Abstract
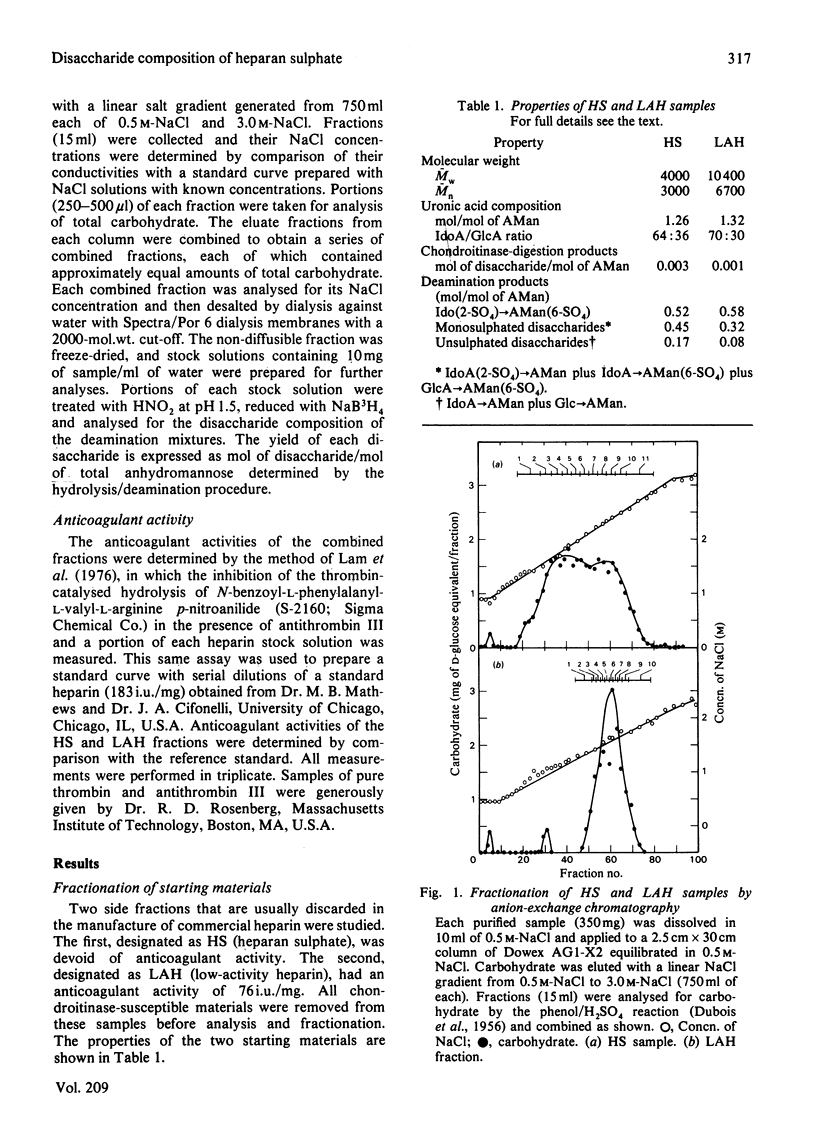
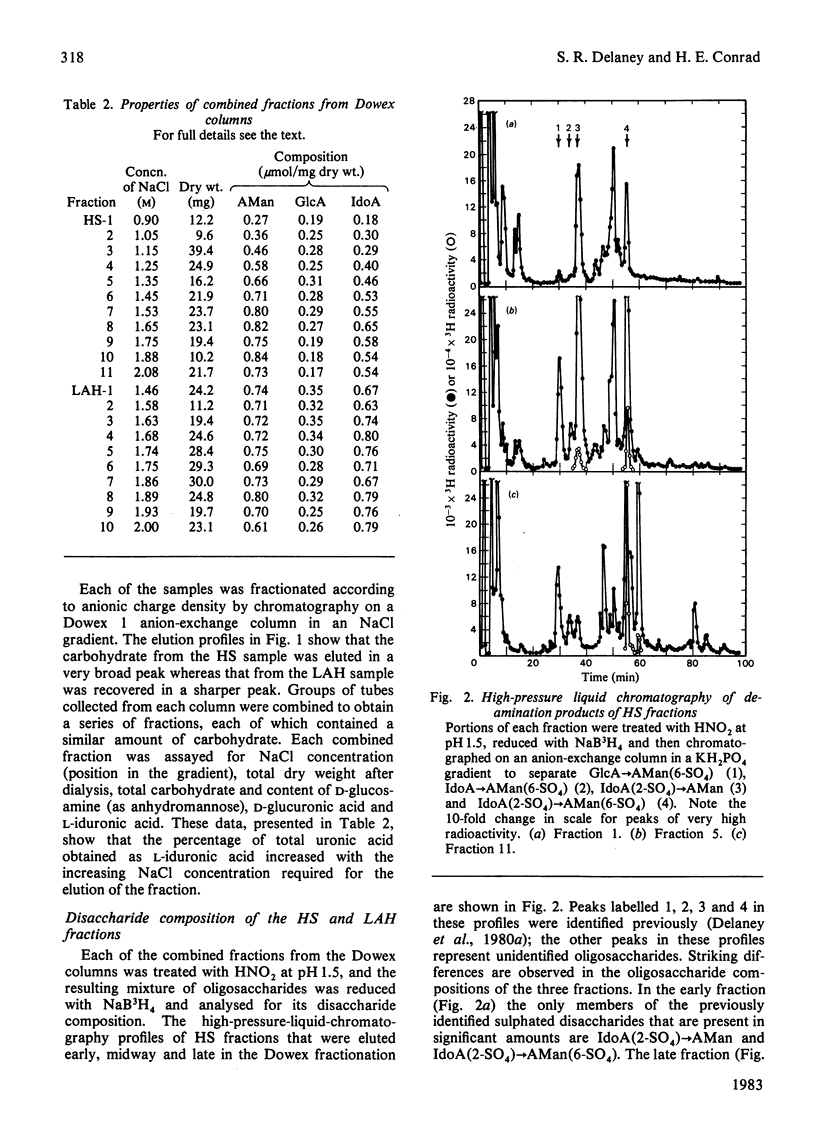
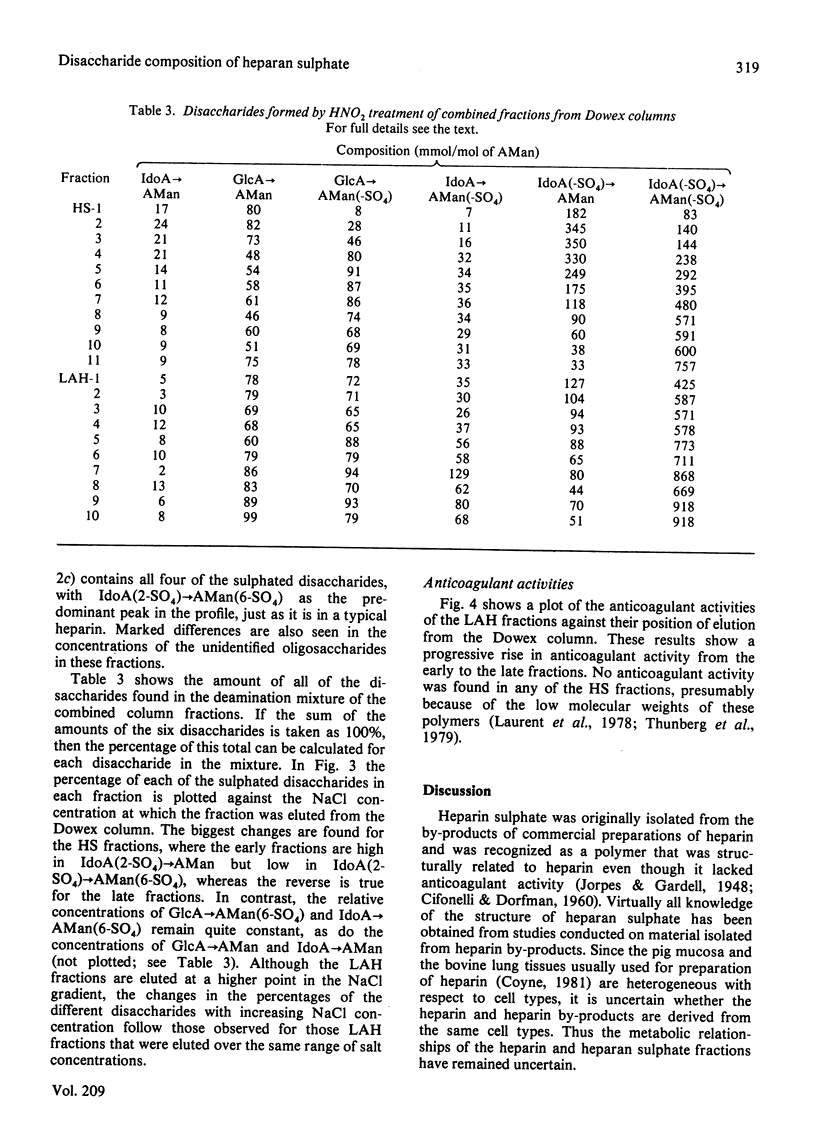
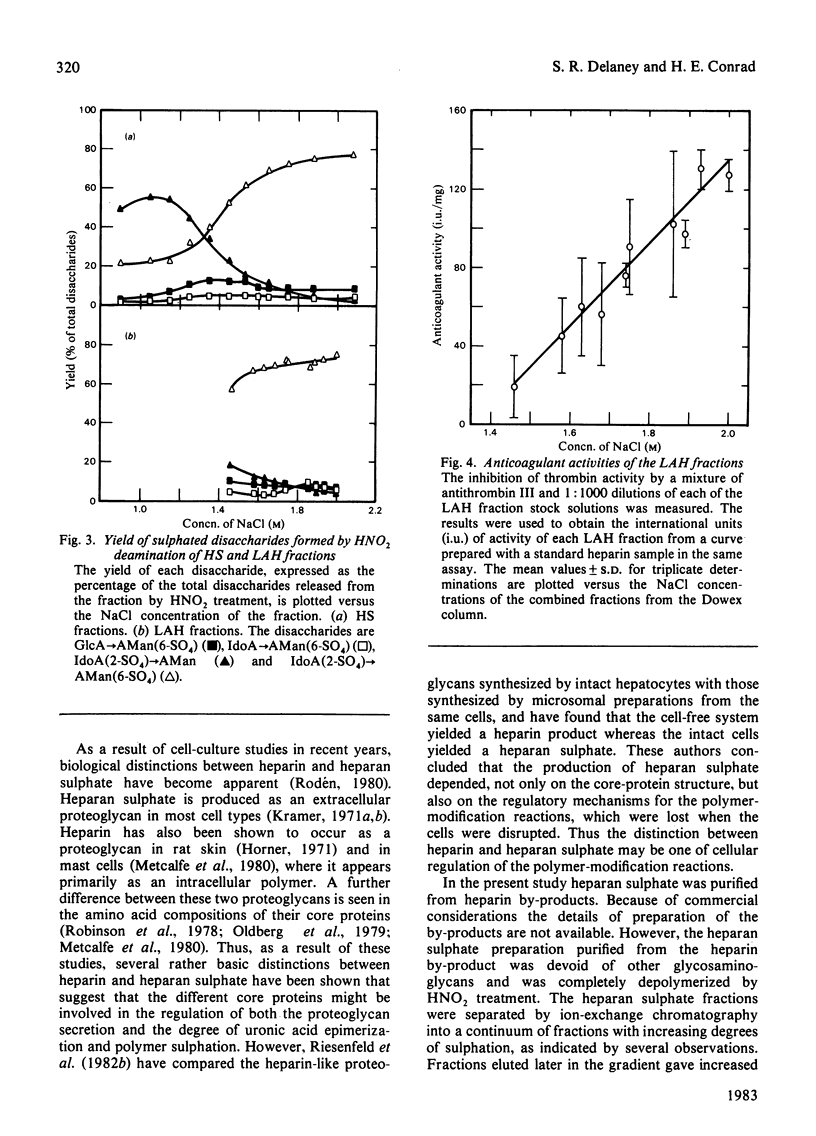
Heparan sulphate by-products from the commercial manufacture of pig mucosal heparin were freed of chondroitin sulphate and fractionated according to anionic density. The fractions were treated with HNO2 at pH 1.5, and the resulting mixtures of oligosaccharides were reduced with NaB3H4 and analysed for their disaccharide composition by paper chromatography and by high-pressure liquid chromatography. The results show that the molar ratio of 2-O-sulpho-alpha-L-iduronosylanhydromannose to 6-O-sulpho-(2-O-sulpho-alpha-L-iduronosyl)anhydromannose decreased from 2.5 to 0.04 as the degree of sulphation of the fractions increased. In contrast, the molar ratio of 6-O-sulpho-(beta-D-glucuronosyl)anhydromannose to 6-O-sulpho-(alpha-L-iduronosyl)anhydromannose was approx. 2.4 in all heparan sulphate fractions and decreased to only half of this value in the most highly sulphated heparin fractions. These results are consistent with biosynthetic studies, which have shown that the N-sulpho-(2-O-sulpho-alpha-L-iduronosyl)D-glucosamine disaccharide is the metabolic precursor of the NO-disulpho-(2-O-sulpho-alpha-L-iduronosyl)-D-glucosamine disaccharide in heparin biosynthesis. The high-pressure liquid chromatography of the heparan sulphate oligosaccharides also revealed a number of unidentified oligosaccharides in the deamination mixtures.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CIFONELLI J. A., DORFMAN A. Properties of heparin monosulfate (heparitin monosulfate). J Biol Chem. 1960 Nov;235:3283–3286. [PubMed] [Google Scholar]
- Cifonelli J. A., King J. A. Structural characteristics of heparan sulfates with varying sulfate contents. Biochemistry. 1977 May 17;16(10):2137–2141. doi: 10.1021/bi00629a014. [DOI] [PubMed] [Google Scholar]
- Cifonelli J. A., King J. The distribution of sulfated uronic acid and hexosamine residues in heparin and heparan sulfate. Connect Tissue Res. 1975;3(1):97–104. doi: 10.3109/03008207509152346. [DOI] [PubMed] [Google Scholar]
- Conrad H. E. The acid lability of the glycosidic bonds of L-iduronic acid residues in glycosaminoglycans. Biochem J. 1980 Nov 1;191(2):355–363. doi: 10.1042/bj1910355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney S. R., Conrad H. E., Glaser J. H. A high-performance liquid chromatography approach for isolation and sequencing of chondroitin sulfate oligosaccharides. Anal Biochem. 1980 Oct;108(1):25–34. doi: 10.1016/0003-2697(80)90689-2. [DOI] [PubMed] [Google Scholar]
- Delaney S. R., Leger M., Conrad H. E. Quantitation of the sulfated disaccharides of heparin by high performance liquid chromatography. Anal Biochem. 1980 Jul 15;106(1):253–261. doi: 10.1016/0003-2697(80)90145-1. [DOI] [PubMed] [Google Scholar]
- Forsee W. T., Rodén L. Biosynthesis of heparin. Transfer of N-acetylglucosamine to heparan sulfate oligosaccharides. J Biol Chem. 1981 Jul 25;256(14):7240–7247. [PubMed] [Google Scholar]
- Hök M., Lindahl U., Hallén A., Bäckström G. Biosynthesis of heparin. Studies on the microsomal sulfation process. J Biol Chem. 1975 Aug 10;250(15):6065–6071. [PubMed] [Google Scholar]
- Jacobsson I., Hök M., Pettersson I., Lindahl U., Larm O., Wirén E., von Figura K. Identification of N-sulphated disaccharide units in heparin-like polysaccharides. Biochem J. 1979 Apr 1;179(1):77–87. doi: 10.1042/bj1790077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson I., Lindahl U. Biosynthesis of heparin. Concerted action of late polymer-modification reactions. J Biol Chem. 1980 Jun 10;255(11):5094–5100. [PubMed] [Google Scholar]
- Kosakai M., Yamauchi F., Yosizawa Z. Isolation and characterization of sulfated disaccharides from the deamination products of porcine heparin (alpha-heparin) and whale heparin (omega-heparin) and a comparison of the deamination products. J Biochem. 1978 Jun;83(6):1567–1575. doi: 10.1093/oxfordjournals.jbchem.a132067. [DOI] [PubMed] [Google Scholar]
- Kraemer P. M. Heparan sulfates of cultured cells. I. Membrane-associated and cell-sap species in Chinese hamster cells. Biochemistry. 1971 Apr 13;10(8):1437–1445. doi: 10.1021/bi00784a026. [DOI] [PubMed] [Google Scholar]
- Lam L. H., Silbert J. E., Rosenberg R. D. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- Laurent T. C., Tengblad A., Thunberg L., Hök M., Lindahl U. The molecular-weight-dependence of the anti-coagulant activity of heparin. Biochem J. 1978 Nov 1;175(2):691–701. doi: 10.1042/bj1750691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Hök M., Thunberg L., Fransson L. A., Linker A. Structure of the antithrombin-binding site in heparin. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3198–3202. doi: 10.1073/pnas.76.7.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Jansson L., Hallén A. Biosynthesis of heparin. II. Formation of sulfamino groups. J Biol Chem. 1973 Oct 25;248(20):7234–7241. [PubMed] [Google Scholar]
- Lindahl U., Bäckström G., Thunberg L., Leder I. G. Evidence for a 3-O-sulfated D-glucosamine residue in the antithrombin-binding sequence of heparin. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6551–6555. doi: 10.1073/pnas.77.11.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker A., Hovingh P. Structural studies of heparitin sulfates. Biochim Biophys Acta. 1975 Apr 7;385(2):324–333. doi: 10.1016/0304-4165(75)90360-8. [DOI] [PubMed] [Google Scholar]
- Linker A. Structure of heparan sulphate oligosaccharides and their degradation by exo-enzymes. Biochem J. 1979 Dec 1;183(3):711–720. doi: 10.1042/bj1830711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmström A., Rodén L., Feingold D. S., Jacobsson I., Bäckström G., Lindahl U. Biosynthesis of heparin. Partial purification of the uronosyl C-5 epimerase. J Biol Chem. 1980 May 10;255(9):3878–3883. [PubMed] [Google Scholar]
- Metcalfe D. D., Smith J. A., Austen K. F., Silbert J. E. Polydispersity of rat mast cell heparin. Implications for proteoglycan assembly. J Biol Chem. 1980 Dec 25;255(24):11753–11758. [PubMed] [Google Scholar]
- Oldberg A., Kjellén L., Hök M. Cell-surface heparan sulfate. Isolation and characterization of a proteoglycan from rat liver membranes. J Biol Chem. 1979 Sep 10;254(17):8505–8510. [PubMed] [Google Scholar]
- Oosta G. M., Gardner W. T., Beeler D. L., Rosenberg R. D. Multiple functional domains of the heparin molecule. Proc Natl Acad Sci U S A. 1981 Feb;78(2):829–833. doi: 10.1073/pnas.78.2.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ototani N., Yosizawa Z. Anticoagulant activity of heparin octasaccharide. J Biochem. 1981 Nov;90(5):1553–1556. doi: 10.1093/oxfordjournals.jbchem.a133625. [DOI] [PubMed] [Google Scholar]
- Prihar H. S., Campbell P., Feingold D. S., Jacobsson I., Jensen J. W., Lindahl U., Rodén L. Biosynthesis of heparin. Hydrogen exchange at carbon 5 of the glucuronosyl residues. Biochemistry. 1980 Feb 5;19(3):495–500. doi: 10.1021/bi00544a016. [DOI] [PubMed] [Google Scholar]
- Riesenfeld J., Hök M., Lindahl U. Biosynthesis of heparin. Assay and properties of the microsomal N-acetyl-D-glucosaminyl N-deacetylase. J Biol Chem. 1980 Feb 10;255(3):922–928. [PubMed] [Google Scholar]
- Riesenfeld J., Hök M., Lindahl U. Biosynthesis of heparin. Concerted action of early polymer-modification reactions. J Biol Chem. 1982 Jan 10;257(1):421–425. [PubMed] [Google Scholar]
- Riesenfeld J., Hözok M., Lindahl U. Biosynthesis of heparan sulfate in rat liver. Characterization of polysaccharides obtained with intact cells and with a cell-free system. J Biol Chem. 1982 Jun 25;257(12):7050–7055. [PubMed] [Google Scholar]
- Riesenfeld J., Thunberg L., Hök M., Lindahl U. The antithrombin-binding sequence of heparin. Location of essential N-sulfate groups. J Biol Chem. 1981 Mar 10;256(5):2389–2394. [PubMed] [Google Scholar]
- Robinson H. C., Horner A. A., Hök M., Ogren S., Lindahl U. A proteoglycan form of heparin and its degradation to single-chain molecules. J Biol Chem. 1978 Oct 10;253(19):6687–6693. [PubMed] [Google Scholar]
- Rosenberg R. D., Armand G., Lam L. Structure-function relationships of heparin species. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3065–3069. doi: 10.1073/pnas.75.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. D., Lam L. Correlation between structure and function of heparin. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1218–1222. doi: 10.1073/pnas.76.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILBERT J. E. INCORPORATION OF 14C AND 3H FROM NUCLEOTIDE SUGARS INTO A POLYSACCHARIDE IN THE PRESENCE OF A CELL-FREE PREPARATION FROM MOUSE MAST CELL TUMORS. J Biol Chem. 1963 Nov;238:3542–3546. [PubMed] [Google Scholar]
- Saito H., Yamagata T., Suzuki S. Enzymatic methods for the determination of small quantities of isomeric chondroitin sulfates. J Biol Chem. 1968 Apr 10;243(7):1536–1542. [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Silbert J. E. Biosynthesis of heparin. IV. N-Deacetylation of a precursor glycosaminoglycan. J Biol Chem. 1967 Nov 10;242(21):5153–5157. [PubMed] [Google Scholar]
- Sugisaka N., Petracek F. J. Rapid molecular size characterization of heparins by high pressure liquid chromatography. Fed Proc. 1977 Jan;36(1):89–92. [PubMed] [Google Scholar]
- Taylor R. L., Shively J. E., Conrad H. E., Cifonelli J. A. Uronic acid composition of heparins and heparan sulfates. Biochemistry. 1973 Sep 11;12(19):3633–3637. doi: 10.1021/bi00743a010. [DOI] [PubMed] [Google Scholar]
- Thunberg L., Bäckström G., Lindahl U. Further characterization of the antithrombin-binding sequence in heparin. Carbohydr Res. 1982 Mar 1;100:393–410. doi: 10.1016/s0008-6215(00)81050-2. [DOI] [PubMed] [Google Scholar]
- Thunberg L., Lindahl U., Tengblad A., Laurent T. C., Jackson C. M. On the molecular-weight-dependence of the anticoagulant activity of heparin. Biochem J. 1979 Jul 1;181(1):241–243. doi: 10.1042/bj1810241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T., Saito H., Habuchi O., Suzuki S. Purification and properties of bacterial chondroitinases and chondrosulfatases. J Biol Chem. 1968 Apr 10;243(7):1523–1535. [PubMed] [Google Scholar]


