Abstract
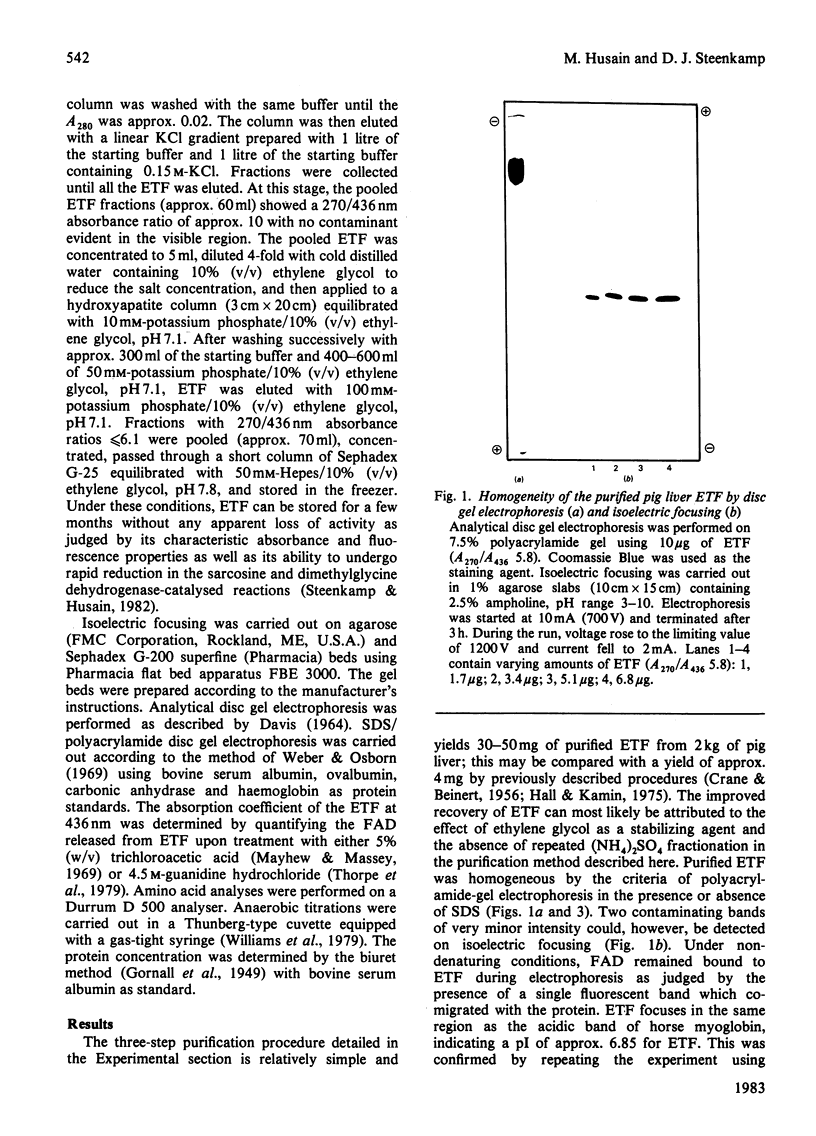
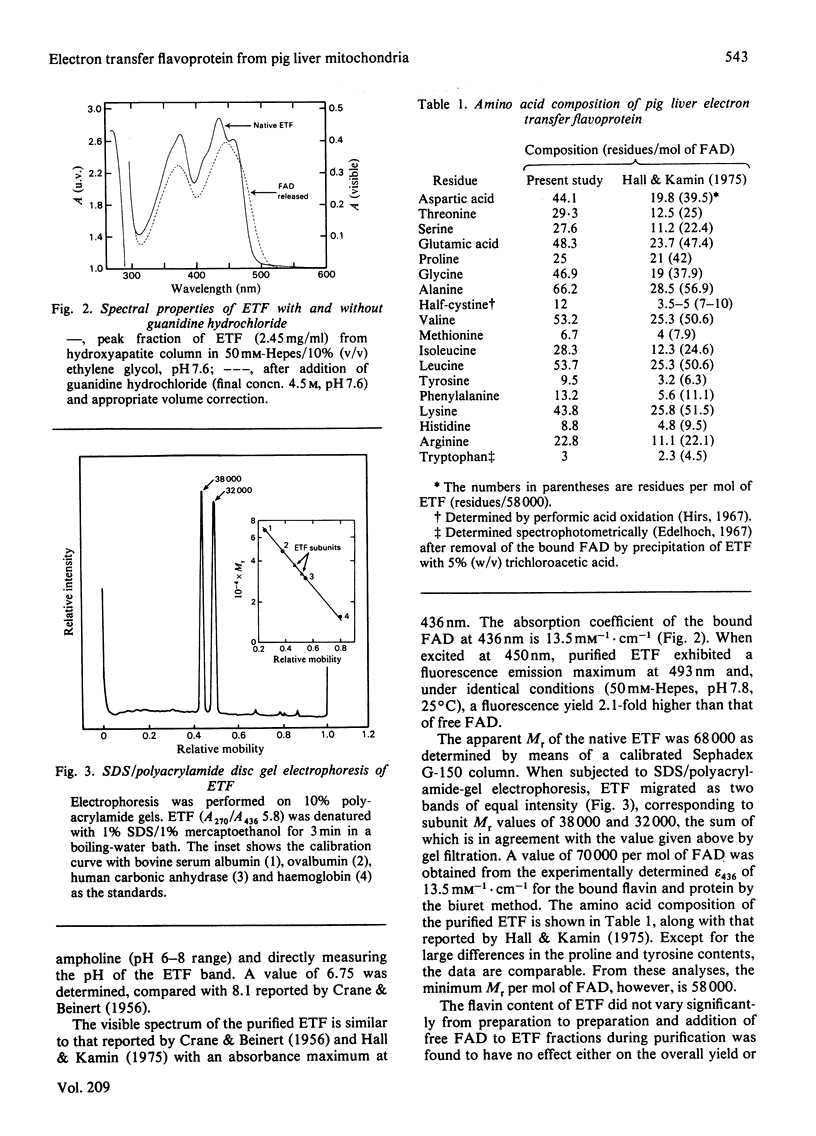
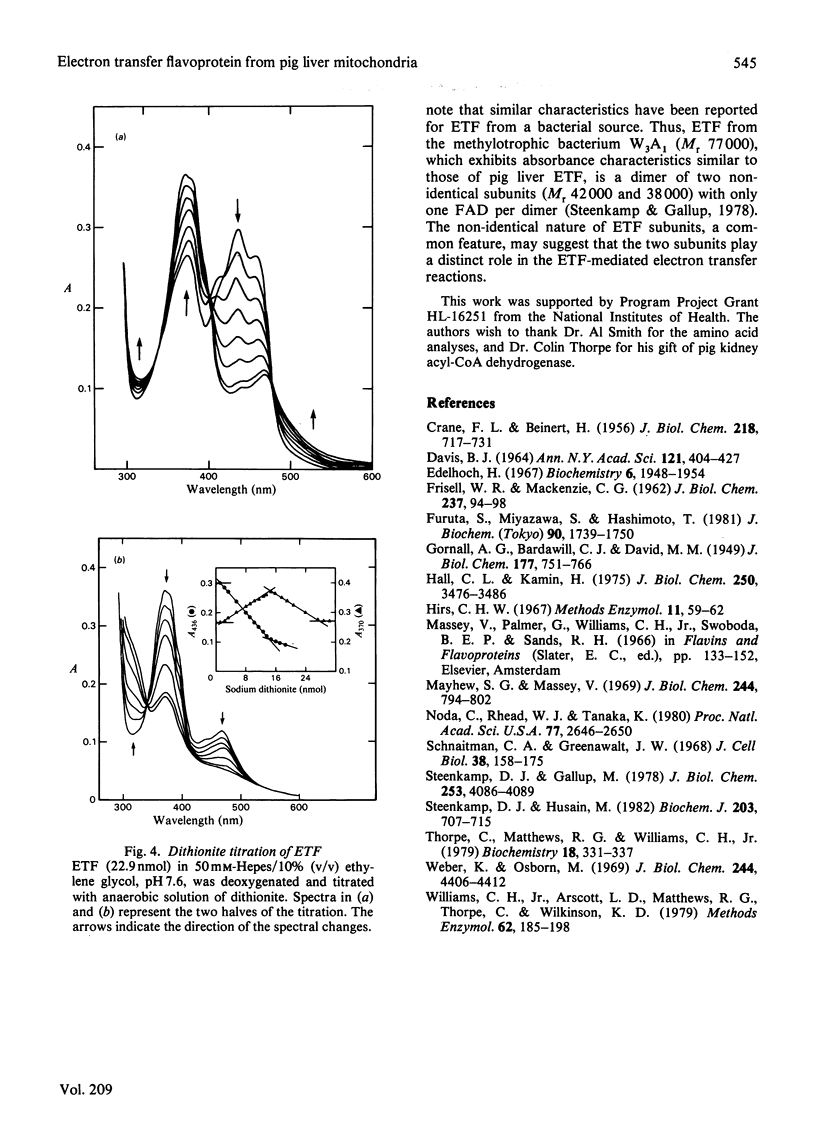
Electron transfer flavoprotein (ETF) from pig liver mitochondria has been purified to homogeneity by a three-step procedure with approx. 10-fold higher yields than previously reported. The purified ETF exhibits an absorption coefficient for the bound FAD of 13.5 mM-1.cm-1 at 436 nm and an isoelectric point of 6.75. Gel filtration, sodium dodecyl sulphate gel electrophoresis and flavin analysis indicate that pig liver ETF is a dimer, composed of non-identical subunits (Mr 38 000 and 32 000) with only one FAD/dimer. Anaerobic reduction by dithionite produces anionic flavin semiquinone as a stable intermediate and establishes the flavin to be the only redox-active chromophore in ETF.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRANE F. L., BEINERT H. On the mechanism of dehydrogenation of fatty acyl derivatives of coenzyme A. II. The electron-transferring flavoprotein. J Biol Chem. 1956 Feb;218(2):717–731. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- FRISELL W. R., MACKENZIE C. G. Separation and purification of sarcosine dehydrogenase and dimethylglycine dehydrogenase. J Biol Chem. 1962 Jan;237:94–98. [PubMed] [Google Scholar]
- Furuta S., Miyazawa S., Hashimoto T. Purification and properties of rat liver acyl-CoA dehydrogenases and electron transfer flavoprotein. J Biochem. 1981 Dec;90(6):1739–1750. doi: 10.1093/oxfordjournals.jbchem.a133651. [DOI] [PubMed] [Google Scholar]
- Hall C. L., Kamin H. The purification and some properties of electron transfer flavoprotein and general fatty acyl coenzyme A dehydrogenase from pig liver mitochondria. J Biol Chem. 1975 May 10;250(9):3476–3486. [PubMed] [Google Scholar]
- Mayhew S. G., Massey V. Purification and characterization of flavodoxin from Peptostreptococcus elsdenii. J Biol Chem. 1969 Feb 10;244(3):794–802. [PubMed] [Google Scholar]
- Noda C., Rhead W. J., Tanaka K. Isovaleryl-CoA dehydrogenase: demonstration in rat liver mitochondria by ion exchange chromatography and isoelectric focusing. Proc Natl Acad Sci U S A. 1980 May;77(5):2646–2650. doi: 10.1073/pnas.77.5.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp D. J., Gallup M. The natural flavorprotein electron acceptor of trimethylamine dehydrogenase. J Biol Chem. 1978 Jun 25;253(12):4086–4089. [PubMed] [Google Scholar]
- Steenkamp D. J., Husain M. The effect of tetrahydrofolate on the reduction of electron transfer flavoprotein by sarcosine and dimethylglycine dehydrogenases. Biochem J. 1982 Jun 1;203(3):707–715. doi: 10.1042/bj2030707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe C., Matthews R. G., Williams C. H., Jr Acyl-coenzyme A dehydrogenase from pig kidney. Purification and properties. Biochemistry. 1979 Jan 23;18(2):331–337. doi: 10.1021/bi00569a016. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Williams C. H., Jr, Arscott L. D., Matthews R. G., Thorpe C., Wilkinson K. D. Methodology employed for anaerobic spectrophotometric titrations and for computer-assisted data analysis. Methods Enzymol. 1979;62:185–198. doi: 10.1016/0076-6879(79)62217-6. [DOI] [PubMed] [Google Scholar]


