Abstract
Purpose
The purpose of this study was to investigate the effects of postoperative complications on long-term survival after laparoscopic surgery for resectable colorectal cancer.
Methods
We retrospectively included 204 patients who underwent laparoscopic surgery for colorectal cancer from January 2016 to June 2020.
Results
Overall, 68 (33.3%) patients had postoperative complications, twelve (17.6%) of which were classified as Clavien–Dindo class 3a or higher. The 5-year overall survival rate of the non-complication and complication groups were 93.0% and 81.7%, respectively (p = 0.048; Kaplan–Meier analysis and log-rank test), and those among patients with stage III disease were 87.0% and 61.3%, respectively (p = 0.045). The 5-year disease-free survival rates were 85.6% and 77.4%, respectively (p = 0.042). Multivariable Cox proportional-hazards analysis revealed that nodal stage (hazard ratio, 8.392; 95% confidence interval, 1.892–37.175; p = 0.005) was an independent prognostic factor for overall survival, and postoperative complications (hazard ratio, 2.996; 95% confidence interval, 1.076–8.340; p = 0.036) were independent prognostic factors for disease-free survival.
Conclusion
Postoperative complications were associated with poor oncological outcomes, especially among patients with stage III colorectal cancer, and independent prognostic factors for disease-free survival.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00384-024-04730-8.
Keywords: Colorectal neoplasm, Postoperative complications, Survival analysis
Introduction
Colorectal cancer (CRC) is one of the most prevalent cancers and the second leading cause of cancer-related death worldwide [1]. Although numerous therapies for CRC are available, surgical resection remains the primary treatment. Despite improvements in surgical techniques over time, postoperative complications continue to be prevalent and pose a substantial challenge for surgeons. The postoperative complication rate following colorectal cancer is 18 to 38% [2–5]. Complications following surgery not only reduce a patient’s quality of life but may lengthen the hospital stay, increase hospital expenses, necessitate additional surgical intervention [6], and delay the administration of adjuvant chemotherapy for CRC [7].
Recently, laparoscopic surgery has gained popularity for CRC. Laparoscopic surgery outperforms open surgery in terms of the length of hospital stay, rate of surgical complications, time to recovery of bowel function, and immunological effects [8–11]. Some studies have suggested that laparoscopic surgery has a positive immunological influence, resulting in superior oncological outcomes to open surgery [9, 12]. The immunological response to cancer after surgery is negatively associated with the viability of undetected tumors and disease recurrence [13]. Although the causes are clearly multifactorial, the postoperative immune response is primarily characterized by immune suppression [14].
The effect of postoperative complications on long-term oncological outcomes has garnered considerable research interest. In the majority of studies, complications were associated with poor oncological outcomes [15, 16]. Moreover, studies on the effect of postoperative complications on oncological outcomes, including metastatic disease, in patients undergoing laparoscopic surgery for resectable CRC are scarce. The purpose of this study was to investigate the effects of postoperative complications on long-term survival after laparoscopic surgery for resectable CRC.
Materials and methods
Ethical considerations
This study was approved by the Institutional Review Board of the Dongsan Medical Center (Daegu, Republic of Korea; IRB no.: 2022‑12‑026–001). Data collection and analysis were conducted in an ethical way, protecting the patients’ rights to privacy. Owing to the retrospective nature of this study, the need for informed consent was waived by the Institutional Review Board.
Study design and setting
We screened a prospectively maintained database for participants’ demographic characteristics and retrospectively obtained data on perioperative outcomes and postoperative complications from their electronical medical records.
Participants
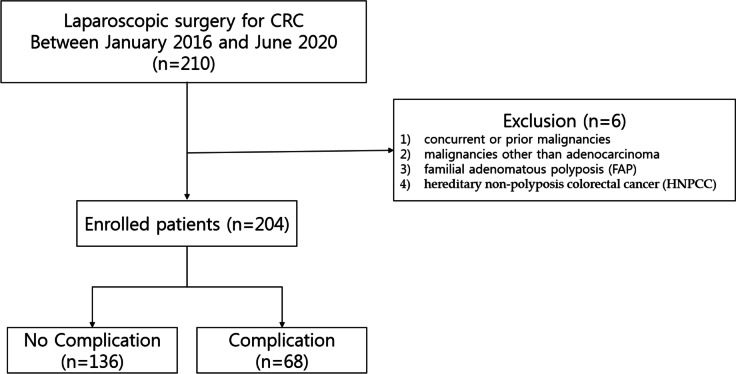
From January 2016 to June 2020, 210 patients underwent surgery for resectable CRC at our institution. Resectable stage IV cancer defined as cases where metastasis has occurred, and surgery could be performed based on the judgment that it is possible to resect a single lung lesion, a single liver lesion, or lesions located in single segment or section. Adjuvant chemotherapy was done for patients who were diagnosed pathologic stage III or IV and stage II with poor prognostic features including poorly differentiated histology, lymphatic/vascular invasion, perineural invasion, bowel obstruction, localized perforation, close, indeterminate, positive margins, high tumor budding, or less than 12 lymph nodes harvest. The exclusion criteria were having (1) concurrent or prior malignancies, (2) malignancies other than adenocarcinoma, (3) familial adenomatous polyposis or hereditary non-polyposis CRC, and (4) patients who underwent (Fig. 1). The patients were divided into a group of 68 with complications and a group of 136 without complications.
Fig. 1.
Flow chart of patient selection according to exclusion criteria. CRC, colorectal cancer
Definitions of variables
Information on patient demographics that we analyzed were age, sex, hypertension, diabetes mellitus, preoperative carcinoembryonic antigen (CEA) concentration, preoperative C-reactive protein (CRP) concentration, American Society of Anesthesiology physical status class, and tumor location. Pathological outcomes analyzed were tumor, node, and metastasis stage, histological features, the numbers of harvested and positive lymph nodes, tumor size, lymphovascular invasion, and perineural invasion. The tumor stage was identified using the American Joint Committee on Cancer categorization method, 8th edition. Over a period of 3 years following the surgical intervention, a combination of serum CEA concentrations, chest radiographic images (conducted every 3 months), and chest/abdominal computed tomographic scans (performed every 6 months) were used to assess the patients’ postoperative clinical status. The follow-up interval was extended to 6 months after 3 years. Overall survival (OS) was defined as the duration between the surgical procedure and either the most recent follow-up appointment or death from any cause. Disease-free survival (DFS) defined as the duration from surgery to the first confirmed recurrence event or death due to disease progression. Local recurrence was defined as any tumor recurrence in the surgical field. Systemic recurrences included local and synchronous systemic recurrences.
Postoperative complications were defined as complications that occurred within 30 days of the primary surgery. Postoperative ileus defined as prolonged absence of gut function which manifests as persistent nausea and/or vomiting with abdominal distention and failure to pass flatus. Dysuria defined as patient who were failed to void after removal of Foley catheter. Cardiac complications including acute myocardial infarction and respiratory complications included atelectasis, pneumonia, and pleural effusion. For patients with multiple complications, the most serious complication was selected as the representative complication. The most serious complication referred to the complication that had the greatest impact on the patient’s treatment plan and length of stay. All complications were classified according to the Clavien–Dindo (CD) classification, which is widely used to grade adverse events that occur as a result of surgical procedures [17].
Preoperative evaluation and surgical treatment
Prior to the surgical intervention, all patients underwent a preoperative assessment, which consisted of colonoscopy, computed tomography of the chest and abdomen, and magnetic resonance imaging of the pelvis. In some cases, positron emission tomography was performed to detect distant metastases. Our surgical approach for CRC treatment adhered to the fundamental principles of complete mesocolic or mesorectal excision and central vessel ligation. The primary tumor was surgically removed via precise dissection of the visceral plane, starting from the parietal fascial layer, with the objective of extracting the entire regional mesocolon in one complete unit.
Statistical analysis
IBM SPSS Statistics for Windows, version 28.0.1.1, was used to conduct all statistical analyses (IBM Corp., Armonk, NY, USA). For continuous variables, data are reported as means ± standard deviations, and categorical variables are reported as frequencies with percentages. The Fisher exact test and the chi-square test were used to assess categorical variables. Mann–Whitney U and independent Student’s t-tests were used to evaluate continuous variables. Statistical significance was set at p < 0.05. Using the log-rank test for univariate analysis, the Kaplan–Meier method was used to construct OS and DFS curves. Variables with differences of p < 0.1 between groups in the univariate analysis were added into the multivariable analysis model to evaluate factors predictive of survival. For multivariable analysis, a Cox proportional-hazards model was used. Individual variables’ effects on patient survival were reported as hazard ratios (HRs) with 95% confidence intervals (CIs).
Results
Patient characteristics
Among all 204 patients, 68 (33.3%) had one or more postoperative complications. Patient demographic characteristics and most clinical characteristics did not significantly differ between the two groups (Table 1). The number of days between surgery and adjuvant treatment was longer for patients with complications than for those without complications (48.93 ± 23.06 vs. 33.32 ± 10.37 days, p = 0.002).
Table 1.
Patients and tumor characteristics
| Complications ( −) (n = 136) | Complications ( +) (n = 68) | p-value | |
|---|---|---|---|
| Age (years) | 65.32 ± 10.237 | 67.22 ± 9.455 | 0.202 |
| Sex | 0.088 | ||
| Male | 88 (64.7%) | 52 (76.5%) | |
| Female | 48 (35.3%) | 16 (23.5%) | |
| Hypertension | 55 (40.4%) | 34 (50.0%) | 0.194 |
| Diabetes mellitus | 28 (20.6%) | 15 (22.1%) | 0.808 |
| Preoperative CEA (ng/mL) | 4.22 ± 7.07 | 9.69 ± 29.47 | 0.139 |
| Preoperative CRP (mg/L) | 0.50 ± 1.11 | 0.78 ± 1.56 | 0.210 |
| ASA physical status class | 0.086 | ||
| I | 42 (30.9%) | 17 (25.0%) | |
| II | 81 (59.6%) | 37 (54.4%) | |
| III | 13 (9.6%) | 14 (20.6%) | |
| Location of tumor | 0.562 | ||
| Right | 31 (22.8%) | 18 (26.5%) | |
| Left | 105 (77.2%) | 50 (73.5%) | |
| Time to adjuvant treatment (days) | 33.32 ± 10.37 | 48.93 ± 23.06 | 0.002 |
| PLR | 64 (47.4%) | 38 (55.9%) | 0.254 |
| NLR | 65 (48.1%) | 39 (57.4%) | 0.216 |
| Perineural invasion | 68 (50.4%) | 34 (50.0%) | 0.960 |
| PIV | 61 (45.2%) | 41 (60.3%) | 0.042 |
| Albumin | 76 (55.9%) | 38 (55.9%) | > 0.99 |
Values are presented as mean ± standard deviation or frequency (%). Statistical significance was set at p < 0.05
ASA American Society of Anesthesiologists, CEA carcinoembryonic antigen, CRP C-reactive protein, NLR neutrophil–lymphocyte ratio, PIV pan-immune inflammation value, PLR platelet-lymphocyte ratio
Postoperative complications
The incidence of postoperative complications and the CD classification is summarized in Table 2. The most common complication was ileus, which occurred in 15 patients (7.4%). Twelve of the 68 patients (17.6%) with complications were classified as CD class 3a or higher.
Table 2.
Postoperative complications
| Variable | Frequency (%) |
|---|---|
| Complication | |
| Ileus | 15 (7.4%) |
| Anastomosis leakage | 11 (5.4%) |
| Dysuria | 10 (4.9%) |
| Chyle leakage | 8 (3.9%) |
| Bleeding (hematochezia) | 8 (3.9%) |
| Surgical site infection | 7 (3.4%) |
| Cardiac complication | 2 (1.0%) |
| PMC | 2 (1.0%) |
| Respiratory complication | 2 (1.0%) |
| Intra-abdominal abscess | 2 (1.0%) |
| Colitis | 1 (0.5%) |
| Clavien–Dindo class | |
| 0 | 0 |
| 1 | 24 (11.8%) |
| 2 | 32 (15.7%) |
| 3a | 2 (1.0%) |
| 3b | 8 (3.9%) |
| 4 | 2 (1.0%) |
Values are presented as mean ± standard deviation or frequency (%)
PMC pseudomembranous colitis
Postoperative pathological outcomes
Table 3 summarizes the postoperative pathological outcomes. The groups did not significantly differ in terms of tumor and nodal stages, histological features, number of retrieved lymph nodes, number of positive lymph nodes, lymphovascular invasion, or perineural invasion. The complication group had larger tumors than the no-complication group (4.06 cm vs. 3.44 cm, p = 0.047).
Table 3.
Postoperative pathological outcomes
| Complications ( −) (n = 136) | Complications ( +) (n = 68) | p-value | |
|---|---|---|---|
| Tumor stage | 0.319 | ||
| Tis–T2 | 64 (47.1%) | 27 (39.7%) | |
| T3–4 | 72 (52.9%) | 41 (60.3%) | |
| Nodal stage | 0.406 | ||
| N0 | 86 (63.2%) | 47 (69.1%) | |
| N1–2 | 50 (36.8%) | 21 (30.9%) | |
| Metastasis | 0.074 | ||
| M0 | 133 (97.8%) | 63 (92.6%) | |
| M1 | 3 (2.2%) | 5 (7.4%) | |
| Histology (n = 134/67) | 0.345 | ||
| Well differentiated | 10 (7.5%) | 3 (4.5%) | |
| Moderately differentiated | 118 (88.1%) | 58 (86.6%) | |
| Poorly differentiated | 6 (4.5%) | 6 (9.0%) | |
| Retrieved LNs | 17.94 ± 9.204 | 20.12 ± 9.286 | 0.114 |
| Retrieved LNs > 12 | 113 (83.1%) | 63 (92.6%) | 0.061 |
| Positive LNs | 1.05 ± 2.165 | 0.81 ± 1.814 | 0.428 |
| Tumor size (cm) | 3.44 ± 2.07 | 4.06 ± 2.19 | 0.047 |
| Lymphovascular invasion | 32 (24.2%) | 22 (32.4%) | 0.221 |
| Perineural invasion | 24 (18.9%) | 17 (25.4%) | 0.294 |
Categorical variables were analyzed using the chi-square and Fisher’s exact tests. Continuous variables were analyzed using the independent t-test and Mann–Whitney U test. Values are presented as mean ± standard deviation or frequency (%). Statistical significance was set at p < 0.05
LN lymph node
Oncological outcomes
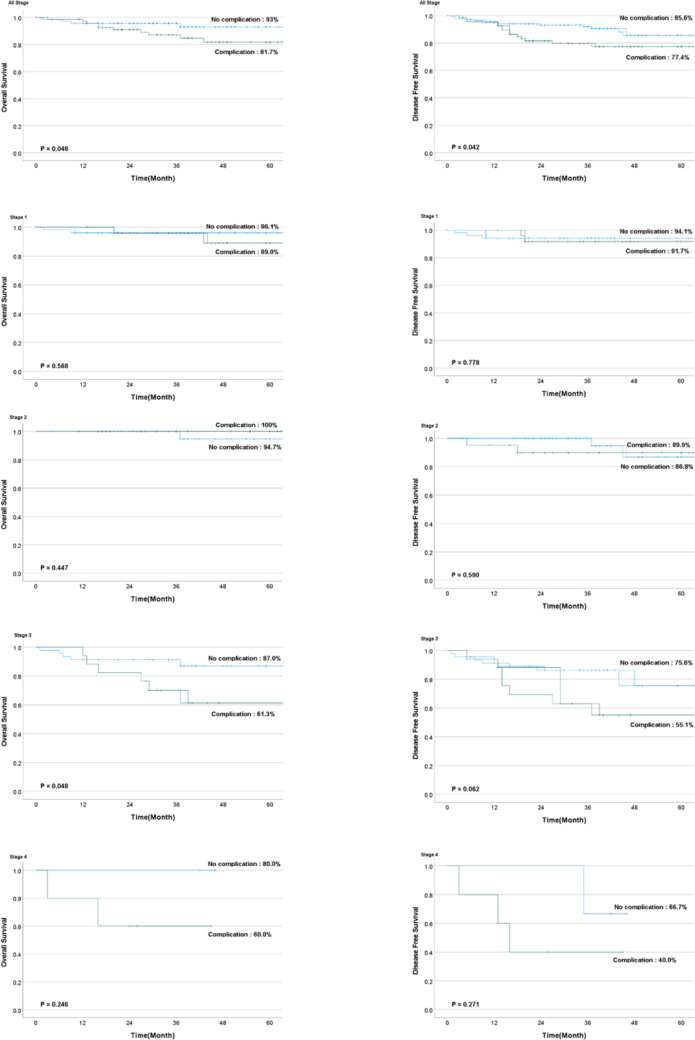
The median follow-up period was similar in the two groups at approximately 38.1 months (Table 4). Overall, the group without complications had better 5-year OS (93.0% vs. 81.3%, p = 0.048) and DFS (85.6% vs. 77.4%, p = 0.042) rates. Subgroup analysis revealed that the no-complications group had a superior OS rate among patients with stage III disease (87.0% vs. 61.3%, p = 0.045) than the group with complications, whereas none of the other subgroups significantly differed in terms of OS or DFS (Fig. 2).
Table 4.
Oncological outcomes
| Complications ( −) (n = 136) | Complications ( +) (n = 68) | p-value | |
|---|---|---|---|
| Median follow-up (months) | 38.10 ± 17.387 | 38.16 ± 17.318 | 0.980 |
| 5-yr OS (%) | 93.0 | 81.7 | 0.048 |
| Stage 1 | 96.1 | 89.0 | 0.568 |
| Stage 2 | 94.7 | 100 | 0.447 |
| Stage 3 | 87.0 | 61.3 | 0.045 |
| Stage 4 | 80.0 | 60.0 | 0.246 |
| 5-yr DFS (%) | 85.6 | 77.4 | 0.042 |
| Stage 1 | 94.1 | 91.7 | 0.778 |
| Stage 2 | 86.8 | 89.9 | 0.590 |
| Stage 3 | 75.6 | 55.1 | 0.062 |
| Stage 4 | 66.7 | 40.0 | 0.271 |
| Recurrence | 8 (5.9%) | 9 (13.2%) | 0.204 |
| Recurrence pattern | 0.113 | ||
| Systemic recurrence | 7 (5.1%) | 6 (8.8%) | 0.311 |
| Local recurrence | 1 (0.7%) | 3 (4.4%) | 0.074 |
The Kaplan–Meier method with the log-rank test was used for survival analysis. Categorical variables were analyzed using the chi-square and Fisher’s exact tests. Continuous variables were analyzed using the independent t-test and Mann–Whitney U test. Values are presented as mean ± standard deviation or frequency (%). Statistical significance was set at p < 0.05
DFS disease-free survival, OS overall survival
Fig. 2.
Kaplan–Meier survival curves for the cumulative risk of recurrence
The two groups did not differ in terms of the recurrence pattern (p = 0.113). The group without complications had seven cases of systemic recurrence, whereas the group with complications had six. In the group with no complications, two patients exhibited liver recurrence, four had lung recurrence, and one had liver and prostate metastases. In the complications group, one patient had liver recurrence, two had lung recurrence, and three had lymph node recurrence. One case of local recurrence occurred in the group without complications, whereas three occurred in the group with complications.
Univariate and multivariable survival analyses of prognostic factors
Upon univariate analysis, complications, sex, preoperative CRP concentration, N stage, and M stage yielded p-values < 0.1 for 5-year OS. Complications, sex, preoperative CEA concentration, and T, N, and M stage yielded p-values < 0.1 for 5-year DFS (Table 5). Multivariable analysis revealed that N stage was an independent prognostic factor for 5-year OS (HR, 8.392; 95% CI, 1.892–37.175; p = 0.005). Complications was independent prognostic factors for 5-year DFS (HR, 2.996; 95% CI, 1.076–8.340; p = 0.036) (Table 6).
Table 5.
Prognostic factors of 5-year survival (univariate analysis)
| Prognostic factor | N | 5-year OS, % | p-value | 5-year DFS, % | p-value |
|---|---|---|---|---|---|
| Complications | 0.048 | 0.042 | |||
| No | 136 | 93.0 | 85.6 | ||
| Yes | 68 | 81.7 | 77.4 | ||
| Age (years) | 0.689 | 0.917 | |||
| ≤ 65 | 89 | 90.2 | 84.7 | ||
| > 65 | 115 | 87.8 | 82.1 | ||
| Sex | 0.060 | 0.016 | |||
| Male | 140 | 85.5 | 79.5 | ||
| Female | 64 | 96.9 | 92.0 | ||
| Pre-op CEA (ng/mL) (n = 201) | 0.126 | 0.058 | |||
| < 5 | 159 | 91.1 | 85.9 | ||
| ≥ 5 | 42 | 82.2 | 76.7 | ||
| Pre-op CRP (mg/L) (n = 154) | 0.043 | 0.623 | |||
| < 0.3 | 99 | 90.0 | 86.6 | ||
| ≥ 0.3 | 55 | 80.3 | 83.7 | ||
| ASA score | 0.440 | 0.807 | |||
| 1 | 59 | 94.9 | 81.9 | ||
| 2 | 118 | 85.6 | 85.8 | ||
| 3 | 27 | 91.5 | 75.1 | ||
| Sidedness | 0.431 | 0.687 | |||
| Right-sided | 49 | 84.2 | 79.4 | ||
| Left-sided | 155 | 90.6 | 84.7 | ||
| T stage | 0.119 | 0.037 | |||
| Tis–T2 | 91 | 92.8 | 92.0 | ||
| T3–4 | 113 | 85.6 | 76.0 | ||
| N stage | < 0.001 | 0.001 | |||
| N0 | 133 | 94.5 | 90.4 | ||
| N1–2 | 71 | 79.0 | 69.5 | ||
| M stage | 0.086 | 0.001 | |||
| M0 | 196 | 89.5 | 85.0 | ||
| M1 | 8 | 75.0 | 46.9 | ||
| Tumor size (cm) (n = 201) | 0.139 | 0.178 | |||
| < 3.4 | 100 | 91.4 | 89.5 | ||
| ≥ 3.4 | 101 | 85.9 | 77.1 |
The Kaplan–Meier method with the log-rank test was used for univariate analysis. Statistical significance was set at p < 0.05
ASA American Society of Anesthesiologists, CEA carcinoembryonic antigen, CRP C-reactive protein, DFS disease-free survival, LN Lymph node, op operation, OS overall survival, PIV pan-immune inflammation value
Table 6.
Prognostic factors of 5-year survival (multivariable analysis)
| Variables | Reference category | Overall survival | Disease-free survival | ||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Complications | 0.092 | 0.036 | |||
| Yes | No | 2.762 (0.848–8.991) | 2.996 (1.076–8.340) | ||
| Sex | 0.727 | 0.141 | |||
| Female | Male | 0.747 (0.145–3.850) | 0.211(0.027–1.673) | ||
| Pre-op CEA (ng/mL) | 0.967 | 0.842 | |||
| ≥ 5 | < 5 | 1.026 (0.301–3.503) | 1.134 (0.331–3.884) | ||
| Pre-op CRP (mg/L) | 0.303 | 0.642 | |||
| ≥ 0.3 | < 0.3 | 1.919 (0.555–6.631) | 0.771 (0.257–2.311) | ||
| T stage | 0.720 | 0.360 | |||
| T3, T4 | T1, T2 | 0.738 (0.140–3.884) | 1.840 (0.498–6.799) | ||
| N stage | 0.005 | 0.098 | |||
| N1, N2 | N0 | 8.392 (1.892–37.175) | 2.584 (0.839–7.954) | ||
| M stage | 0.847 | 0.920 | |||
| M0 | M1 | 0.844 (0.150–4.754) | 1.094 (0.188–6.371) | ||
Cox proportional-hazards models were used for statistical analysis. The effects of individual variables on patient survival were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). Statistical significance was set at p < 0.05
CEA carcinoembryonic antigen, CRP C-reactive protein, op operation, PIV pan-immune inflammation value
Discussion
This study demonstrated that postoperative complications were associated with poor long-term oncological outcomes, particularly in patients with stage III disease. The interval between operation and postoperative adjuvant treatment was longer for patients with complications than for those without complications. Postoperative complications were independent prognostic factors for 5-year DFS in patients who underwent laparoscopic surgery for resectable CRC.
The prognostic importance of postoperative complications in CRC has previously been investigated [15, 18]. Cienfuegos et al. [19] reported that patients with major complications had a significantly lower OS than those with no complications at both 5 and 10 years after resection. Liu et al. [18] analyzed 4599 patients with CRC and demonstrated that complications after CRC surgery affected survival. Fransgaard et al. [20] included patients undergoing surgery for CRC and receiving adjuvant chemotherapy and discovered no correlation between postoperative complications and DFS, recurrence-free survival, or overall mortality. In the present study, the complication group had significantly lower 5-year OS and DFS than the non-complication group, and postoperative complications were independent prognostic factors for DFS after laparoscopic surgery for resectable CRC, corroborating the results of numerous earlier studies.
Adjuvant chemotherapy is associated with improved survival of patients with stage III CRC, a node-positive disease [10, 21, 22]. In the subgroup analyses in our study, the 5-year OS rate of the complication group was significantly lower only among patients with stage III disease, and the number of days between surgery and adjuvant therapy was significantly longer for patients with complications than it was for those without complications. Despite the small number of patients with stage III disease in this study, we think that the difference in OS between patients with complications and those without complications among patients with stage III disease is due to delayed adjuvant treatment owing to complications rather than to the complications themselves. Fransgaard et al. [20] also reported that postoperative complications were not associated with the long-term prognosis after adjusting for the time to adjuvant chemotherapy. However, additional research on the relationships among postoperative complications, delayed adjuvant chemotherapy, and oncological outcomes is required, specifically among patients with stage III disease.
In the present study, multivariable analysis revealed that N stage was an independent prognostic factor for 5-year OS (HR, 8.392; 95% CI, 1.892–37.175; p = 0.005), and postoperative complications were independent prognostic factors for 5-year DFS (HR, 2.996; 95% CI, 1.076–8.340; p = 0.036). Considering these results, postoperative complications are thought to have a more significant impact on recurrence as well as overall survival. It is believed that reducing surgical and medical postoperative complications through advancements in surgical techniques, prehabilitation based on nutritional risk, and improvements in intraoperative and postoperative management may lead to improved oncologic outcomes.
The oncological outcomes of colorectal cancer (CRC) are significantly influenced by the immune status of a patient [9, 23–26]. The enhanced preservation of immune function with laparoscopic surgery is believed to contribute to superior long-term outcomes, including a reduced likelihood of cancer recurrence and metastasis [9, 27]. However, there has been little research on whether postoperative complications affect survival in colorectal cancer patients who underwent laparoscopic surgery. Park et al. investigated the influence of postoperative complications on the long-term oncologic prognosis following laparoscopic low anterior resection for rectal cancer [28]. They reported that complication group exhibited a higher rate of local recurrence compared to the non-complication group. Sugamata et al. [29] retrospectively examined patients who underwent laparoscopic elective resection with negative resection margins for stage I–III colorectal cancer. They reported that the occurrence of surgical site infection, including anastomotic leak and pathological stage, was an independent co-factor for relapse-free survival. In the current study, we concluded that postoperative complications affect long-term oncologic outcomes in patients undergoing laparoscopic surgery for colorectal cancer based on our data.
This study had several limitations. First, it was a single-center, retrospective study with small sample size. Second, in the study, the median follow-up period was 38.1 months. Therefore, this could be a limitation of the study. At last, the effects of long-term complications (> 1 month after surgery) on oncological outcomes were not analyzed as the relevant data had not been captured. Therefore, a prospective study involving a larger number of patients is warranted.
Conclusion
Following laparoscopic surgery for resectable CRC in this study, postoperative complications were associated with poor oncological outcomes, particularly in patients with stage III disease, and were independent prognostic factors for 5-year DFS.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
JaeEun Lee and SungUk Bae prepare the Conceptualization, Formal analysis, Methodology. JaeEun Lee, SungUk Bae, KyeongEui Kim wrote original draft. KyeongEui Kim, WoonKyung Jeong, SungKyu Baek review and edit the manuscript. Finally, all authors reviewed and approved the final manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This study was presented as an oral presentation for surgical residents at the Annual Congress of KSS 2023 & 75th Congress of the Korean Surgical Society, Seoul, Korea.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424 [DOI] [PubMed] [Google Scholar]
- 2.Kim S, Bae SU, Jeong WK, Baek SK, Son YG (2023) Effect of intracorporeal anastomosis on postoperative ileus after laparoscopic right colectomy. Ann Surg Treat Res 104(3):156–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song WJ, Bae SU, Jeong WK, Baek SK (2022) A propensity score-matched analysis of advanced energy devices and conventional monopolar device for colorectal cancer surgery: comparison of clinical and oncologic outcomes. Ann Surg Treat Res 103(5):290–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park CH, Bae SU, Jeong WK, Baek SK (2021) Early and late clinico-pathologic outcomes of minimally invasive total mesorectal excision for rectal cancer: a propensity score-matched comparison of robotic and laparoscopic approaches. Int J Med Robot 17(6):e2324 [DOI] [PubMed] [Google Scholar]
- 5.Bae SU, Yang SY, Min BS (2019) Totally robotic modified complete mesocolic excision and central vascular ligation for right-sided colon cancer: technical feasibility and mid-term oncologic outcomes. Int J Colorectal Dis 34(3):471–479 [DOI] [PubMed] [Google Scholar]
- 6.Vonlanthen R, Slankamenac K, Breitenstein S, Puhan MA, Muller MK, Hahnloser D, Hauri D, Graf R, Clavien PA (2011) The impact of complications on costs of major surgical procedures: a cost analysis of 1200 patients. Ann Surg 254(6):907–913 [DOI] [PubMed] [Google Scholar]
- 7.Nowakowski M, Pisarska M, Rubinkiewicz M, Torbicz G, Gajewska N, Mizera M, Major P, Potocki P, Radkowiak D, Pedziwiatr M (2018) Postoperative complications are associated with worse survival after laparoscopic surgery for non-metastatic colorectal cancer - interim analysis of 3-year overall survival. Wideochir Inne Tech Maloinwazyjne 13(3):326–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durak D, Alkurt EG, Turhan VB, Tutan B, Sahiner IT, Kendirci M (2022) Comparison of short-term results of laparoscopic and open surgeries for colorectal cancer: a single-center experience. Cureus 14(5):e24635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bae SU, Saklani AP, Lim DR, Kim DW, Hur H, Min BS, Baik SH, Lee KY, Kim NK (2014) Laparoscopic-assisted versus open complete mesocolic excision and central vascular ligation for right-sided colon cancer. Ann Surg Oncol 21(7):2288–2294 [DOI] [PubMed] [Google Scholar]
- 10.Bae SU, Yang CS, Kim S et al (2019) Long-term oncologic outcomes of laparoscopic versus open resection following stent insertion for obstructing colon cancer: a multi-center retrospective study. Surg Endosc 33(12):3937–3944 [DOI] [PubMed] [Google Scholar]
- 11.Bae SU, Park JS, Choi YJ, Lee MK, Cho BS, Kang YJ, Park JS, Kim CN (2014) The role of hand-assisted laparoscopic surgery in a right hemicolectomy for right-sided colon cancer. Ann Coloproctol 30(1):11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacy AM, Garcia-Valdecasas JC, Delgado S, Castells A, Taura P, Pique JM, Visa J (2002) Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet 359(9325):2224–2229 [DOI] [PubMed] [Google Scholar]
- 13.Goldfarb Y, Sorski L, Benish M, Levi B, Melamed R, Ben-Eliyahu S (2011) Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg 253(4):798–810 [DOI] [PubMed] [Google Scholar]
- 14.Wang G, Jiang Z, Zhao K, Li G, Liu F, Pan H, Li J (2012) Immunologic response after laparoscopic colon cancer operation within an enhanced recovery program. J Gastrointest Surg 16(7):1379–1388 [DOI] [PubMed] [Google Scholar]
- 15.Warps AK, Tollenaar R, Tanis PJ, Dekker JWT, Dutch ColoRectal A (2022) Postoperative complications after colorectal cancer surgery and the association with long-term survival. Eur J Surg Oncol 48(4):873–882 [DOI] [PubMed] [Google Scholar]
- 16.Oh CK, Huh JW, Lee YJ et al (2020) Long-term oncologic outcome of postoperative complications after colorectal cancer surgery. Ann Coloproctol 36(4):273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu XY, Zhang B, Kang B, Cheng YX, Yuan C, Tao W, Wei ZQ, Peng D (2022) The effect of complications on oncological outcomes of colorectal cancer patients after primary surgery: a propensity score matching analysis. Front Oncol 12:857062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cienfuegos JA, Baixauli J, Beorlegui C, Ortega PM, Granero L, Zozaya G, Hernandez Lizoain JL (2018) The impact of major postoperative complications on long-term outcomes following curative resection of colon cancer. Int J Surg 52:303–308 [DOI] [PubMed] [Google Scholar]
- 20.Fransgaard T, Caspar Thygesen L, Gogenur I (2021) The impact of postoperative complications and delay of adjuvant chemotherapy on oncological outcomes in patients with colorectal cancer. Colorectal Dis 23(5):1132–1140 [DOI] [PubMed] [Google Scholar]
- 21.Andre T, de Gramont A, Vernerey D et al (2015) Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol 33(35):4176–4187 [DOI] [PubMed] [Google Scholar]
- 22.Fukui Y, Hida K, Hoshino N et al (2022) Oncologic benefit of adjuvant chemotherapy for locally advanced rectal cancer after neoadjuvant chemoradiotherapy and curative surgery with selective lateral pelvic lymph node dissection: an international retrospective cohort study. Eur J Surg Oncol 48(7):1631–1637 [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Yuan R, Wu X, He X, Zeng Y, Fan X, Wang L, Wang J, Lan P, Wu X (2016) A novel immune marker model predicts oncological outcomes of patients with colorectal cancer. Ann Surg Oncol 23(3):826–832 [DOI] [PubMed] [Google Scholar]
- 24.Seo I, Lee HW, Byun SJ, Park JY, Min H, Lee SH, Lee JS, Kim S, Bae SU (2021) Neoadjuvant chemoradiation alters biomarkers of anticancer immunotherapy responses in locally advanced rectal cancer. J Immunother Cancer 9(3):e001610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim KE, Bae SU, Jeong WK, Baek SK (2022) Impact of preoperative visceral fat area measured by bioelectrical impedance analysis on clinical and oncologic outcomes of colorectal cancer. Nutrients 14(19):3971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae SU, Jeong WK, Baek SK, Kim NK, Hwang I (2018) Prognostic impact of programmed cell death ligand 1 expression on long-term oncologic outcomes in colorectal cancer. Oncol Lett 16(4):5214–5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bohne A, Grundler E, Knuttel H, Furst A, Volkel V (2023) Influence of laparoscopic surgery on cellular immunity in colorectal cancer: a systematic review and meta-analysis. Cancers (Basel) 15(13):3381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park EJ, Baik SH, Kang J, Hur H, Min BS, Lee KY, Kim NK (2016) The impact of postoperative complications on long-term oncologic outcomes after laparoscopic low anterior resection for rectal cancer. Medicine (Baltimore) 95(14):e3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugamata N, Okuyama T, Takeshita E et al (2022) Surgical site infection after laparoscopic resection of colorectal cancer is associated with compromised long-term oncological outcome. World J Surg Oncol 20(1):111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.