Abstract
Background
Early exposure to general anaesthetics for multiple surgeries or procedures might negatively affect brain development. Recent studies indicate the importance of microbiota in the development of stress-related behaviours. We determined whether repeated anaesthesia and surgery in early life cause gut microbiota dysbiosis and anxiety-like behaviours in rats.
Methods
Sprague Dawley rats received skin incisions under sevoflurane 2.3 vol% three times during the first week of life. After 4 weeks, gut microbiota, anxiety-related behaviours, hippocampal serotonergic activity, and plasma stress hormones were tested. Subsequently, we explored the effect of faecal microbiota transplantation from multiple anaesthesia/surgery exposed rats after administration of a cocktail of antibiotics on anxiety-related behaviours.
Results
Anxiety-like behaviours were observed in rats with repeated anaesthesia/surgery exposures: In the OF test, multiple anaesthesia/surgery exposures induced a decrease in the time spent in the centre compared to the Control group (P<0.05, t=3.05, df=16, Cohen’s d=1.44, effect size=0.58). In the EPM test, rats in Multiple AS group travelled less (P<0.05, t=5.09, df=16, Cohen’s d=2.40, effective size=0.77) and spent less time (P<0.05, t=3.58, df=16, Cohen’s d=1.69, effect size=0.65) in the open arms when compared to the Control group. Repeated exposure caused severe gut microbiota dysbiosis, with exaggerated stress response (P<0.01, t=4.048, df=16, Cohen's d=−1.91, effect size=−0.69), a significant increase in the hippocampal concentration of 5-hydroxytryptamine (5-HT) and 5-hydroxyindoleacetic acid (5-HIAA) (P<0.05; for 5-HT: t=3.33, df=18, Cohen’s d=−1.49, effect size=−0.60; for 5-HIAA: t=3.12, df=18, Cohen’s d=−1.40, effect size=−0.57), and changes in gene expression of serotonergic receptors later in life (for Htr1a: P<0.001, t=4.49, df=16, Cohen’s d=2.24, effect size=0.75; for Htr2c: P<0.01, t=3.72, df=16, Cohen’s d=1.86, effect size=0.68; for Htr6: P<0.001, t=7.76, df=16, Cohen’s d=3.88, effect size=0.89). Faecal microbiota transplantation led to similar anxiety-like behaviours and changes in the levels of 5-hydroxytryptamine and 5-hydroxyindoleacetic acid.
Conclusions
Gut microbiota dysbiosis caused by early repeated exposure to anaesthesia and surgery affects long-term anxiety emotion behaviours in rats.
Keywords: anaesthesia, anxiety, brain development, dysbiosis, gut microbiota, microbiota-gut-brain axis, surgery
Editor's key points.
-
•
Early exposure to general anaesthesia has been shown to negatively affect brain development in animal models.
-
•
Given the importance of the gut microbiota to the development of stress-related behaviours, the authors determined whether anaesthesia and surgery in early life cause gut dysbiosis and anxiety-like behaviours in rats.
-
•
Multiple exposures of neonatal rats to sevoflurane and surgery led to subsequent anxiety-like behaviours later in life and changes in gut microbiota, stress hormones, and the hippocampal serotonergic system.
-
•
Faecal microbiota transfer from multiple anaesthesia/surgery exposed rats to naive rats reproduced the anxiety-like behaviours and changes in the serotonin system, indicating an important role for effects on the gut microbiota in developmental neurotoxicity.
General anaesthesia is required when infants and young children receive invasive procedures. However, preclinical studies and clinical trials show that exposure to general anaesthesia and surgery during early brain development can be associated with long-term alterations in cognition and behaviours.1, 2, 3, 4 A recent study indicated that early repeated exposure to inhalation anaesthetics in infant non-human primates led to an anxious phenotype and social behavioural changes.5, 6, 7 However, the exact mechanisms underlying general anaesthesia- and surgery-related long-term behavioural changes are unclear.
Strong evidence indicates that differences in gut microbiota can influence behaviours and lead to stress-related disorders later in life.8, 9, 10 Recent studies have reported gut microbiota dysbiosis after exposure to anaesthesia/surgery in both juvenile and aged rodents.11, 12, 13, 14 These data suggest that abnormalities in gut microbiota might underlie subsequent behavioural changes caused by anaesthesia/surgery. The mechanisms underlying communication along the microbiota–gut–brain axis are being studied,15 and the endocrine system could play an important role.16 Moreover, the gut microbiota contribute to the developing serotonergic system, which is particularly relevant to stress, anxiety, and depression,17,18 and the microbiome–gut–brain axis regulates the hippocampal serotonergic system during early life.19
We aimed to elucidate possible correlations between multiple anaesthesia/surgery exposures early in life and changes in gut microbiota in juvenile rats. We also examined faecal microbiota transplantation to verify the role of gut microbiota in anxiety-related behaviours.
Methods
Anaesthesia and surgery
Animal use was approved by the Institutional Animal Care and Use Committee at Sun Yat-sen University (Guangzhou, China). Male and female Sprague Dawley rats at postnatal day 6 (PND 6, 16–17 g) were used with all efforts made to minimise the number of animals used and any suffering. The room was illuminated with a 12-h light–dark cycle, and the room temperature was maintained at 20–22°C. Rats had ad libitum access to water and food.
The pups at PND 6 were randomly divided into the air-treated control group (Control group) and the multiple sevoflurane/surgery exposure group (Multiple AS group). Moreover, multiple anaesthesia exposed rats without surgery (Multiple A group) were added for additional comparison. We cross-fostered rats before weaning and co-housed rats after weaning to eliminate the microbiota cage effect.20 The anaesthesia exposure model was performed as reported previously.21 Rats in the Multiple AS group were placed in a plastic container and exposed sevoflurane to 2.3 vol% continuously for 4 h using air as a carrier with the total gas flow of 2 L min−1 on PND 6, 7, and 8. Gas levels in the chamber were monitored using a gas monitor (Detex-Ohmeda, Louisville, KY, USA). Rats in the Control group were placed in a similar container and exposed only to air for 4 h. Pups in the Multiple AS group were subjected to a back-incision procedure 10 min after induction with minor modification22 (details provided in Supplementary methods).
Tissue harvest and faeces collection
On PND 35, faecal pellets from all groups were collected for microbiome analysis (n=9 per group) and subsequent faecal microbiota transfer (n=10 per group). On the same day, after faeces collection, nine rats were used for behavioural tests, while 10 rats were killed under deep pentobarbital (200 mg kg−1, i. p.). The hippocampus was removed, cooled on ice, snap-frozen, and stored for assessing anxiety-related neurotransmitters and 5-hydroxytryptamine (5-HT) receptors using liquid chromatography–mass spectrometry analysis and quantitative polymerase chain reaction (qPCR) analysis, respectively (see Supplementary material). Finally, plasma was collected from 10 rats at baseline and 10 after stress to measure novel environment stress-induced corticosterone. All samples were frozen at −80°C until used.
Anxiety-related behavioural tests
On PND 35, open field test and elevated plus maze test were used. All behavioural tests were carried out by investigators blind to treatment conditions. We also collected data and analysed sex differences in behavioural tests on juvenile rats. The detailed protocols of behavioural tests are presented in Supplementary material.
Measurement of corticosterone after novel environment stress
Rats were individually removed from the home cage to a novel cage of 33×16×13 cm for 30 min before sacrifice. Baseline and post-stress corticosterone were assessed with separate groups of rats to avoid the stress caused by blood draws (cross-section design). Blood samples (2–3 ml) were collected into prechilled 4-ml centrifuge tubes containing ethylenediaminetetraacetic acid (7.2 mg) and placed on ice. Samples were centrifuged at 1500 g for 10 min in a refrigerated centrifuge (at 4°C). Plasma samples were then pipetted off and processed immediately. Corticosterone levels were analysed using a commercial ELISA kit (Cat# EIA-4164, DRG Diagnostics, RRID:AB_2636819, Germany) as described in Supplementary material.
Microbiome analysis
DNA extraction with the PowerFecal DNA/RNA Kit (Cat#80244, QIAGEN, Dusseldorf, Germany) and quantification of faecal bacteria were performed according to the manufacturer's instructions (see Supplementary material). Alpha diversity was applied in analysing the complexity of species diversity for a sample through four indices, including Observed species, Chao 1, Shannon and Simpson. Beta diversity analysis was used to evaluate differences of samples in species complexity using analysis of similarity (ANOSIM). Weighted UniFrac distance matrices and visualisation by principal components analysis (PCoA) were calculated. Based on the relative abundance of species at each classification level in OUT table, R software was used to draw the heat map. Linear discriminant analysis effect size, which emphasises both statistical significance and biological relevance, was used to find biomarkers of each group using R software.
Faecal microbiota transfer
Faecal collection and faecal microbiota transfer were conducted according to previous studies (see Supplementary material).23,24 Anxiety-related behavioural tests and levels of 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) were assessed 4 weeks after faecal microbiota transfer.
To confirm the effect of antibiotics, we compared 10 rats with normal drinking water for 2 weeks with 10 randomly chosen antibiotic-treated rats for bacterial load determined by semiquantitative real time–PCR and alpha diversity. We also performed qPCR targeting 16s rRNA of certain major bacteria before behavioural tests to make sure faecal microbiota transfer was successful after antibiotic treatment. The 5-HT receptor antagonist ziprasidone (ZIP, Cat#CP88059, GlpBio, Montclair, CA, USA) was administered i. p. to investigate the relationship between faecal microbiota transfer phenotype and 5-HT receptors. Rats in the FMT-Multi+ZIP (n=10) received ZIP (2.5 mg kg−1 daily) for 7 days, based on previous studies.25
Statistics
GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA, USA) was used for statistical analysis and figure creation. Data were tested for normality using Wilke Shapiro test, but are presented as mean (standard error of the mean) to facilitate visual comparisons, whereas non-normally distributed variables are shown as median and inter-quartile range. Comparisons of mean values of two independent groups were performed using the Student's t-test or Mann–Whitney U-test. For comparisons of the mean values of three or more independent groups, we used one-way analysis of variance (anova) followed by Tukey post hoc test.
For microbiota analysis, alpha diversity was calculated with QIIME. Beta diversity analysis was done using ANOSIM. Weighted UniFrac distance matrices and visualisation by PCoA were calculated. R software was used to draw the heat map based on the relative abundance of species at each classification level. P-values ≤0.05 were considered statistically significant.
Data repository
The 16SrRNA gene sequence data were deposited at NCBI under Bioproject: PRJNA597811, available from https://www.ncbi.nlm.nih.gov/bioproject/597811.
Results
Anxiety-like behaviour
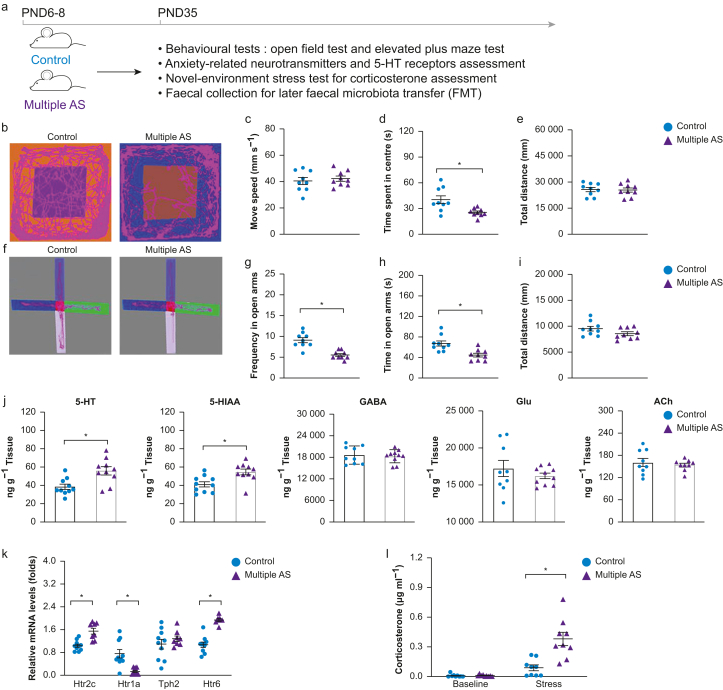
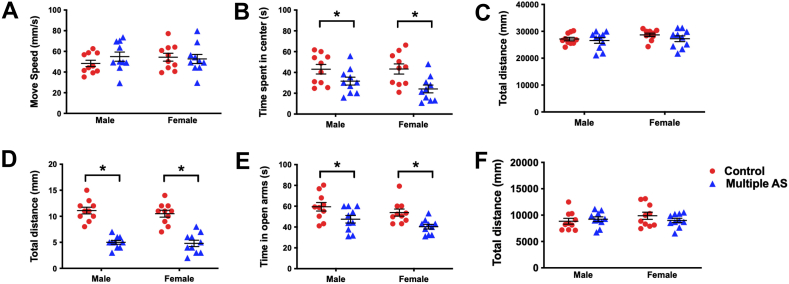
We determined the effects of multiple anaesthesia/surgery on later anxiety-like behaviours in rats. In the open field test (Fig 1 b–e), multiple anaesthesia/surgery exposures induced a decrease in the time spent in the centre compared with the Control group (Fig. 1d, P<0.05, t=3.05, df=6, Cohen's d=1.44). No significant difference was found between groups in movement speed (Fig. 1c, P>0.05, t=0.58, df=16, Cohen's d=0.27) and distance (Fig. 1e, P>0.05, t=0.15, df=16, Cohen's d=0.07). In the elevated plus maze test (Fig. 1e–h), rats in the Multiple AS group travelled less (Fig. 1g, P<0.05, t=5.09, df=16, Cohen's d=2.40) and spent less time (Fig. 1h, P<0.05, t=3.58, df=16, Cohen's d=1.69) in the open arms compared with the Control group. The total travel distance did not differ between groups (Fig. 1i, P>0.05, t=1.66, df=16, Cohen's d=0.78). There were no significant differences between male and female rats (Supplementary Fig. S1).
Fig 1.
Impact of multiple anaesthesia/surgery exposure on anxiety-like behaviours, hippocampal 5-HT transmission and stress response in juvenile rats. (a) Illustration of experimental design. (b–i) Multiple anaesthesia/surgery exposures resulted in anxiety-like behaviours shown in the open field test (OF, b–e) and elevated plus maze test (EPM, f–i). (b) Representative routes of rats in the OF test. No statistical difference was found between the two groups in distance moved (c) and total distance (e), whereas rats in the Multiple AS group showed a significant reduction in time spent in the centre of the open field (d). (f) Representative routes of rats in the EPM test between different groups. Rats in the Multiple AS group exhibited a markedly lower number of open-arm entries (g) along with less time spent in the open arms (h). (j) Hippocampal 5-HT, 5-HIAA, GABA, Glu, and ACh concentrations evaluated using liquid chromatography–mass spectrometry. Significantly elevated 5-HT and 5-HIAA levels in the hippocampus were detected in the Multiple AS group. (k) Quantitative real-time polymerase chain reaction analysis of major subtypes of 5-HT receptors (Htr1a, Htr2c, Tph2, and Htr6) in the hippocampus relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The mRNA levels of Htr1a, Htr2c, and Htr6 in the hippocampus significantly changed after multiple anaesthesia/surgery exposures. (l) Cortisol concentrations at baseline and after stress of the two groups were analysed via enzyme-linked immunosorbent assay. The cortisol concentration in the Multiple AS group after stress was significantly higher than in the control group. n=10 for each group. Data are shown as the mean (sem) of triplicate wells in three different experiments. ∗P<0.05. 5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, 5-hydroxytryptamine; ACh, acetylcholine; GABA, gamma-aminobutyric acid; Glu, glutamic acid; Multiple AS, multiple sevoflurane/surgery exposure; PND, postnatal day.
Hippocampal 5-HT levels and serotonergic receptor gene expression
We screened anxiety-related neurotransmitters in juvenile rats exposed to multiple anaesthesia/surgery (Fig 1g). Of the five neurotransmitters tested, only 5-HT and its metabolite 5-HIAA showed significant differences in the Multiple AS group compared with the Control group (P<0.05; for 5-HT: t=3.33, df=18, Cohen's d=−1.49; for 5-HIAA: t=3.12, df=18, Cohen's d=−1.40). No significant differences were found in the levels of gamma-aminobutyric acid (GABA), glutamic acid (Glu), and acetylcholine (Ach) (P>0.05; for GABA: t=0.41, df=16, Cohen's d=0.19; for Glu: t=0.73, df=16, Cohen's d=0.38; for ACh: t=0.55, df=16, Cohen's d=0.26). Relative mRNA levels of the three major 5-HT receptors (Htr1a, Htr2c, and Htr6) were altered (Fig. 1k, P<0.05). Among them, Htr2c and Htr6 were increased, whereas Htr1a was decreased in the Multiple AS group. However, the 5-HT synthetic enzyme (Tph2) was not affected (P>0.05).
Hypothalamic–pituitary–adrenal axis reactivity
To assess a potential association between the endocrine system and multiple anaesthesia/surgery exposures in neonatal rats, we measured corticosterone after novel environment stress. There was no significant difference between groups in baseline corticosterone (Fig. 1l, P>0.05). After stress, the level of corticosterone in the Multiple AS group was higher than in the Control group (P<0.01), suggesting exaggerated hypothalamic–pituitary–adrenal (HPA) axis reactivity.
Changes in the gut microbiome
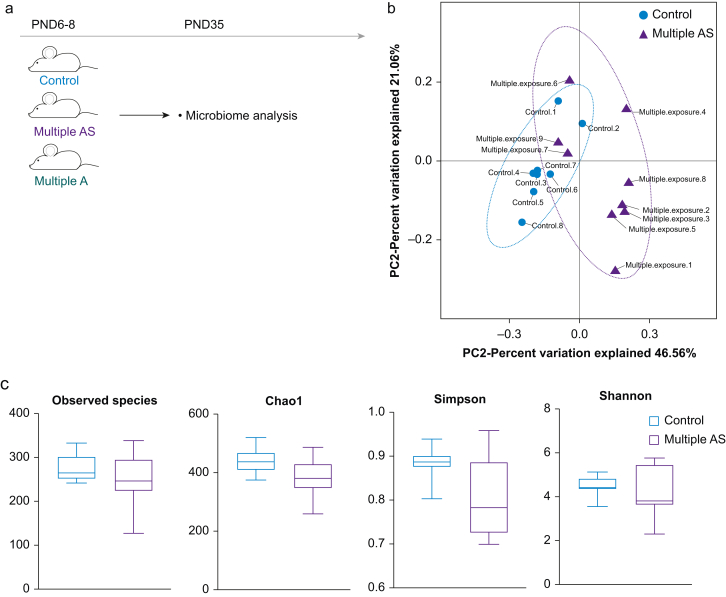
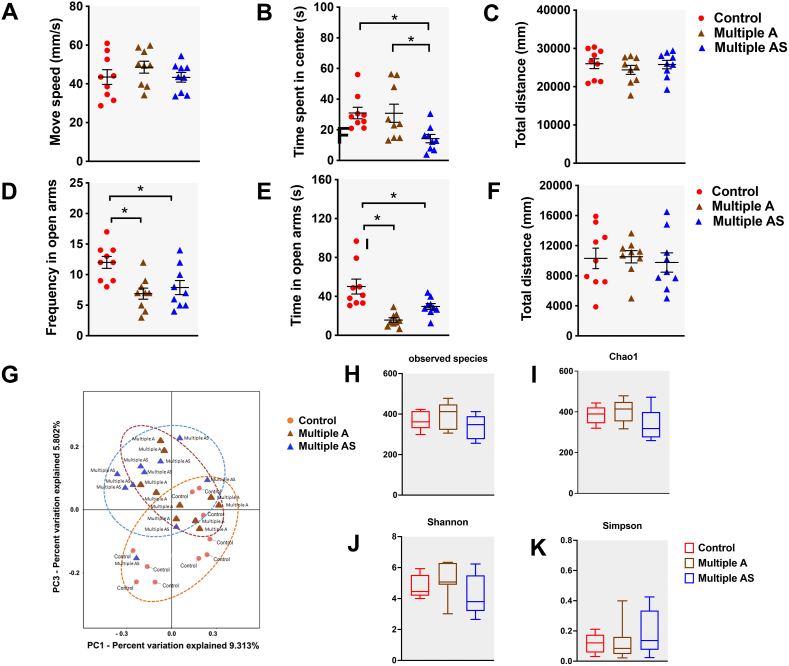
We examined whether multiple anaesthesia/surgery exposures induced long-term changes in the gut microbiome 4 weeks after exposure. The overall composition of the gut microbiome was different between the two groups. We analysed the principal component in the gut microbiome of both groups by PCoA based on the weighted UniFrac distance on operational taxonomic units (OTU) (Fig 2b). We performed permutational multivariate anova analyses and found sufficient evidence to reject the hypothesis that rats exposed multiply to sevoflurane were similar to those exposed to air. Alpha diversity was then quantified by observed species, Chao 1, and Shannon and Simpson. No statistical difference between groups (Fig 2c, Pobserved species= 0.26, PChao1=0.06, PShannon=0.28, PSimpson=0.12, all P>0.05) was found, since the indices used here relate to OTU richness, evenness, and rare species. The results show that both groups shared similar phenotypes in OUT richness, evenness, and rare species.
Fig 2.
Early exposures to anaesthesia/surgery changed the composition of the gut microbiome in juvenile rats. (a) Illustration of experimental design. (b) Principal components analysis (PCoA) of weighted UniFrac distance representing beta diversity of the gut microbiota in rats of both Control (red) and Multiple AS groups (green). The first two axes are represented with principal coordinate axis 1 (46.6% variability) and principal coordinate axis 2 (21.1% variability). (c) Indices representing alpha diversity of the gut microbiota between Control and Multiple AS groups. The analysis of alpha diversity indices showed no statistical difference between groups (Pobserved species=0.26, PChao1=0.06, PShannon=0.28, PSimpson=0.12, all P>0.05). (d–g) Differences in the microbiota composition at the phylum (d and e) and family levels (f and g) in rats from the Control and Multiple AS groups. Bar graphs show individual rat (d) and mean (e) relative abundance of the major phyla. Bar graphs show individual rat (f) and mean (g) relative abundance of the major families. (h and i) Differences in the microbiota composition at the phylum and family levels in rats from the Control, Multiple A, and Multiple AS groups. Bar graphs showed mean relative abundance of the major phyla (h) and major families (i). Multiple A, multiple anaesthesia without surgery; Multiple AS, multiple sevoflurane/surgery exposure; PND, postnatal day.
Changes of predominant bacteria (>20%) in the two groups are clearly represented at the phylum (Fig 2d and e) and family levels (Fig 2f and g). In terms of the abundance at the phylum level, the predominant bacteria (>20%) were Bacteroidetes (0.38 [0.05]) and Firmicutes (0.53 [0.06]) in the Control group, whereas Bacteroidetes (0.37 [0.05]), Firmicutes (0.26 [0.05]), and Verrucomicrobia (0.26 [0.07]) predominated in the Multiple AS group. Compared with the Control group, the relative abundance of Firmicutes was decreased (P=0.002), whereas Verrucomicrobia was increased (P=0.018). At the family level, there were changes in the predominant bacteria Lactobacillaceae and Verrucomicrobiaceae. In the Control group, the predominant bacteria were Lactobacillaceae (0.37 [0.06]) and S24-7 (0.29 [0.04]), whereas in the Multiple AS group they were Verrucomicrobiaceae (0.25 [0.07]) and S24-7 (0.23 [0.03]). The relative abundance of Lactobacillaceae was decreased (P<0.01), whereas that of Verrucomicrobiaceae was increased (P=0.018).
Changes in anxiety-like behaviours and gut microbiome composition
We included a Multiple A group to differentiate the individual contributions of anaesthesia and the surgical procedure to the anxiety-related phenotype and changes in gut microbiome. In the open field test, rats in both the Control group and the Multiple A group showed more time spent in the centre than the Multiple AS group (Supplementary Fig. S2b, P<0.05). In the elevated plus maze test, rats in the Multiple A group and Multiple AS group showed less frequency and time in open arms compared with the Control group (Supplementary Fig. S2d and e, P<0.05). The other indices between the three groups had no significant differences (Supplementary Fig. S2a, c, f, P>0.05).
Changes in the predominant bacteria in the three groups are clearly represented at the phylum (Fig 2h) and family levels (Fig 2i). In terms of abundance at the phylum level, the trend of the Multiple A group is basically the same as that of the Multiple AS group, and no significant difference was found between the Multiple A group and the Multiple AS group (P>0.05). However, at the family level, changes in composition of Enterobacteriaceae, Helicobacteraceae, Lachnospiraceae, Lactobacillaceae, Moraxellaceae, Prevotellaceae, Ruminococcaceae, and Paraprevotellaceae were found between groups (P<0.05). Among them, four bacteria, including Lachnospiraceae, Lactobacillaceae, Ruminococcaceae, and Paraprevotellaceae, showed differences between the Multiple A group and the Multiple AS group (P<0.05). See Supplementary materials for detailed analysis.
Gut microbiota transfer showed similar anxiety-like behaviours
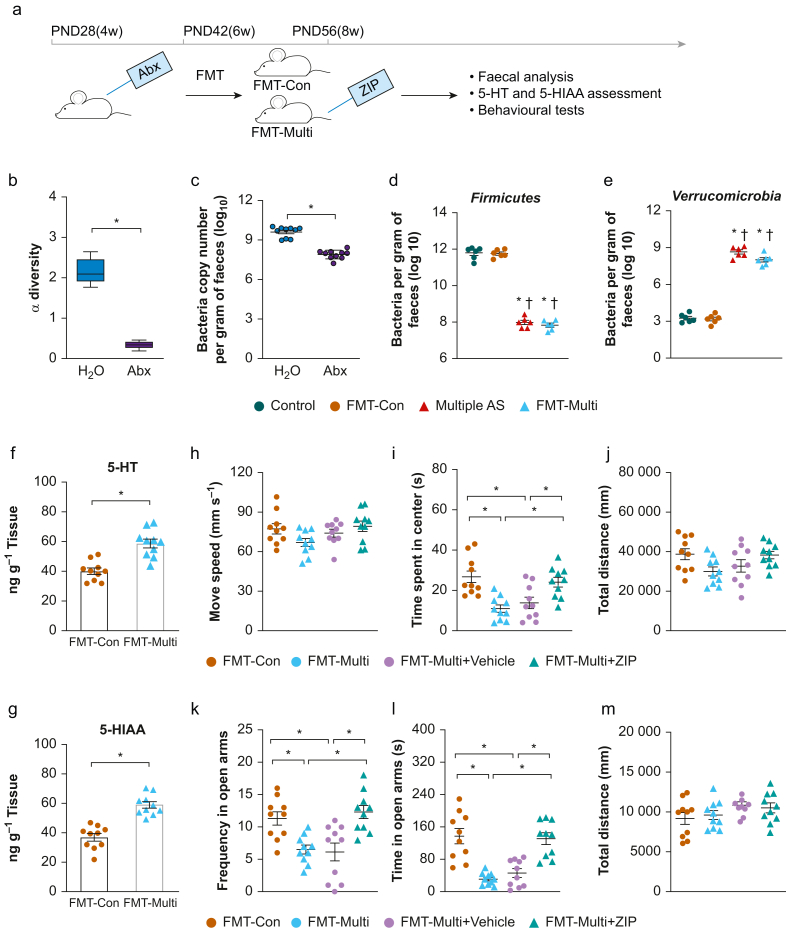
To determine the link between multiple anaesthetic/surgery exposure-induced changes in behavioural and gut microbiota, we applied faecal microbiota transplantation to antibiotic-treated rats. To verify the antibiotic effect, we performed bacterial alpha diversity of microbiota and load assessment. Feeding mice antibiotics reduced alpha diversity of microbiota (P<0.05, Fig 3b) and bacterial load (P<0.05, Fig 3c) by semiquantitative real-time PCR. Quantifications of two major bacteria in high proportion, including Firmicutes and Verrucomicrobia, were assessed by qPCR targeting 16s rRNA extracted from faeces after faecal microbiota transplantation (Fig 3d and e). There was no significant difference in the levels of the above bacteria between the Control and FMT-Con groups, or the Multiple AS and FMT-Multi groups (P>0.05). The results confirmed that the bacteria colonised the recipient rats. The levels of 5-HT and 5-HIAA were increased in the FMT-Multi group compared with the FMT-Con group (Fig. 3f and g, P<0.05; for 5-HT: t=5.16, df=18, Cohen's d=-2.31; for 5-HIAA: t=6.60, df=18, Cohen's d=−2.95).
Fig 3.
Antibiotic-treated rats treated with gut microbiota transfer showed similar anxiety-like behaviours. (a) Illustration of experimental design. (b) Feeding mice antibiotics reduced alpha diversity of microbiota (P<0.05). (c) Feeding mice antibiotics reduced bacterial load (P<0.05) by semiquantitative real-time polymerase chain reaction (PCR). (d and e) Quantification of Firmicutes spp. and Verrucomicrobia spp. by quantitative PCR in 16s rRNA extracted from faeces after faecal microbiota transfer (FMT) (n=6 per group, ∗P<0.05 vs Control group, †P<0.05 vs FMT-Con group). (f and g) Levels of 5-HT and 5-HIAA were increased in the FMT-Multi group compared with the FMT-Con group (P<0.05). (h–j) Open Field Test (OF test) of FMT-Con, FMT-Multi, FMT-Multi+Vehicle, and FMT-Multi+ZIP groups. Compared with the FMT-Con group and the FMT-Multi+ZIP group, the FMT-Multi and FMT-Multi+Vehicle groups exhibited markedly less time in the centre (i, P<0.05). No statistical differences were found between groups in movement speed (h) and total distance (j, P>0.05). (k–m) Elevated plus maze test (EPM) of different groups. No statistical difference was found between groups in total distance (m, P>0.05), whereas the FMT-Multi and FMT-Multi+Vehicle groups showed a reduction in frequency (k) and time spent in the open arm (l, P<0.05). One-way analysis of variance followed by Tukey post hoc test. n=10 for each group, ∗P<0.05.5-HIAA, 5-hydroxyindoleacetic acid; 5-HT, 5-hydroxytryptamine; Abx, antibiotic mix in the drinking water for 2 weeks; Multiple AS, multiple sevoflurane/surgery exposure; PND, postnatal day; ZIP, ziprasidone 2.5 mg kg−1 i. p. daily for 7 days.
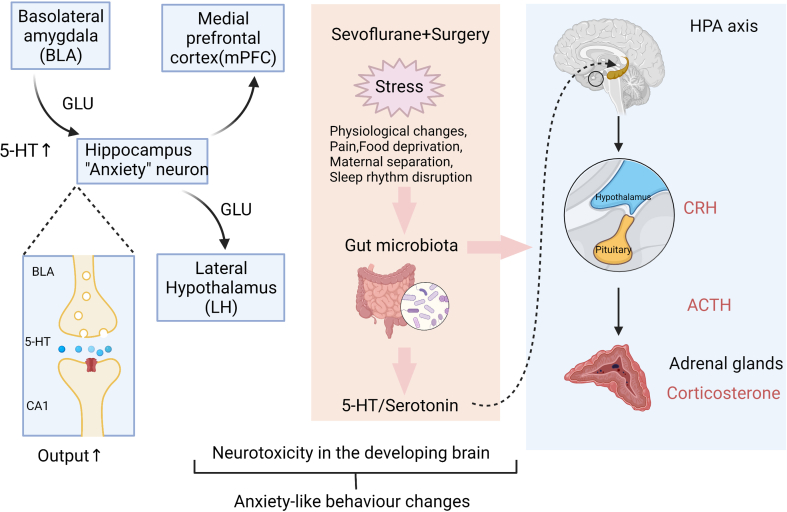
In the open field test (Fig. 3h–j), compared with the FMT-Con and FMT-Multi+ZIP groups, rats in the FMT-Multi and FMT-Multi+Vehicle groups exhibited markedly less time in the centre (P<0.05). No difference was found between groups in movement speed and total distance (P>0.05). For the elevated plus maze test (Fig. 3k–m), rats in the FMT-Multi and FMT-Multi+Vehicle groups showed a reduction in frequency and time spent in the open arm (P<0.05). No statistical difference was found between groups in total distance (P>0.05). Supplementary Figure S3 shows a proposed mechanism for the gut–brain axis interactions based on our study.
Discussion
We show that multiple early-life anaesthesia/surgery exposures are associated with long-term gut microbiota dysbiosis and increased anxiety in juvenile rats. Among the anxiety-related neurotransmitters, 5-HT and 5-HIAA were increased in the hippocampus. The mRNA expression of serotonergic receptors in hippocampus was decreased and plasma corticosterone was elevated in exposed rats. Moreover, antibiotic-pretreated rats with gut microbiota transfer showed similar anxiety-like behaviours, and changes in levels of 5-HT, indicating a modulatory influence of gut microbiota on long-term anxiety behaviour in rats.
A number of cohort studies2,3,26 and preclinical evidence21,27, 28, 29 indicate the potential neurotoxicity and behaviour effects after early anaesthesia/surgery exposure. More specifically, it is suggested that repeated exposures, but not a single exposure, are associated with subsequent impaired neurodevelopment and abnormal social behaviours.30 The long-term effect of early anaesthesia/surgery exposure on neurocognitive behaviours in later life, including anxiety, remains elusive. A recent study showed that repeated sevoflurane exposure in early life alters emotional reactivity in primates,5 which is consistent with our results showing that early repeated exposures led to later anxiety-like behaviours in juvenile rats. A recent study found that early exposure to midazolam, N2O, and isoflurane results in long-lasting anxiety-related behaviours in both male and female rats.31 Interestingly, their results further showed that females were more affected than males. However, other studies have reported that male rats exposed to sevoflurane alone may be more susceptible.32, 33, 34 Our study showed no sex differences. This suggests that the different result lies in different exposure models, which needs to be answered in further studies.
Maternal, genetic, and environmental factors contribute to neonatal rodent microbial colonisation.35 We strictly controlled these factors among groups before any manipulation. Our results indicate that repeated anaesthesia and surgery in early life can cause gut microbiota dysbiosis 4 weeks after exposure. A recent investigation also showed abnormal composition of gut microbiota and delirium-like behaviour after anaesthesia/surgery in adult mice.36 Another preclinical study revealed that juvenile rats showed changes in gut microbiota after a single neonatal exposure to isoflurane.14 Compared with controls, the abundances of Firmicutes and Lachnospiraceae were increased in exposed rats, whereas Bacteroidetes and Bacteroidaceae were decreased. Our results showed that, in multiple anaesthesia/surgery exposed rats, the relative abundance of Firmicutes and Lachnospiraceae was decreased, whereas Bacteroidetes and Bacteroidaceae did not significantly change. The discrepancy may lie in different animal exposure models, indicating that the gut microbiome is largely influenced by different environmental settings.
An increasing number of studies suggest that exposure to surgery and anaesthesia is associated with adverse cognitive development.37, 38, 39 In conjunction with these studies assessing the deleterious effects of anaesthesia and surgery, we tried to dissociate their potential effects. Compared with the Mutiple AS group, the Multiple A group showed similar but milder anxiety-like behaviours and changes in the gut microbiome, indicating that surgery itself contributes to the adverse effect on cognitive development. Our findings are in line with other studies, emphasising the role that nociceptive stimulation may play in the perioperative period.39, 40, 41
Anaesthesia/surgery exposure during a vulnerable period is a focus of anaesthetic research.42 We chose PND 6–8, as it aligns with the majority of works performed on PND 7 rats.27 Our results show that PND 6–8 is a vulnerable window for changes in the gut microbiota. To determine the causal relationship of the gut microbiome and behavioural changes, faecal microbiota transfer was applied to 4-week-old rats during the window of opportunity to influence microbiome composition.35 After 4 weeks of antibiotic pretreatment and microbiota transfer, 8-week-old rats still showed anxiety-like behaviours, which in turn emphasises the important role gut microbiota play in the behavioural changes. The mechanism may relate to central changes in the serotonergic system based on our findings.
Recent studies have reported the bidirectional relationship between gut microbiota and mood disorders.43 We detected some specific alterations in gut microbiome that compare to those found in patients with mood disorders. In healthy humans, Firmicutes and Bacteroidetes make up >90% of the intestinal microbiota.44 A Major Depression Disorder (MDD) study showed decreased Firmicutes and increased Proteobacteria in patients.45 Aizawa and colleagues46 found that beneficial gut bacteria such as Lactobacillus were also reduced in MDD patients. Naseribafrouei and colleagues47 revealed a lower abundance of Lachnospiraceae in depressed patients. Our results show some similarities: the relative abundance of Firmicutes, Lachnospiraceae, and Lactobacillus decreased, whereas Proteobacteria increased, in exposed rats.
Among the mechanisms of communications between stress and microbiota, alterations in the HPA axis function and serotonergic system are widely discussed.48 By measuring corticosterone, our results indicate an earlier activation of the HPA axis in rats with multiple anaesthesia/surgery exposures. In our screen of changes in anxiety-related neurotransmitters, including ACh, monoamine γ-aminobutyric acid, and glutamate/glutamine in the hippocampus,49 only 5-HT changed significantly. Serotonin is known for its central contributions to anxiety and depression. Clarke and colleagues19 first reported that germ-free mice display anxiety-like behaviour and have increased levels of hippocampal 5-HT and 5-HIAA. Our results are consistent with this. Moreover, the effective treatment of the 5-HT receptor antagonist ZIP in reversing the behavioural changes showed a close relationship between gut microbiota and the CNS serotonergic system.
We found gene expression changes in three serotonergic receptors in hippocampus, including Htr1a, Htr2c, and Htr6. Studies have provided direct evidence concerning the role of 5-HT1a and 5-HT2c receptors in the regulation of anxiety,49,50 which produce anxiolytic and anxiogenic effects, respectively. We found that 5-HT1a receptors decreased and 5-HT2c receptors increased. These receptor-based differences in serotonergic regulation of emotional behaviour could contribute to anxiety. We also observed no change in the Tph2 gene, which encodes tryptophan hydroxylase, the rate-limiting enzyme in the synthesis of 5-HT. Tph2 has been shown to impact on emotion-related mood/anxiety disorders.51 Our results indicate that there must be other factors that affect hippocampal 5-HT content in our experimental setting.
Given the critical relationship between serotonergic systems and anxiety,52 we propose that early anaesthesia/surgery exposures, as a stressor, induce gut microbiota dysbiosis and inappropriate development of the CNS serotonergic system, which would later lead to anxiety behaviours. It has long been known that stress influences the composition of the gut microbiome.53 The putative environmental changes, such as anaesthetic- and surgery-related physiological changes, could work as perioperative stress, and lead to a persistent impact. There is evidence to support the idea that early-life environmental stress could have consequential long-term effects.54
Our study provides new insights on the role of gut microbiota as an intermediary between the HPA axis and central serotonergic system. Gut microbiota, the HPA, and the hippocampal serotonergic system are all particularly relevant to anxiety.55 Activation of the HPA axis during early life has been related to an altered serotonergic system, and postnatal microbial colonisation.56,57 We proposed an underlying mechanism whereby perioperative stress alters the gut microbiota, and consequently affects the serotonergic system and HPA axis. Going forward, the bidirectional communication between microbiota and the CNS influences stress reactivity. We conclude that visceral signals originating from gut microbiota are transmitted to the brain, leading to anxiety-like behaviours.
Authors' contributions
Study supervision: XF
Study design/planning: XZ, XX, DHL, KYC, WH, XF
Study conduct: XX, XC, LL, YW
Data analysis: XZ, XX, YW, XYY, WX, WDL
Data interpretation: XZ, DHL, KYC
Drafting of manuscript: XZ, DHL, XX, WH, XF
Revision of manuscript: XZ, SQS, DHL, KYC, XF
Declarations of interest
The authors declare that they have no conflicts of interest.
Funding
National Natural Science Foundation of China (81571032, 81870829, and 82071224 to X.F., 81701047 to X.Z.), and Major Project of Basic and Applied Basic Research Foundation of Guangdong Province (2019B1515120054 to X.F.).
Acknowledgements
The authors thank Zerong You from Centre for Translational Pain Research, Massachusetts General Hospital (Boston, MA, USA) for useful discussions. The authors also thank Rao Fu from the Department of Anatomy, Zhongshan School of Medicine, Sun Yat-sen University for his helpful advice on neural circuit discussions.
Handling editor: Hugh C Hemmings Jr
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2022.06.039.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. S1.
Supplementary Fig. S2.
Supplementary Fig. S3.
References
- 1.Glatz P., Sandin R.H., Pedersen N.L., Bonamy A.K., Eriksson L.I., Granath F. Association of anesthesia and surgery during childhood with long-term academic performance. JAMA Pediatr. 2017;171 doi: 10.1001/jamapediatrics.2016.3470. [DOI] [PubMed] [Google Scholar]
- 2.Hu D., Flick R.P., Zaccariello M.J., et al. Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population-based birth cohort. Anesthesiology. 2017;127:227–240. doi: 10.1097/ALN.0000000000001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham M.R., Brownell M., Chateau D.G., Dragan R.D., Burchill C., Fransoo R.R. Neurodevelopmental assessment in kindergarten in children exposed to general anesthesia before the age of 4 years: a retrospective matched cohort study. Anesthesiology. 2016;125:667–677. doi: 10.1097/ALN.0000000000001245. [DOI] [PubMed] [Google Scholar]
- 4.Johnson S.C., Pan A., Li L., Sedensky M., Morgan P. Neurotoxicity of anesthetics: mechanisms and meaning from mouse intervention studies. Neurotoxicol Teratol. 2019;71:22–31. doi: 10.1016/j.ntt.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raper J., De Biasio J.C., Murphy K.L., Alvarado M.C., Baxter M.G. Persistent alteration in behavioural reactivity to a mild social stressor in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br J Anaesth. 2018;120:761–767. doi: 10.1016/j.bja.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman K., Robertson N.D., Dissen G.A., et al. Isoflurane anesthesia has long-term consequences on motor and behavioral development in infant rhesus macaques. Anesthesiology. 2017;126:74–84. doi: 10.1097/ALN.0000000000001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neudecker V., Perez-Zoghbi J.F., Coleman K., et al. Infant isoflurane exposure affects social behaviours, but does not impair specific cognitive domains in juvenile non-human primates. Br J Anaesth. 2021;126:486–499. doi: 10.1016/j.bja.2020.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quagliariello A., Del Chierico F., Russo A., et al. Gut microbiota profiling and gut-brain crosstalk in children affected by pediatric acute-onset neuropsychiatric syndrome and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. Front Microbiol. 2018;9:675. doi: 10.3389/fmicb.2018.00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felice V.D., O'Mahony S.M. The microbiome and disorders of the central nervous system. Pharmacol Biochem Behav. 2017;160:1–13. doi: 10.1016/j.pbb.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Christian L.M., Galley J.D., Hade E.M., Schoppe-Sullivan S., Kamp Dush C., Bailey M.T. Gut microbiome composition is associated with temperament during early childhood. Brain Behav Immun. 2015;45:118–127. doi: 10.1016/j.bbi.2014.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serbanescu M.A., Mathena R.P., Xu J., et al. General anesthesia alters the diversity and composition of the intestinal microbiota in mice. Anesth Analg. 2019;129:e126. doi: 10.1213/ANE.0000000000003938. –e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liufu N., Liu L., Shen S., et al. Anesthesia and surgery induce age-dependent changes in behaviors and microbiota. Aging (Albany NY) 2020;12 doi: 10.18632/aging.102736. 1965–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhan G., Hua D., Huang N., et al. Anesthesia and surgery induce cognitive dysfunction in elderly male mice: the role of gut microbiota. Aging (Albany NY) 2019;11:1778–1790. doi: 10.18632/aging.101871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L., Yang X., Wu H. Juvenile rats show altered gut microbiota after exposure to isoflurane as neonates. Neurochem Res. 2019;44:776–786. doi: 10.1007/s11064-018-02707-y. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., Mian M.F., Neufeld K.M., Forsythe P. CD4(+)CD25(+) T cells are essential for behavioral effects of Lactobacillus rhamnosus JB-1 in male BALB/c mice. Brain Behav Immun. 2020;88:451–460. doi: 10.1016/j.bbi.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 16.Kountouras J., Zavos C., Polyzos S.A., Deretzi G. The gut-brain axis: interactions between Helicobacter pylori and enteric and central nervous systems. Ann Gastroenterol. 2015;28:506. [PMC free article] [PubMed] [Google Scholar]
- 17.Gaspar P., Cases O., Maroteaux L. The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci. 2003;4:1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 18.Leonard B.E. The HPA and immune axes in stress: the involvement of the serotonergic system. Eur Psychiatry. 2005;20(Suppl 3):S302–S306. doi: 10.1016/s0924-9338(05)80180-4. [DOI] [PubMed] [Google Scholar]
- 19.Clarke G., Grenham S., Scully P., et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 20.Witjes V.M., Boleij A., Halffman W. Reducing versus embracing variation as strategies for reproducibility: the microbiome of laboratory mice. Animals (Basel) 2020;10:2415. doi: 10.3390/ani10122415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng X., Liu J.J., Zhou X., et al. Single sevoflurane exposure decreases neuronal nitric oxide synthase levels in the hippocampus of developing rats. Br J Anaesth. 2012;109:225–233. doi: 10.1093/bja/aes121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed M., Kumar G., Navarro G., et al. Systemic siRNA nanoparticle-based drugs combined with radiofrequency ablation for cancer therapy. PLoS One. 2015;10 doi: 10.1371/journal.pone.0128910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Connor K.M., Lucking E.F., Golubeva A.V., et al. Manipulation of gut microbiota blunts the ventilatory response to hypercapnia in adult rats. EBioMedicine. 2019;44:618–638. doi: 10.1016/j.ebiom.2019.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piewngam P., Zheng Y., Nguyen T.H., et al. Pathogen elimination by probiotic Bacillus via signalling interference. Nature. 2018;562:532–537. doi: 10.1038/s41586-018-0616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdul-Monim Z., Reynolds G.P., Neill J.C. The effect of atypical and classical antipsychotics on sub-chronic PCP-induced cognitive deficits in a reversal-learning paradigm. Behav Brain Res. 2006;169:263–273. doi: 10.1016/j.bbr.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 26.Sun L.S., Li G., Miller T.L., et al. Association between a single general anesthesia exposure before age 36 months and neurocognitive outcomes in later childhood. JAMA. 2016;315:2312–2320. doi: 10.1001/jama.2016.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walters J.L., Paule M.G. Review of preclinical studies on pediatric general anesthesia-induced developmental neurotoxicity. Neurotoxicol Teratol. 2017;60:2–23. doi: 10.1016/j.ntt.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X., da Li W., Yuan B.L., et al. Lithium treatment prevents apoptosis in neonatal rat hippocampus resulting from sevoflurane exposure. Neurochem Res. 2016;41:1993. doi: 10.1007/s11064-016-1909-x. –2005. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Z.B., Yang X.Y., Yuan B.L., et al. Sevoflurane-induced down-regulation of hippocampal oxytocin and arginine vasopressin impairs juvenile social behavioral abilities. J Mol Neurosci. 2015;56:70–77. doi: 10.1007/s12031-014-0468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilder R.T., Flick R.P., Sprung J., et al. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diana P., Joksimovic S.M., Faisant A., Jevtovic-Todorovic V. Early exposure to general anesthesia impairs social and emotional development in rats. Mol Neurobiol. 2020;57:41–50. doi: 10.1007/s12035-019-01755-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cabrera O.H., Gulvezan T., Symmes B., Quillinan N., Jevtovic-Todorovic V. Sex differences in neurodevelopmental abnormalities caused by early-life anaesthesia exposure: a narrative review. Br J Anaesth. 2020;124 doi: 10.1016/j.bja.2019.12.032. e81–e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu C., Tan S., Zhang J., et al. Anesthesia with sevoflurane in neonatal rats: developmental neuroendocrine abnormalities and alleviating effects of the corticosteroid and Cl(-) importer antagonists. Psychoneuroendocrinology. 2015;60:173–181. doi: 10.1016/j.psyneuen.2015.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ju L.S., Yang J.J., Morey T.E., et al. Role of epigenetic mechanisms in transmitting the effects of neonatal sevoflurane exposure to the next generation of male, but not female, rats. Br J Anaesth. 2018;121:406–416. doi: 10.1016/j.bja.2018.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laukens D., Brinkman B.M., Raes J., De Vos M., Vandenabeele P. Heterogeneity of the gut microbiome in mice: guidelines for optimizing experimental design. FEMS Microbiol Rev. 2016;40:117–132. doi: 10.1093/femsre/fuv036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J., Bi J.J., Guo G.J., et al. Abnormal composition of gut microbiota contributes to delirium-like behaviors after abdominal surgery in mice. CNS Neurosci Ther. 2019;25:685–696. doi: 10.1111/cns.13103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terrando N., Monaco C., Ma D., Foxwell B.M., Feldmann M., Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A. 2010;107:20518–20522. doi: 10.1073/pnas.1014557107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren Q., Peng M., Dong Y., et al. Surgery plus anesthesia induces loss of attention in mice. Front Cell Neurosci. 2015;9:346. doi: 10.3389/fncel.2015.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Broad K.D., Kawano G., Fierens I., et al. Surgery increases cell death and induces changes in gene expression compared with anesthesia alone in the developing piglet brain. PLoS One. 2017;12 doi: 10.1371/journal.pone.0173413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shu Y., Zhou Z., Wan Y., et al. Nociceptive stimuli enhance anesthetic-induced neuroapoptosis in the rat developing brain. Neurobiol Dis. 2012;45:743–750. doi: 10.1016/j.nbd.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 41.Alam A., Hana Z., Jin Z., Suen K.C., Ma D. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–556. doi: 10.1016/j.ebiom.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jevtovic-Todorovic V. Exposure of developing brain to general anesthesia: what is the animal evidence? Anesthesiology. 2018;128:832–839. doi: 10.1097/ALN.0000000000002047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang T.T., Lai J.B., Du Y.L., Xu Y., Ruan L.M., Hu S.H. Current understanding of gut microbiota in mood disorders: an update of human studies. Front Genet. 2019;10:98. doi: 10.3389/fgene.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Qin J., Li R., Raes J., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang H., Ling Z., Zhang Y., et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 46.Aizawa E., Tsuji H., Asahara T., et al. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord. 2016;202:254–257. doi: 10.1016/j.jad.2016.05.038. [DOI] [PubMed] [Google Scholar]
- 47.Naseribafrouei A., Hestad K., Avershina E., et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26:1155–1162. doi: 10.1111/nmo.12378. [DOI] [PubMed] [Google Scholar]
- 48.Rogers G.B., Keating D.J., Young R.L., Wong M.L., Licinio J., Wesselingh S. From gut dysbiosis to altered brain function and mental illness: mechanisms and pathways. Mol Psychiatry. 2016;21:738–748. doi: 10.1038/mp.2016.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engin E., Treit D. The role of hippocampus in anxiety: intracerebral infusion studies. Behav Pharmacol. 2007;18:365–374. doi: 10.1097/FBP.0b013e3282de7929. [DOI] [PubMed] [Google Scholar]
- 50.Li Q., Luo T., Jiang X., Wang J. Anxiolytic effects of 5-HT(1)A receptors and anxiogenic effects of 5-HT(2)C receptors in the amygdala of mice. Neuropharmacology. 2012;62:474–484. doi: 10.1016/j.neuropharm.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inoue H., Yamasue H., Tochigi M., et al. Effect of tryptophan hydroxylase-2 gene variants on amygdalar and hippocampal volumes. Brain Res. 2010;1331:51–57. doi: 10.1016/j.brainres.2010.03.057. [DOI] [PubMed] [Google Scholar]
- 52.O'Mahony S.M., Clarke G., Borre Y.E., Dinan T.G., Cryan J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res. 2015;277:32–48. doi: 10.1016/j.bbr.2014.07.027. [DOI] [PubMed] [Google Scholar]
- 53.Tannock G.W., Savage D.C. Influences of dietary and environmental stress on microbial populations in the murine gastrointestinal tract. Infect Immun. 1974;9:591–598. doi: 10.1128/iai.9.3.591-598.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cox L.M., Yamanishi S., Sohn J., et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158:705–721. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leonard B.E. HPA and immune axes in stress: involvement of the serotonergic system. Neuroimmunomodulation. 2006;13:268–276. doi: 10.1159/000104854. [DOI] [PubMed] [Google Scholar]
- 56.Ohta K., Miki T., Warita K., et al. Prolonged maternal separation disturbs the serotonergic system during early brain development. Int J Dev Neurosci. 2014;33:15–21. doi: 10.1016/j.ijdevneu.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Sudo N., Chida Y., Aiba Y., et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558:263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.