaker or ICD (%) | 83 (29.3%) | 16 (24.6%) | 21 (31.1%) | 23 (27.4%) | 23 (34.3%) | 0.617 | 0.304 |
| Dialysis (%) | 20 (7.1%) | 4 (6.2%) | 4 (6.0%) | 4 (4.8%) | 8 (11.9%) | 0.347 | 0.075 |
| Serum hemoglobin (g/dL) | 11.5 ± 2.2 | 12.3 ± 2.0 | 11.4 ± 2.4 | 11.4 ± 1.8 | 11.0 ± 2.3 | 0.006 | 0.009 |
| Serum creatinine (mg/dL) | 1.2 (1.0-1.7) | 1.1 (0.9-1.5) | 1.3 (1.0-1.7) | 1.2 (1.0-1.8) | 1.2 (1.0-1.9) | 0.430 | 0.971 |
| Prior 2-week NYHA functional class (%) | 0.628 | 0.236 | |||||
| I | 8 (2.9%) | 2 (3.2%) | 1 (1.6%) | 1 (1.2%) | 4 (6.1%) | ||
| II | 49 (17.7%) | 10 (15.9%) | 12 (18.8%) | 17 (20.2%) | 10 (15.2%) | ||
| III | 183 (66.1%) | 41 (65.1%) | 42 (65.6%) | 59 (70.2%) | 41 (62.1%) | ||
| IV | 37 (13.4%) | 10 (15.9%) | 9 (14.1%) | 7 (8.3%) | 11 (16.7%) | ||
| Echocardiographic characteristics | |||||||
| Etiology of MR (%) | 0.421 | 0.300 | |||||
| Primary | 157 (55.5%) | 42 (64.6%) | 33 (49.3%) | 50 (59.5%) | 32 (47.8%) | ||
| Secondary | 111 (39.2%) | 20 (30.8%) | 31 (46.3%) | 30 (35.7%) | 30 (44.8%) | ||
| Mixed | 15 (5.3%) | 3 (4.6%) | 3 (4.5%) | 4 (4.8%) | 5 (7.5%) | ||
| Baseline MR severity (%) | 0.264 | 0.780 | |||||
| Moderate-to-severe | 54 (19.1%) | 14 (21.5%) | 17 (25.4%) | 11 (13.1%) | 12 (17.9%) | ||
| Severe | 229 (80.9%) | 51 (78.5%) | 50 (74.6%) | 73 (86.9%) | 55 (82.1%) | ||
| Baseline TMPG (mm Hg) | 2.4 ± 1.3 | 1.7 ± 0.7 | 2.1 ± 1.0 | 2.5 ± 1.2 | 3.2 ± 1.5 | <0.001 | <0.001 |
| Baseline mitral valve area, cm2 | 5.3 ± 1.6 | 5.7 ± 1.5 | 5.4 ± 1.6 | 5.2 ± 1.6 | 4.9 ± 1.4 | 0.014 | 0.013 |
| MAC (%) | 0.032 | 0.007 | |||||
| None | 199 (70.3%) | 53 (81.5%) | 47 (70.1%) | 58 (69.0%) | 41 (61.2%) | ||
| Mild | 53 (18.7%) | 11 (16.9%) | 15 (22.4%) | 16 (19.0%) | 11 (16.4%) | ||
| Moderate | 18 (6.4%) | 1 (1.5%) | 2 (3.0%) | 7 (8.3%) | 8 (11.9%) | ||
| Severe | 13 (4.6%) | 0 (0.0%) | 3 (4.5%) | 3 (3.6%) | 7 (10.4%) | ||
| LVEF (%) | 51.4 ± 14.7 | 52.0 ± 14.0 | 51.0 ± 15.1 | 51.9 ± 14.6 | 50.5 ± 15.0 | 0.917 | 0.582 |
| LA volume index (mL/m2) | 63.3 ± 26.6 | 66.1 ± 28.6 | 65.2 ± 28.5 | 62.1 ± 23.4 | 61.2 ± 26.6 | 0.670 | 0.411 |
| LVEDVi (mL/m2) | 87.8 ± 36.8 | 95.5 ± 45.8 | 88.5 ± 31.3 | 85.5 ± 28.1 | 83.9 ± 44.5 | 0.718 | 0.542 |
| LVESVi (mL/m2) | 45.2 ± 28.2 | 46.7 ± 27.8 | 49.5 ± 31.6 | 41.0 ± 22.0 | 46.7 ± 33.8 | 0.708 | 0.823 |
| PA systolic pressure (mm Hg) | 52.8 ± 17.7 | 50.4 ± 16.8 | 47.6 ± 15.5 | 55.9 ± 17.4 | 56.2 ± 19.5 | 0.019 | 0.101 |
| Tricuspid regurgitation severity (%) | 0.277 | 0.364 | |||||
| None/trace | 69 (24.4%) | 12 (18.5%) | 12 (17.9%) | 25 (29.8%) | 20 (29.9%) | ||
| Mild | 105 (37.1%) | 29 (44.6%) | 31 (46.3%) | 26 (31.0%) | 19 (28.4%) | ||
| Moderate | 80 (28.3%) | 15 (23.1%) | 18 (26.9%) | 26 (31.0%) | 21 (31.3%) | ||
| Severe | 29 (10.2%) | 9 (13.8%) | 6 (9.0%) | 7 (8.3%) | 7 (10.4%) | ||
Values are mean ± SD, n (%), or median (IQR).
BMI = body mass index; CABG = coronary artery bypass graft; ICD = intracardiac cardioverter defibrillator; LA = left atrium; LVEDVi = left ventricular end-diastolic volume index; LVEF = left ventricular ejection fraction; LVESVi = left ventricular end-systolic volume index; MAC = mitral annular calcification; MR = mitral regurgitation; MV = mitral valve; PA = pulmonary artery; PCI = percutaneous coronary intervention; STS = Society of Thoracic Surgeons; TEER = transcatheter edge-to-edge repair; TMPG = transmitral pressure gradient.
Procedural characteristics
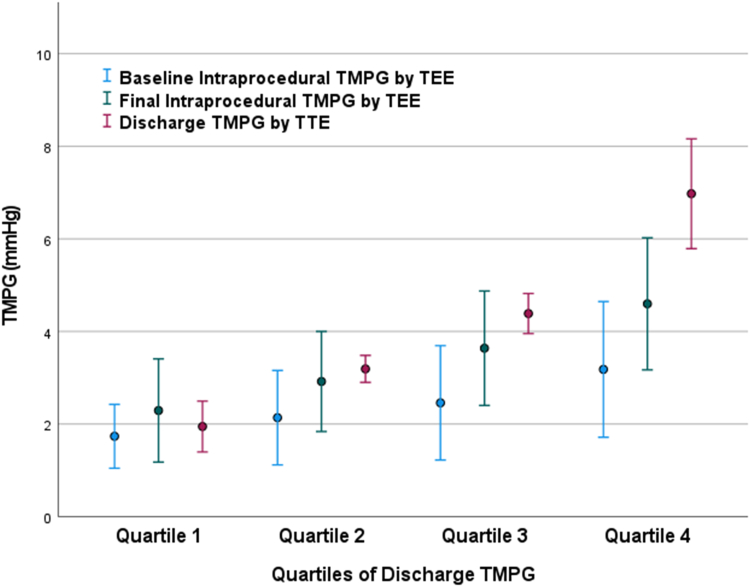
There was no statistically significant difference in the number of clips used or residual MR across the groups (Table 2). Among patients with quartiles 1 through 4, the mean final intraprocedural TMPG was 2.3 ± 1.1 mm Hg, 2.9 ± 1.1 mm Hg, 3.6 ± 1.2 mm Hg, and 4.6 ± 1.4 mm Hg, respectively. Residual TMPG on predischarge TTE was higher than during the procedure across the quartiles, as follows: 1.9 ± 0.5 mm Hg, 3.2 ± 0.3 mm Hg, 4.3 ± 0.4 mm Hg, and 7.0 ± 1.1 mm Hg, respectively (Figure 1). Among patients in the fourth quartile, the mean TMPG was 6.6 ± 2.7 mm Hg at 30 days (N = 216), and 5.5 ± 2.5 mm Hg at 1 year (N = 141).
Table 2.
Procedural Characteristics and Outcomes According to Quartiles of Residual Post-TEER TMPG at Discharge
|
P Value |
|||||||
|---|---|---|---|---|---|---|---|
| Overall (N = 283) | Quartile 1 (n = 65) | Quartile 2 (n = 67) | Quartile 3 (n = 84) | Quartile 4 (n = 67) | Overall | Q4 vs Q1-3 | |
| Number of clips (%) | 0.721 | 0.402 | |||||
| 1 | 149 (52.7%) | 35 (53.8%) | 38 (56.7%) | 45 (53.6%) | 31 (46.3%) | ||
| 2 | 119 (42.0%) | 28 (43.1%) | 27 (40.3%) | 33 (39.3%) | 31 (46.3%) | ||
| ≥3 | 15 (5.3%) | 2 (3.1%) | 2 (3.0%) | 6 (7.1%) | 5 (7.5%) | ||
| Final intraprocedural TMPG (mm Hg) | 3.4 ± 1.5 | 2.3 ± 1.1 | 2.9 ± 1.1 | 3.6 ± 1.2 | 4.6 ± 1.4 | <0.001 | <0.001 |
| Discharge TMPG (mm Hg) | 4.1 ± 1.9 | 1.9 ± 0.5 | 3.2 ± 0.3 | 4.3 ± 0.4 | 7.0 ± 1.1 | <0.001 | <0.001 |
| 30-day TMPG (mm Hg) (N = 216) | 4.5 ± 2.3 | 3.1 ± 1.7 | 3.5 ± 1.2 | 4.6 ± 1.8 | 6.6 ± 2.7 | <0.001 | <0.001 |
| 1-year TMPG (mm Hg) (N = 141) | 4.3 ± 2.2 | 3.8 ± 2.3 | 3.6 ± 1.7 | 4.4 ± 2.1 | 5.5 ± 2.5 | <0.001 | 0.002 |
| Residual MR at discharge (%) | 0.637 | 0.430 | |||||
| None/trace | 52 (18.4%) | 11 (16.9%) | 11 (16.4%) | 19 (22.6%) | 11 (16.4%) | ||
| Mild | 182 (64.3%) | 41 (63.1%) | 48 (71.6%) | 48 (57.1%) | 45 (67.2%) | ||
| Moderate | 40 (14.1%) | 11 (16.9%) | 6 (9.0%) | 15 (17.9%) | 8 (11.9%) | ||
| Moderate-to-severe | 6 (2.1%) | 1 (1.5%) | 2 (3.0%) | 2 (2.4%) | 1 (1.5%) | ||
| Severe | 3 (1.1%) | 1 (1.5%) | 0 (0.0%) | 0 (0.0%) | 2 (3.0%) | ||
| Residual moderate or less MR at discharge (%) | 274 (96.8%) | 63 (96.9%) | 65 (97.0%) | 82 (97.6%) | 64 (95.5%) | 0.908 | 0.488 |
| 30-day moderate or less MR (%) (N = 255) | 235 (92.5%) | 55 (93.2%) | 56 (94.9%) | 70 (90.9%) | 54 (91.5%) | 0.824 | 0.740 |
| 1-year moderate or less MR (%) (N = 145) | 167 (92.3%) | 36 (87.8%) | 40 (93.0%) | 54 (93.1%) | 37 (94.9%) | 0.658 | 0.491 |
Values are n (%) or mean ± SD.
Abbreviations as in Table 1.
Figure 1.
Comparison of Baseline, Final Intraprocedural, Discharge, and 30-Day TMPG Among Patients With Different Quartiles of TMPG at Discharge
The error bars represent 1 SD around the mean. TMPG = transmitral mean pressure gradient; TTE = transthoracic echocardiogram.
Predictors of TMPG after M-TEER
We found that the number of deployed clips, baseline TMPG, younger age, female sex, body mass index, prior surgical ring, mitral annular calcification, and smaller valve area were associated with higher residual TMPG after multivariable adjustment. The use of wider or longer clips did not predict elevated TMPG (Table 3).
Table 3.
Linear Regression Analysis for Predictors of Elevated TMPG After Mitral TEER
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| Coefficient (95% CI) | P Value | Coefficient (95% CI) | P Value | |
| Age, per 10-y increase | −0.33 (−0.53, −0.12) | 0.002 | −0.32 (−0.46, −0.08) | 0.005 |
| Female | 0.65 (0.19-1.10) | 0.005 | 0.65 (0.28-1.10) | 0.001 |
| White race | −0.10 (−0.64, 0.46) | 0.748 | - | - |
| Body mass index | 0.06 (0.02-0.09) | 0.003 | 0.04 (0.01-0.07) | 0.015 |
| Atrial fibrillation/flutter | −0.67 (−1.14, −0.24) | 0.003 | −0.19 (−0.67, 0.12) | 0.175 |
| Prior surgical ring | 1.06 (0.27-1.85) | 0.008 | 1.00 (0.30-1.67) | 0.005 |
| Secondary MR | 0.32 (−0.21-0.72) | 0.277 | - | - |
| MAC severity, per grade | 0.51 (0.25-0.79) | <0.001 | 0.41 (0.16-0.65) | <0.001 |
| Baseline LVEF | −0.01 (−0.02, 0.01) | 0.610 | - | - |
| Baseline TR severity, per grade | −0.06 (−0.32, 0.16) | 0.512 | - | - |
| Baseline mitral valve area, per cm2 | −0.23 (−0.37, −0.09) | 0.002 | −0.22 (−0.33, −0.06) | 0.005 |
| Baseline intraprocedural TMPG, per mm Hg | 0.69 (0.49-0.82) | <0.001 | 0.60 (0.40-0.70) | <0.001 |
| Number of deployed clips | 0.38 (0.02-0.76) | 0.039 | 0.77 (0.47-1.13) | <0.001 |
| Wider clips | 0.01 (−0.49, 0.50) | 0.988 | - | - |
| Longer clips | −0.20 (−0.66, 0.26) | 0.392 | - | - |
Variables with P <0.20 in the univariable analysis were considered eligible to be entered into the multivariable model. Variables with P < 0.05 in the multivariable analysis were considered significant predictors of elevated TMPG after mitral TEER.
LA = left atrial; TR = tricuspid regurgitation; other abbreviations as in Table 1.
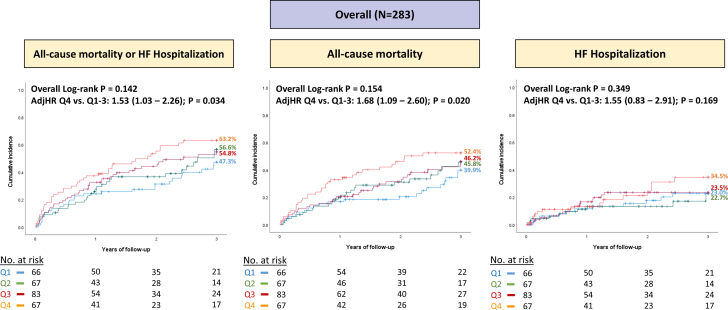
Primary and secondary endpoints
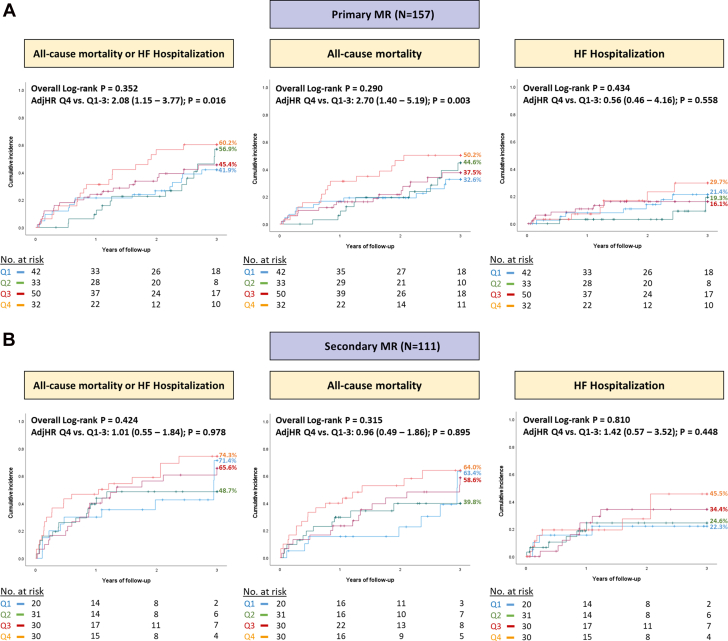
Patients with higher residual TMPG in the fourth quartile had an increased risk of the composite endpoint of all-cause mortality or heart failure hospitalization at 3 years after M-TEER in comparison with those with TMPG quartiles 1 to 3 (adjusted HR [adjHR]: 1.53; 95% CI: 1.03-2.26; P = 0.034) (Central Illustration). Similarly, the risk of individual secondary endpoint of all-cause mortality was also higher with TMPG quartile 4 (adjHR: 1.68; 95% CI: 1.09-2.60; P = 0.020), but there was no difference in the risk of heart failure hospitalization (adjHR: 1.55; 95% CI: 0.83-2.91; P = 0.169) (Figure 2). Next, we performed a subgroup analysis of outcomes according to the mechanism of MR. Among patients with primary MR, similar findings were seen with higher risk of all-cause mortality or heart failure hospitalization (adjHR: 2.08; 95% CI: 1.15-3.77; P = 0.016), and all-cause mortality (adjHR: 2.70; 95% CI: 1.40-5.19; P = 0.003) in patients with quartile 4 versus quartiles 1 to 3 (Figure 3A). On the other hand, the association between elevated TMPG and outcomes did not reach statistical significance in patients with secondary MR (Figure 3B).
Central Illustration.
Association Between Predischarge TMPG in Quartiles 1 to 4 and Outcomes After M-TEER
Impact of transmitral mean pressure gradient (TMPG) on the primary composite outcome of all-cause mortality or heart failure hospitalization at 3 years after M-TEER in (A) all patients; (B) primary mitral regurgitation; and (C) secondary mitral regurgitation. Patients with mixed etiology were excluded from the subgroup analyses shown in (B and C) (N = 15). The panels show adjusted survival analyses stratified by quartiles 1 to 3 versus quartile 4. The variables used for multivariable adjustment are shown in Supplemental Tables 1 to 3. M-TEER = mitral transcatheter edge-to-edge repair.
Figure 2.
Comparison of Primary Composite Outcome and Secondary Individual Endpoints After M-TEER According to Quartiles of TMPG
The panels show unadjusted Kaplan-Meier curves with overall log-rank P values across the 4 quartiles, as well as the multivariable adjusted HRs comparing quartiles 1 to 3 versus quartile 4. M-TEER = mitral transcatheter edge-to-edge repair; other abbreviation as in Figure 1.
Figure 3.
Comparison of Outcomes After M-TEER According to the Mechanism of MR, Including Primary MR and Secondary MR
Patients with mixed etiology of mitral regurgitation were excluded from this analysis (N = 15). (A and B) Show unadjusted Kaplan-Meier curves with overall log-rank P values across the 4 quartiles, as well as the multivariable adjusted HRs comparing quartiles 1 to 3 versus quartile 4. MR = mitral regurgitation; other abbreviation as in Figure 2.
Finally, patients with the worst hemodynamic profile including TMPG quartile 4 and moderate or greater residual MR had an increased risk of all-cause mortality or heart failure hospitalization in comparison with those with TMPG quartiles 1 to 3 who had less than moderate MR (adjHR: 5.44; 95% CI: 2.39-12.39; P < 0.001). Similar findings were noted in patients with primary MR, but there were no significant differences in patients with secondary MR (Supplemental Figure 1).
Discussion
In this single-center experience, we examined the association between residual TMPG after M-TEER at discharge and outcomes. The main study findings are as follows: patients with higher TMPG in the fourth quartile were found to have an increased risk of the composite endpoint of all-cause mortality or heart failure hospitalization, as well as the individual endpoint of all-cause mortality at 3 years after M-TEER. Similar observations were seen in patients with primary MR, however, the association between increased residual TMPG and outcomes did not reach statistical significance among patients with secondary MR. Additionally, patients who had a combination of elevated TMPG in the highest quartile and moderate or greater MR had poor outcomes. Finally, an elevated baseline TMPG correlated with increased TMPG after M-TEER.
The American Society of Echocardiography guidelines use different mean TMPG cutoffs to define abnormal prosthetic valve function including ≥5 mm Hg for regurgitant valves and >10 mm Hg for stenotic valves.10 The Mitral Valve Academic Research Consortium adopted a cutoff of 5 mm Hg as a criterion for stenosis of implanted mitral devices.11 However, this cutoff of 5 mm Hg may be too low and its applicability may vary based on several factors including the type of procedure, the etiology of mitral valve dysfunction, and hemodynamic parameters.3 Currently, there is no definitive consensus regarding the use of a specific TMPG cutoff in patients undergoing M-TEER for prognostication since prior publications have presented conflicting results based on different cutoffs.
Baseline TMPG and small mitral valve area have been shown to predict elevated residual TMPG after M-TEER.3,12 Other studies showed that patients with elevated pre- and post-TEER TMPG were more likely to be women, with a higher prevalence of mitral annular calcification, and a higher number of deployed mitral clips.5,13,14 In the present analysis, all these factors were important predictors of elevated TMPG after M-TEER, in addition to younger age, body mass index, and prior surgical ring. Multimodality imaging may play a role in identifying patients at risk of developing post-TEER mitral stenosis. Certain preprocedural parameters identified by multidetector computed tomography may be associated with high TMPG after M-TEER including smaller mitral annulus area, mitral annulus diameters, and mitral valve orifice area.15 Another study showed that mitral annulus area and leaflet area measured by 3-dimensional TEE were stronger predictors of elevated TMPG after M-TEER than baseline TMPG or mitral valve orifice area in patients with secondary MR.16 Efforts should be made to identify patients at risk of elevated TMPG after M-TEER and try to mitigate that risk by selecting the appropriate candidates for the procedure, deploying fewer clips if applicable, and consider transcatheter mitral valve replacement if anatomically suitable in those at high risk for developing significant mitral stenosis after M-TEER.
Furthermore, we demonstrate that there are differences between final intraprocedural TMPG by TEE, and predischarge TMPG by TTE, which are likely attributed to being under anesthesia, and having lower heart rate during the procedure.17,18 Interestingly, the differences between intraoperative and discharge TMPG became more apparent upon progression from quartile 1 to quartile 4. This trend may be explained by the observation that patients in the higher quartiles exhibited elevated body mass index, a greater degree of mitral annular calcification, and smaller valve area. These factors, which may influence left atrial compliance, could potentially lead to more noticeable differences between intraprocedural and postprocedural TMPG when loading conditions change after the procedure.
Elevated TMPG has been linked with worse outcomes after M-TEER, but the evidence has not been consistent, and it may vary according to the mechanism of MR, which may not only entail primary versus secondary but also the underlying etiologies for each disease process.3 In primary MR, the left ventricular function and compliance are often normal, and higher TMPG after M-TEER may be more indicative of smaller valve area or residual MR.3 Several studies reported an association between postprocedural TMPG and outcomes in patients with primary MR, however different cutoffs were used to define an elevated gradient. In an analysis of 104 patients with primary MR, a TMPG >4.4 mm Hg was associated with worse outcomes at 2 years.4 Another analysis of 265 patients showed that a TMPG ≥5 mm Hg was associated with death or heart failure rehospitalization at 5 years.5 On the other hand, there was another study that showed no association between increased TMPG and all-cause mortality or heart failure hospitalization at 2 years in 419 patients with primary MR. However, that study analyzed patients according to TMPG quartiles, and those in the fourth quartile had higher heart rates, greater residual MR, and less comorbidities. Also, the primary outcomes were separated at 2 years and a longer follow-up may have been needed to reach significance.6 Our study showed that patients with primary MR and higher TMPG in the fourth quartile had higher risk of the composite outcome of all-cause mortality or heart failure hospitalization, which was mainly related to increased mortality, but there were no differences in heart failure hospitalization after multivariable adjustment.
Furthermore, a recent study from the TVT (Transcatheter Valve Therapy) registry involving 19,088 patients with primary MR compared outcomes based on procedural success defined as achieving moderate or less residual MR, with TMPG of <10 mm Hg. This was associated with significantly lower mortality rates compared with unsuccessful procedures. Patients who had both TMPG ≤5 mm Hg and mild or less MR had the lowest rates of mortality and heart failure hospitalization.19 In our cohort, we found a similar observation where patients with higher TMPG in the fourth quartile who also had moderate or greater residual MR after the procedure had an increased risk of adverse events in comparison with those with TMPG quartiles 1 to 3 and less than moderate MR. It is worth mentioning that the number of our patients in the worst hemodynamic profile group was very small.
With regard to secondary MR, patients have diminished left ventricular function and compliance, along with elevated diastolic pressures which may result in a lower TMPG after M-TEER. Therefore, interpreting differences according to TMPG in the setting of secondary MR may be more complex and prone to variability.3 Several studies reported no association between postprocedural TMPG and outcomes in secondary MR using different cutoffs. A post hoc study of 250 patients with secondary MR from the COAPT (Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation) trial by Halaby and colleagues stratified TMPG according to quartiles, similar to the present analysis, and similarly they showed no association with all-cause mortality or heart failure hospitalization at 2 years.7 Two other analyses of 151 and 445 patients with secondary MR showed similar findings of no relationship between TMPG >4.4 mm Hg and >5 mm Hg, respectively, and outcomes at intermediate to long-term follow-up.4,5 One possible explanation for these findings could be that the benefits obtained with reduction of functional MR may outweigh the negative effect of mild to moderate mitral stenosis after M-TEER.5 On the contrary, a multicenter registry from Italy analyzed 570 patients with secondary MR and found that postprocedural TMPG ≥4 mm Hg was an independent predictor of adverse events at 2 years.20 Another single-center study of 268 patients with secondary MR from Germany suggested that TMPG >4.4 mm Hg by echocardiography or >5 mm Hg invasively predicted worse outcomes at 2 years.21 It is possible that in our study, a larger number of patients and longer follow-up may have been necessary to show a difference with higher TMPG in secondary MR.
Based on the current available evidence, Hahn and Hausleiter emphasized on the importance of mitral stenosis assessment after M-TEER in their editorial. This can be achieved by reporting preprocedural and postprocedural TMPG with respective heart rate, and by measuring the mitral valve area using 3-dimensional planimetry. They also recommended to aim for TMPG <5 mm Hg, along with mild or less residual MR and mitral valve area >2 cm2 especially in patients with primary MR.3 However, obtaining accurate mitral valve area measurements after M-TEER may be challenging, as the valve anatomy may be distorted or thickened, with multiple orifices due to the presence of the clip. Future studies with larger numbers of patients are required to identify the TMPG cutoff associated with worse outcomes after M-TEER. A better understanding of this cutoff may help in selecting appropriate candidates for the procedure, optimize the procedure, and again, considering transcatheter mitral valve replacement for those at highest risk for developing mitral stenosis.
Study Limitations
Our findings should be interpreted within the context of certain limitations including being single-center, retrospective, and observational with a relatively small number of patients especially in the subgroups analyses according to the mechanism of MR. Additionally, the echocardiograms were not reviewed by an external core laboratory given the retrospective nature of the study, however, all echocardiograms were exclusively interpreted by experienced level-3 echocardiographers at our center. Furthermore, our study included different generations of the MitraClip device which may create bias. Other commercially available devices were not used at our institution and outcomes may differ with them given the differences in design. Due to the small number of patients with secondary MR, our ability to detect statistically significant differences among the groups was limited. As a result, our conclusion that higher postclip TMPG are not associated with outcomes in this population may be affected. It is also possible we may have missed some hospitalizations for heart failure in patients who presented to outside hospitals. Finally, external validation of our findings with larger prospective studies is important.
Conclusions
Elevated mitral valve gradient after M-TEER was associated with higher all-cause mortality or heart failure hospitalizations at intermediate follow-up. This effect was mainly seen among patients with primary MR and was mainly related to increased mortality. Future studies may be needed to better understand the relationship between TMPG and outcomes after M-TEER and identify an optimal cutoff that operators should try to stay under at the conclusion of the procedure.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Our study builds up on the available literature and shows that patients with higher predischarge TMPG in the fourth quartile had worse outcomes at 3 years after M-TEER. Upon stratifying patients according to the mechanism of MR, this association persisted in patients with primary MR, but did not reach statistical significance among those with secondary MR.
TRANSLATIONAL OUTLOOK: When planning future studies, it is important to understand the effect of residual TMPG on outcomes according to the mechanism of MR and identify an optimal TMPG cutoff to target during the procedure.
Funding support and author disclosures
Dr Atkins is a consultant for WL Gore & Associates. Dr Reardon is a consultant for Medtronic, Boston Scientific, Abbott, and WL Gore & Associates. Dr Kleiman is a local principal investigator in trials sponsored by Boston Scientific, Medtronic, Abbott, and Edwards Lifesciences. Dr Goel is a consultant for Medtronic, WL Gore & Associates, and JC Medical; and is on the Speakers Bureau for Abbott Structural Heart. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and a figure, please see the online version of this paper.
Supplementary Data
References
- 1.Feldman T., Foster E., Glower D.D., et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med. 2011;364:1395–1406. doi: 10.1056/NEJMoa1009355. [DOI] [PubMed] [Google Scholar]
- 2.Stone G.W., Lindenfeld J., Abraham W.T., et al. Transcatheter mitral-valve repair in patients with heart failure. N Engl J Med. 2018;379:2307–2318. doi: 10.1056/NEJMoa1806640. [DOI] [PubMed] [Google Scholar]
- 3.Hahn R.T., Hausleiter J. Transmitral gradients following transcatheter edge-to-edge repair: are mean gradients meaningful? JACC Cardiovasc Interv. 2022;15:946–949. doi: 10.1016/j.jcin.2022.02.018. [DOI] [PubMed] [Google Scholar]
- 4.Patzelt J., Zhang W., Sauter R., et al. Elevated mitral valve pressure gradient is predictive of long-term outcome after percutaneous edge-to-edge mitral valve repair in patients with degenerative mitral regurgitation (MR), but not in functional MR. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koell B., Ludwig S., Weimann J., et al. Long-term outcomes of patients with elevated mitral valve pressure gradient after mitral valve edge-to-edge repair. JACC Cardiovasc Interv. 2022;15:922–934. doi: 10.1016/j.jcin.2021.12.007. [DOI] [PubMed] [Google Scholar]
- 6.Yoon S.H., Makar M., Kar S., et al. Prognostic value of increased mitral valve gradient after transcatheter edge-to-edge repair for primary mitral regurgitation. JACC Cardiovasc Interv. 2022;15:935–945. doi: 10.1016/j.jcin.2022.01.281. [DOI] [PubMed] [Google Scholar]
- 7.Halaby R., Herrmann H.C., Gertz Z.M., et al. Effect of mitral valve gradient after MitraClip on outcomes in secondary mitral regurgitation: results from the COAPT trial. JACC Cardiovasc Interv. 2021;14:879–889. doi: 10.1016/j.jcin.2021.01.049. [DOI] [PubMed] [Google Scholar]
- 8.Zoghbi W.A., Enriquez-Sarano M., Foster E., et al. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 9.Baumgartner H., Hung J., Bermejo J., et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am Soc Echocardiogr. 2009;22:1–23. doi: 10.1016/j.echo.2008.11.029. [DOI] [PubMed] [Google Scholar]
- 10.Zoghbi W.A., Chambers J.B., Dumesnil J.G., et al. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound: a report from the American society of echocardiography's guidelines and standards committee and the task force on prosthetic valves, developed in conjunction with the American college of cardiology cardiovascular imaging committee, cardiac imaging committee of the American heart association, the European association of echocardiography, a registered branch of the European society of cardiology, the Japanese society of echocardiography and the Canadian society of echocardiography, endorsed by the American college of cardiology foundation, American heart association, European association of echocardiography, a registered branch of the European society of cardiology, the Japanese society of echocardiography, and Canadian society of echocardiography. J Am Soc Echocardiogr. 2009;22:975–1014. doi: 10.1016/j.echo.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Stone G.W., Adams D.H., Abraham W.T., et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: Part 2: endpoint definitions: a consensus document from the mitral valve academic Research Consortium. J Am Coll Cardiol. 2015;66:308–321. doi: 10.1016/j.jacc.2015.05.049. [DOI] [PubMed] [Google Scholar]
- 12.Lugo-Fagundo N., Pierre K., Adedinsewo D., et al. The impact of baseline transmitral diastolic mean gradient on left atrial pressure reduction in patients undergoing transcatheter mitral valve edge-to-edge repair. Cathet Cardiovasc Interv. 2023;101:605–609. doi: 10.1002/ccd.30577. [DOI] [PubMed] [Google Scholar]
- 13.Ueyama H., Block P.C. Can patients be selected for transcatheter edge-to-edge repair based on the baseline transmitral gradient? Cathet Cardiovasc Interv. 2023;101:830–831. doi: 10.1002/ccd.30607. [DOI] [PubMed] [Google Scholar]
- 14.Oguz D., Padang R., Rashedi N., et al. Risk for increased mean diastolic gradient after transcatheter edge-to-edge mitral valve repair: a quantitative three-dimensional transesophageal echocardiographic analysis. J Am Soc Echocardiogr. 2021;34:595–603.e2. doi: 10.1016/j.echo.2021.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Kaewkes D., Patel V., Ochiai T., et al. Usefulness of computed tomography to predict mitral stenosis after transcatheter mitral valve edge-to-edge repair. Am J Cardiol. 2021;153:109–118. doi: 10.1016/j.amjcard.2021.05.025. [DOI] [PubMed] [Google Scholar]
- 16.Kato Y., Okada A., Amaki M., et al. Three-dimensional echocardiography for predicting mitral stenosis after MitraClip for functional mitral regurgitation. J Echocardiogr. 2022;20:151–158. doi: 10.1007/s12574-022-00564-x. [DOI] [PubMed] [Google Scholar]
- 17.Kuperstein R., Raibman-Spector S., Canetti M., et al. Influence of anesthesia on hemodynamic assessment of mitral stenosis severity. Cardiol J. 2022;29:245–251. doi: 10.5603/CJ.a2021.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehr M., Taramasso M., Besler C., et al. 1-Year outcomes after edge-to-edge valve repair for symptomatic tricuspid regurgitation. JACC Cardiovasc Interv. 2019;12:1451–1461. doi: 10.1016/j.jcin.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Makkar R.R., Chikwe J., Chakravarty T., et al. Transcatheter mitral valve repair for degenerative mitral regurgitation. JAMA. 2023;329:1778–1788. doi: 10.1001/jama.2023.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Felice F., Paolucci L., Musto C., et al. Outcomes in patients with high transmitral gradient after mitral valve transcatheter edge-to-edge repair for mitral regurgitation. Am J Cardiol. 2022;182:46–54. doi: 10.1016/j.amjcard.2022.07.036. [DOI] [PubMed] [Google Scholar]
- 21.Neuss M., Schau T., Isotani A., Pilz M., Schöpp M., Butter C. Elevated mitral valve pressure gradient after MitraClip implantation deteriorates long-term outcome in patients with severe mitral regurgitation and severe heart failure. JACC Cardiovasc Interv. 2017;10:931–939. doi: 10.1016/j.jcin.2016.12.280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.