Abstract
Full-depth plugs of adult human articular cartilage were cut into serial slices from the articular surface and analysed for their glycosaminoglycan content. The amount of chondroitin sulphate was highest in the mid-zone, whereas keratan sulphate increased progressively through the depth. Proteoglycans were isolated from each layer by extraction with 4M-guanidinium chloride followed by centrifugation in 0.4M-guanidinium chloride/CsCl at a starting density of 1.5 g/ml. The efficiency with which proteoglycans were extracted depended on slice thickness, and extraction was complete only when cartilage from each zone was sectioned at 20 microns or less. When thick sections (250 microns) were extracted, hyaluronic acid was retained in the tissue. Most of the proteoglycans, extracted from each layer under optimum conditions, could interact with hyaluronic acid to form aggregates, although the extent of aggregation was less in the deeper layers. Two pools of proteoglycan were identified in all layers by gel chromatography (Kav. 0.33 and 0.58). The smaller of these was rich in keratan sulphate and protein, and gradually increased in proportion through the cartilage depth. Chondroitin sulphate chain size was constant in all regions. The changes in composition and structure observed were consistent with the current model for hyaline-cartilage proteoglycans and were similar to those observed with increasing age in human articular cartilage.
Full text
PDF

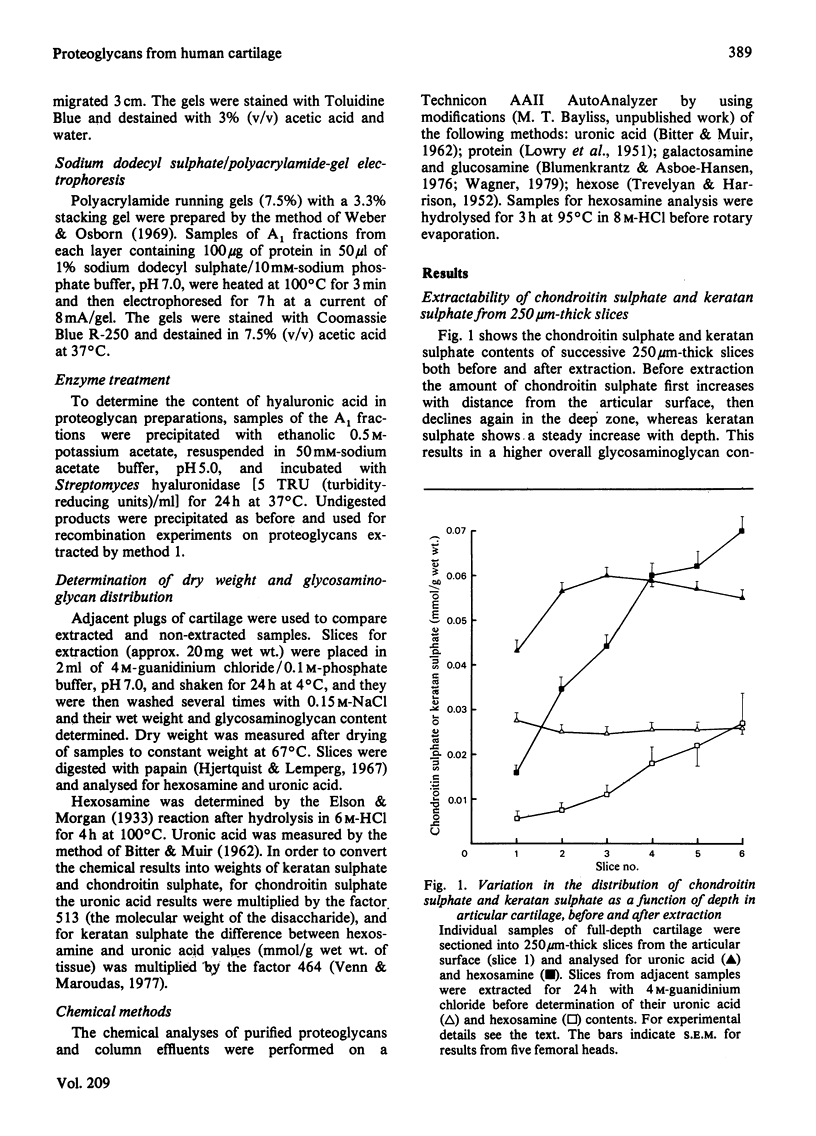

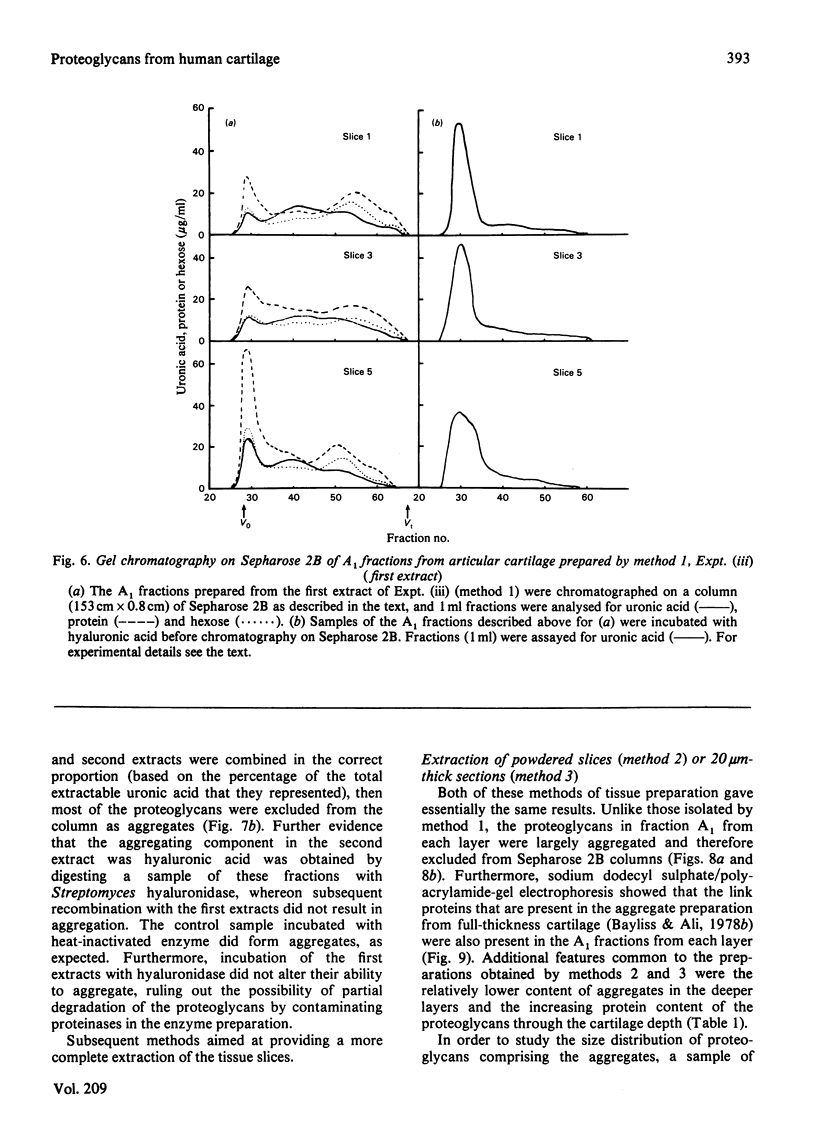
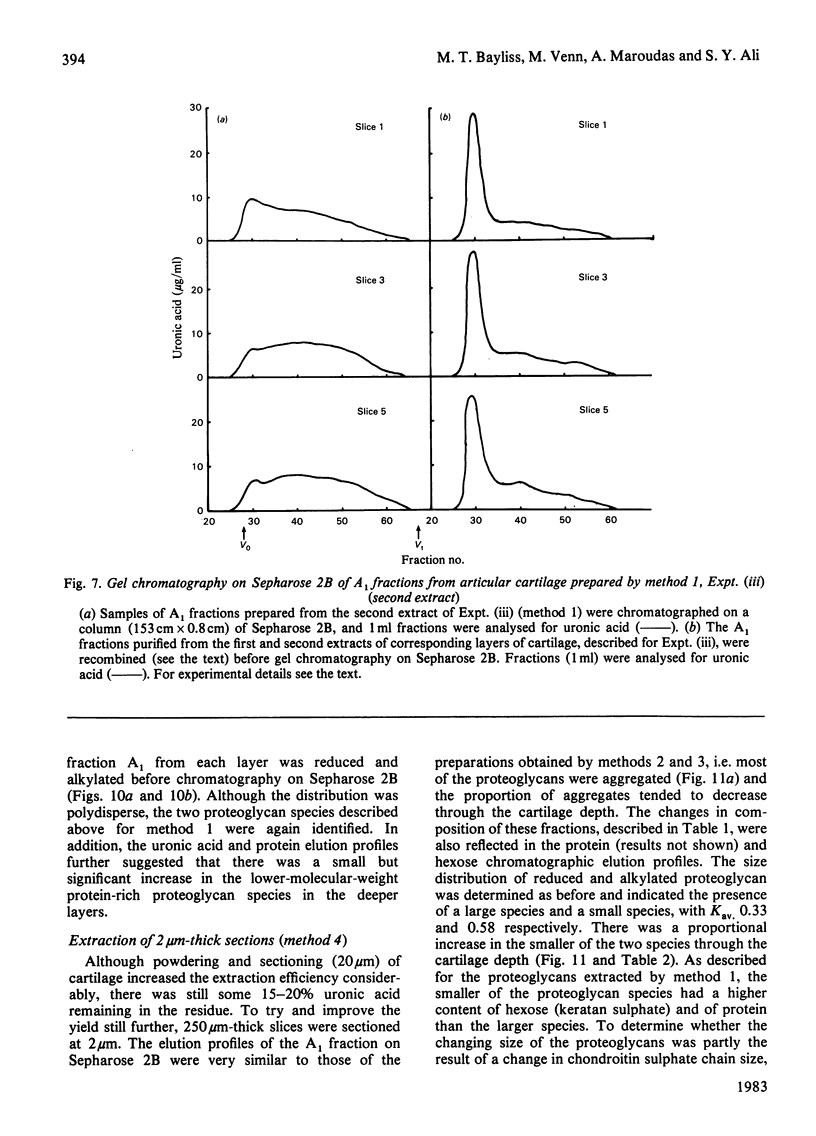
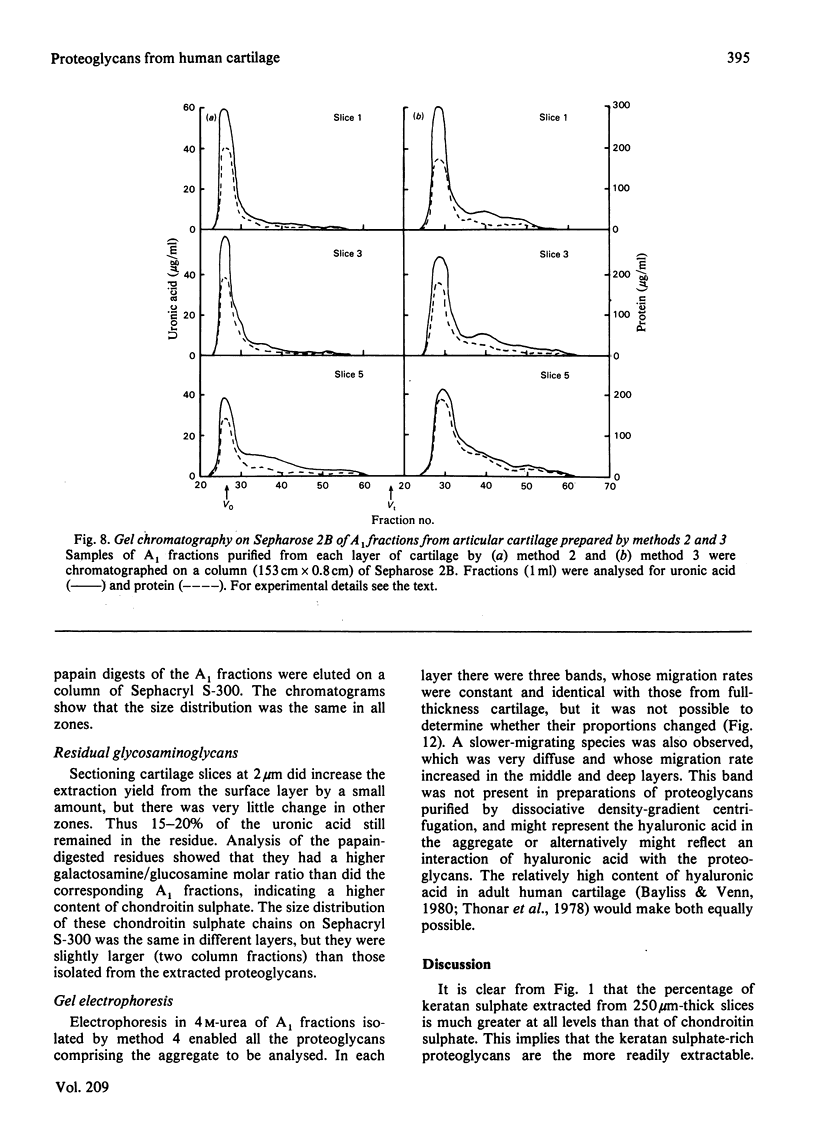
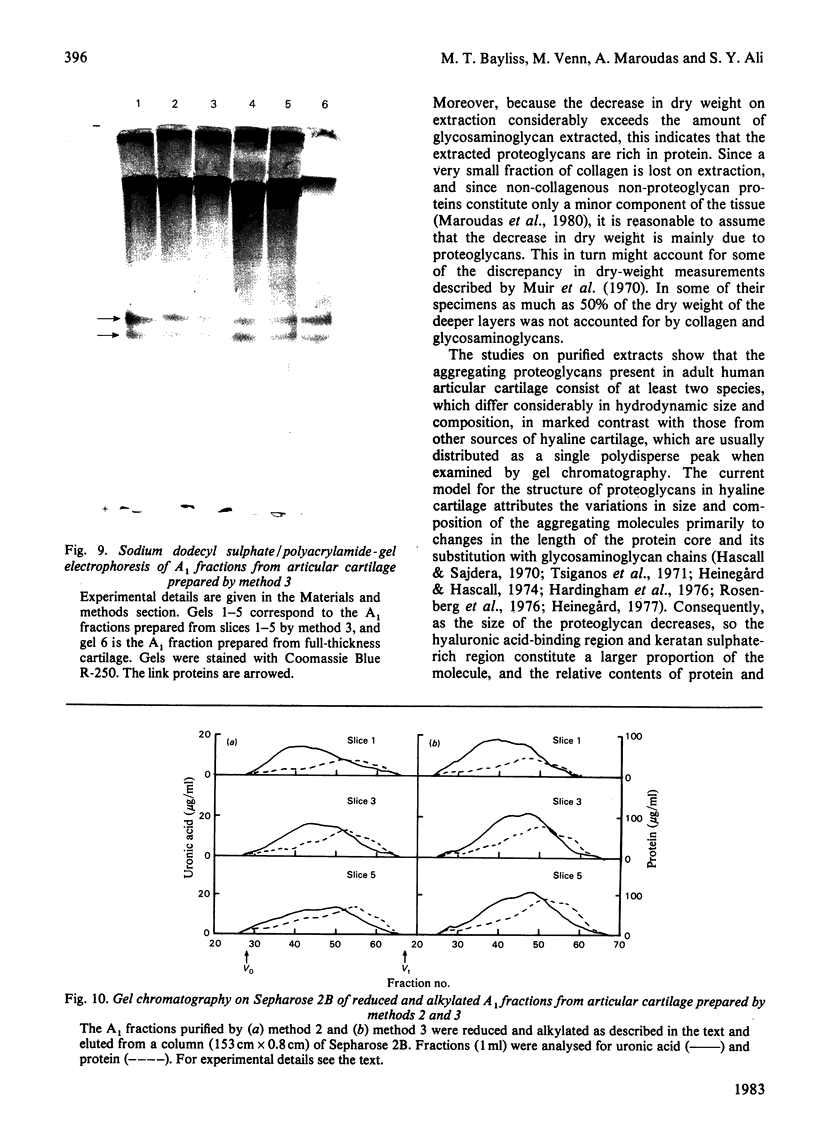



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
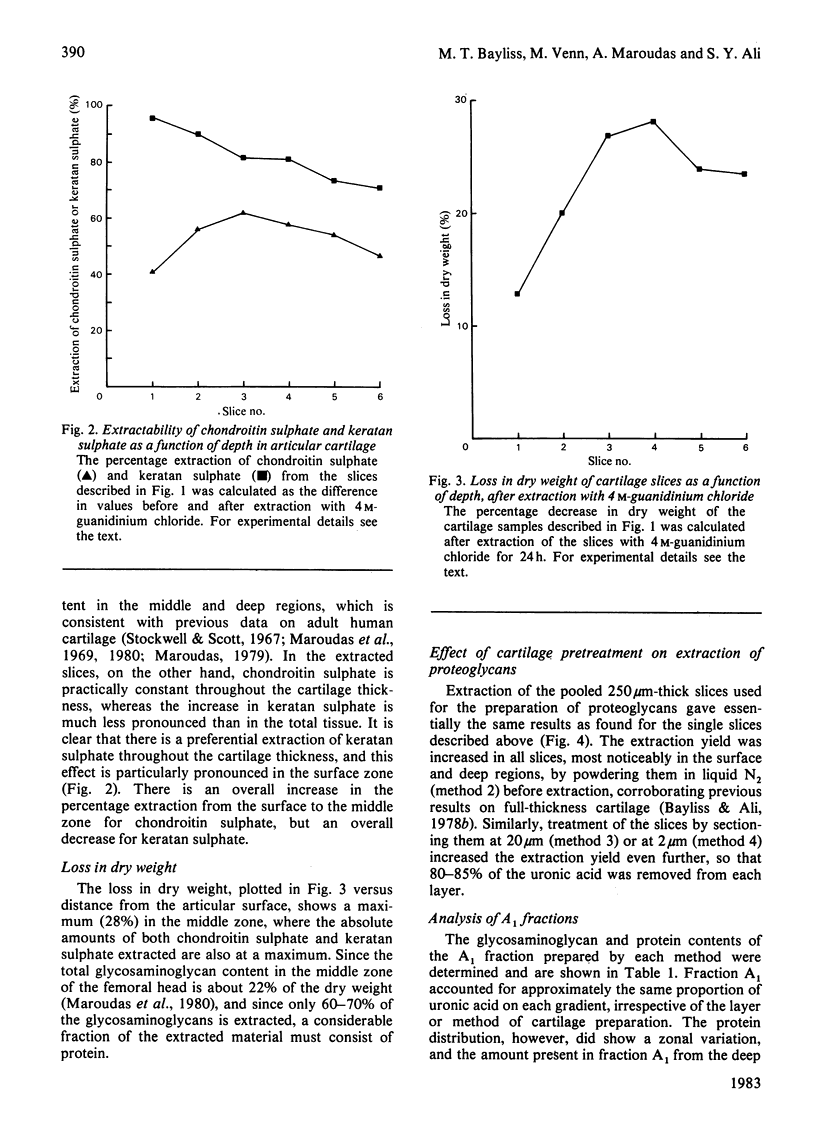
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Age-related changes in the composition and structure of human articular-cartilage proteoglycans. Biochem J. 1978 Dec 15;176(3):683–693. doi: 10.1042/bj1760683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Isolation of proteoglycans from human articular cartilage. Biochem J. 1978 Jan 1;169(1):123–132. doi: 10.1042/bj1690123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. An assay for total hexosamine and a differential assay for glucosamine and galactosamine. Clin Biochem. 1976 Dec;9(6):269–274. doi: 10.1016/s0009-9120(76)80075-6. [DOI] [PubMed] [Google Scholar]
- Bouvet J. P., Ramadier J. O., Auquier L. Hétérogénéité electrophorétique des protéoglycans du cartilage humain normal ou arthrosique: anomalies du cartilage néoformé. Rev Rhum Mal Osteoartic. 1978 Dec;45(12):719–724. [PubMed] [Google Scholar]
- Elson L. A., Morgan W. T. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27(6):1824–1828. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L., Sayers D. C., Ali S. Y. A method of preparation of cartilage for the study of its enzymes. J Med Lab Technol. 1967 Oct;24(4):306–308. [PubMed] [Google Scholar]
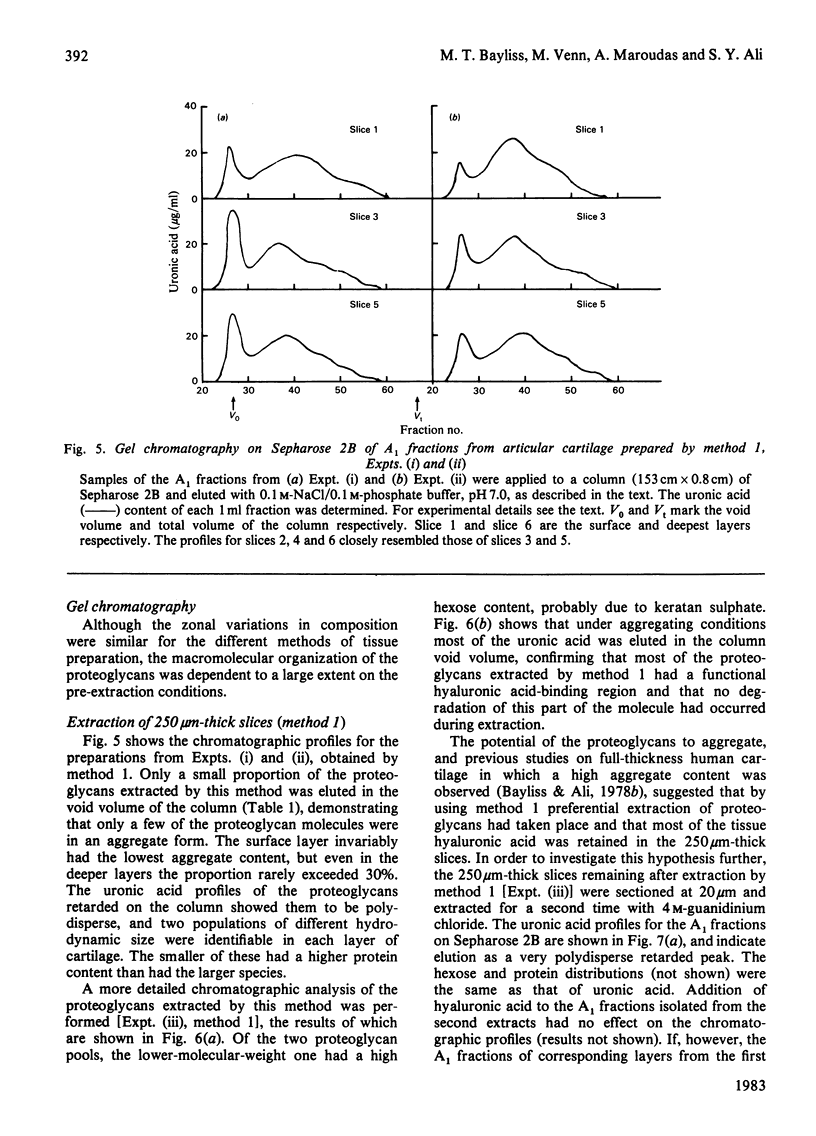
- Franzén A., Inerot S., Hejderup S. O., Heinegård D. Variations in the composition of bovine hip articular cartilage with distance from the articular surface. Biochem J. 1981 Jun 1;195(3):535–543. doi: 10.1042/bj1950535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Ewins R. J., Muir H. Cartilage proteoglycans. Structure and heterogeneity of the protein core and the effects of specific protein modifications on the binding to hyaluronate. Biochem J. 1976 Jul 1;157(1):127–143. doi: 10.1042/bj1570127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. Hyaluronic acid in cartilage and proteoglycan aggregation. Biochem J. 1974 Jun;139(3):565–581. doi: 10.1042/bj1390565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Physical properties and polydispersity of proteoglycan from bovine nasal cartilage. J Biol Chem. 1970 Oct 10;245(19):4920–4930. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Hjertquist S. O., Lemperg R. Studies of autologous diced costal cartilage transplant. 3. With special regard to glycosaminoglycans, hydroxyproline, calcium and 35S-sulphate incorporation in vitro after intramuscular implantation. Acta Soc Med Ups. 1967;72(3):173–198. [PubMed] [Google Scholar]
- Inerot S., Heinegård D., Audell L., Olsson S. E. Articular-cartilage proteoglycans in aging and osteoarthritis. Biochem J. 1978 Jan 1;169(1):143–156. doi: 10.1042/bj1690143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempson G. E., Spivey C. J., Swanson S. A., Freeman M. A. Patterns of cartilage stiffness on normal and degenerate human femoral heads. J Biomech. 1971 Dec;4(6):597–609. doi: 10.1016/0021-9290(71)90049-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsson S. E., Lemperg R. K. The glycosaminoglycans of the different layers of bovine articular cartilage in relation to age. II. Incorporation of 35s-sulphate in vitro into different fractions of chondroitin sulphate. Calcif Tissue Res. 1974;15(3):253–267. doi: 10.1007/BF02059061. [DOI] [PubMed] [Google Scholar]
- Lohmander S., Hjerpe A. Proteoglycans of mineralizing rib and epiphyseal cartilage. Biochim Biophys Acta. 1975 Sep 8;404(1):93–109. doi: 10.1016/0304-4165(75)90151-8. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Lippiello L. The glycosaminoglycans of normal and arthritic cartilage. J Clin Invest. 1971 Aug;50(8):1712–1719. doi: 10.1172/JCI106660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A., Bayliss M. T., Venn M. F. Further studies on the composition of human femoral head cartilage. Ann Rheum Dis. 1980 Oct;39(5):514–523. doi: 10.1136/ard.39.5.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A., Evans H., Almeida L. Cartilage of the hip joint. Topographical variation of glycosaminoglycan content in normal and fibrillated tissue. Ann Rheum Dis. 1973 Jan;32(1):1–9. doi: 10.1136/ard.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroudas A., Muir H., Wingham J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochim Biophys Acta. 1969 May 6;177(3):492–500. doi: 10.1016/0304-4165(69)90311-0. [DOI] [PubMed] [Google Scholar]
- McDevitt C. A., Muir H. Gel electrophoresis of proteoglycans and glycosaminoglycans on large-pore composite polyacrylamide-agarose gels. Anal Biochem. 1971 Dec;44(2):612–622. doi: 10.1016/0003-2697(71)90250-8. [DOI] [PubMed] [Google Scholar]
- Muir H., Bullough P., Maroudas A. The distribution of collagen in human articular cartilage with some of its physiological implications. J Bone Joint Surg Br. 1970 Aug;52(3):554–563. [PubMed] [Google Scholar]
- Pasternack S. G., Veis A., Breen M. Solvent-dependent changes in proteoglycan subunit conformation in aqueous guanidine hydrochloride solutions. J Biol Chem. 1974 Apr 10;249(7):2206–2211. [PubMed] [Google Scholar]
- Pearson J. P., Mason R. M. Heterogeneity of proteoglycans from adult human costal cartilage [proceedings]. Biochem Soc Trans. 1978;6(1):244–246. doi: 10.1042/bst0060244. [DOI] [PubMed] [Google Scholar]
- Peyron J. G., Stanescu R., Stanescu V., Maroteaux P. Distribution électrophorétique particulière des populations de protéoglycanes dans les zones de régénération du cartilage arthrosique et étude de leur collagène. Rev Rhum Mal Osteoartic. 1978 Oct;45(10):569–576. [PubMed] [Google Scholar]
- Reihanian H., Jamieson A. M., Tang L. H., Rosenberg L. Hydrodynamic properties of proteoglycan subunit from bovine nasal cartilage. Self-association behavior and interaction with hyaluronate studied by laser light scattering. Biopolymers. 1979 Jul;18(7):1727–1747. doi: 10.1002/bip.1979.360180711. [DOI] [PubMed] [Google Scholar]
- Rosenberg L., Wolfenstein-Todel C., Margolis R., Pal S., Strider W. Proteoglycans from bovine proximal humeral articular cartilage. Structural basis for the polydispersity of proteoglycan subunit. J Biol Chem. 1976 Oct 25;251(20):6439–6444. [PubMed] [Google Scholar]
- Roughley P. J. The degradation of cartilage proteoglycans by tissue proteinases. Proteoglycan heterogeneity and the pathway of proteolytic degradation. Biochem J. 1977 Dec 1;167(3):639–646. doi: 10.1042/bj1670639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., White R. J., Santer V. Comparison of proteoglycans extracted from high and low weight-bearing human articular cartilage, with particular reference to sialic acid content. J Biol Chem. 1981 Dec 25;256(24):12699–12704. [PubMed] [Google Scholar]
- Stockwell R. A., Scott J. E. Distribution of acid glycosaminoglycans in human articular cartilage. Nature. 1967 Sep 23;215(5108):1376–1378. doi: 10.1038/2151376a0. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonar E. J., Sweet M. B., Immelman A. R., Lyons G. Hyaluronate in articular cartilage: age-related changes. Calcif Tissue Res. 1978 Nov 10;26(1):19–21. doi: 10.1007/BF02013228. [DOI] [PubMed] [Google Scholar]
- Tsiganos C. P., Hardingham T. E., Muir H. Proteoglycans of cartilage: an assessment of their structure. Biochim Biophys Acta. 1971 Feb 16;229(2):529–534. doi: 10.1016/0005-2795(71)90216-9. [DOI] [PubMed] [Google Scholar]
- Venn M., Maroudas A. Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Ann Rheum Dis. 1977 Apr;36(2):121–129. doi: 10.1136/ard.36.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner W. D. A more sensitive assay discriminating galactosamine and glucosamine in mixtures. Anal Biochem. 1979 Apr 15;94(2):394–396. doi: 10.1016/0003-2697(79)90379-8. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]