Abstract
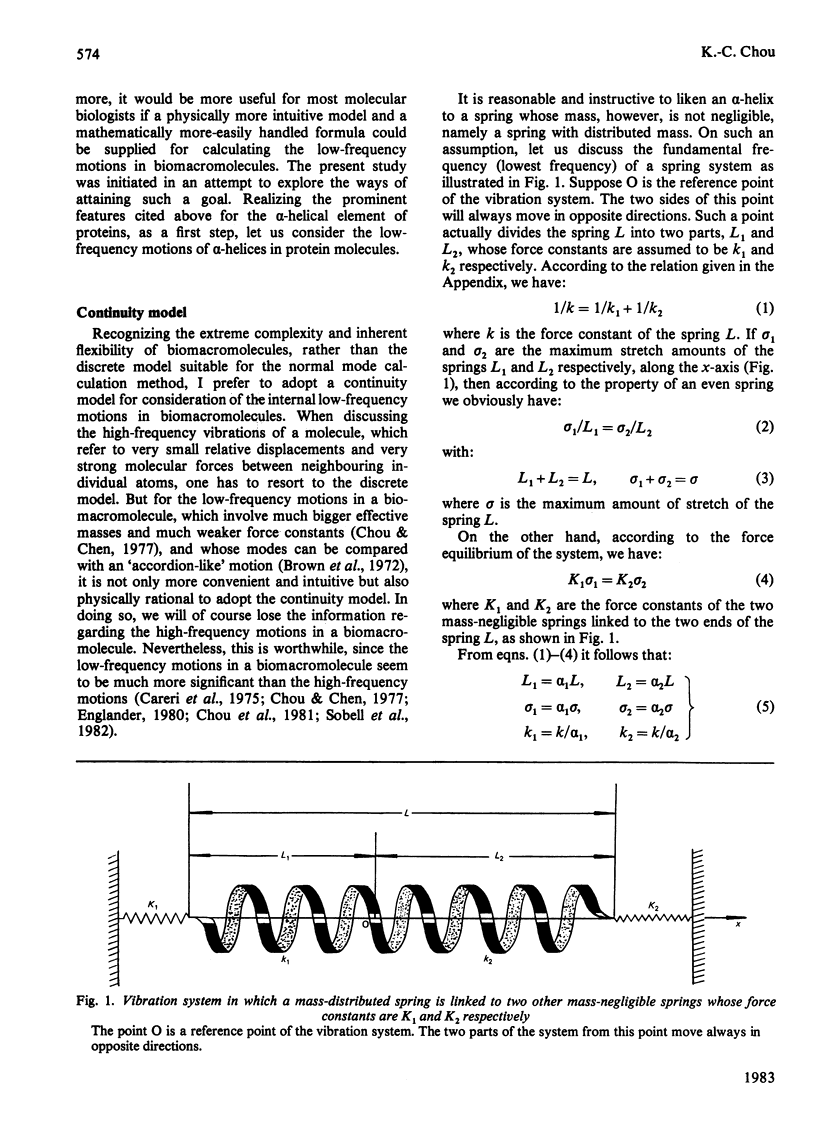
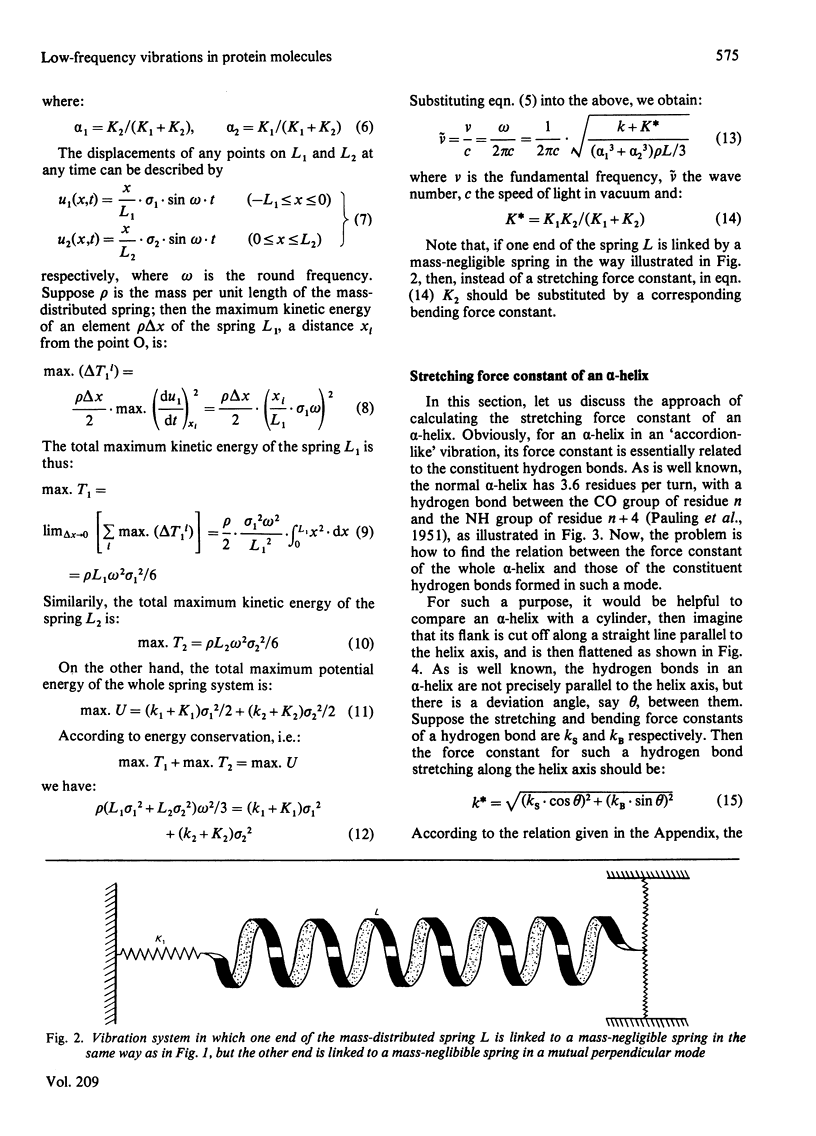
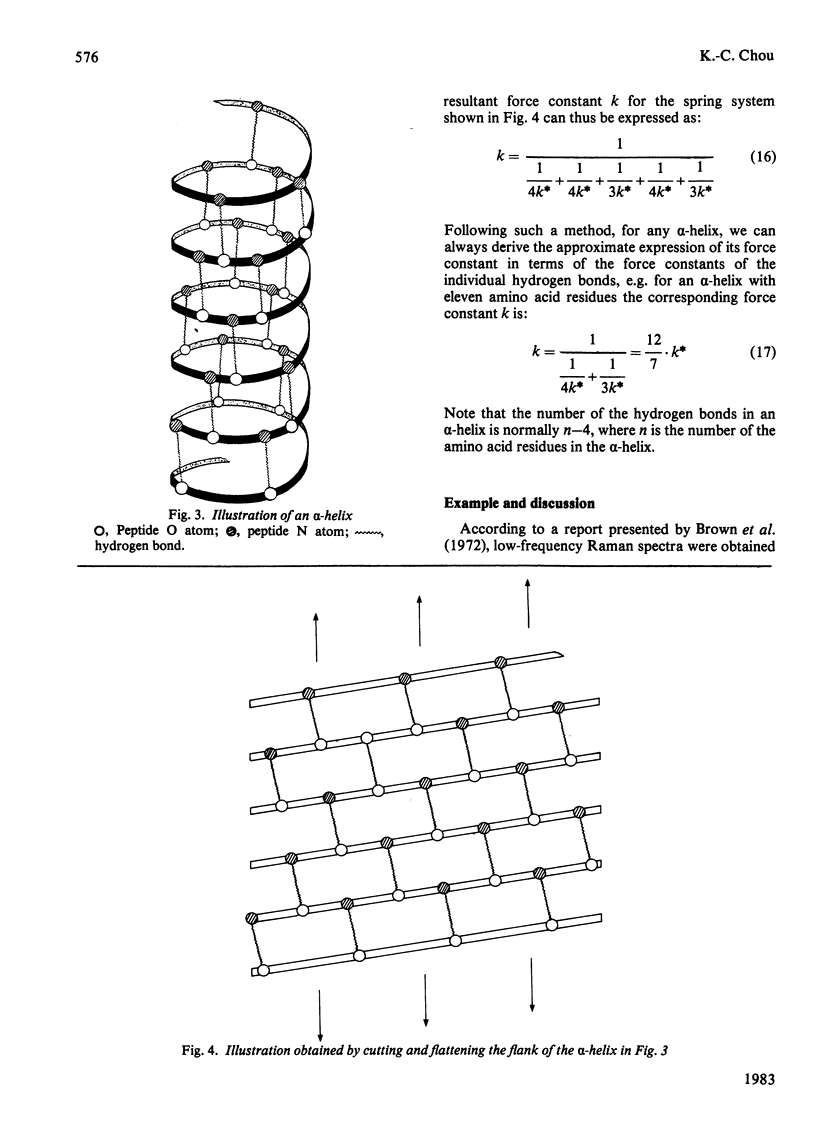
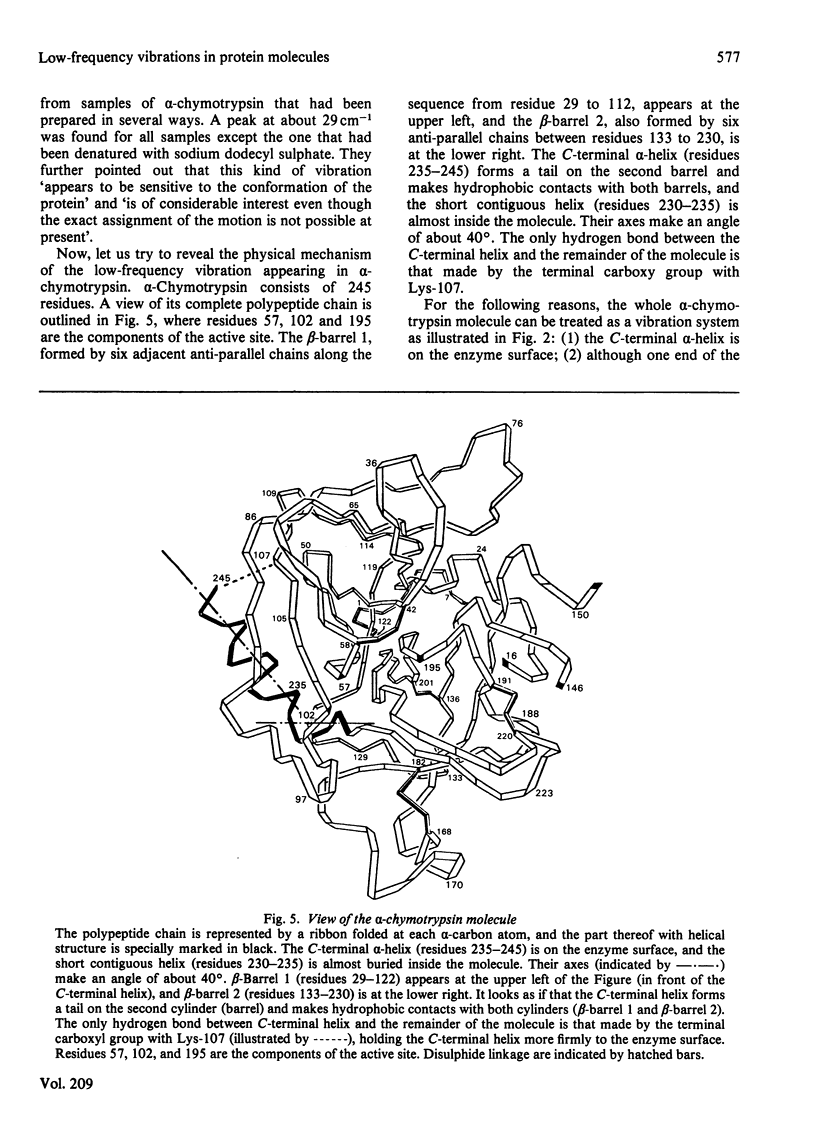
A physically intuitive and mathematically easily handled formula is presented for calculating the low-frequency vibrations of helical structures in protein molecules. alpha-Chymotrypsin is taken as an example, and the calculated result shows precise agreement with observations of the low-frequency Raman spectra. As reflected in the formula, this kind of low frequency is very sensitive to the conformation of a biomacromolecule, and can therefore serve as a vehicle to investigate the mechanism of action of a biomacromolecule from the viewpoint of dynamics. On this basis a feasible experiment is suggested by which one can examine the relationship between a presumed mode of low-frequency vibration in a biomacromolecule and its activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birktoft J. J., Blow D. M. Structure of crystalline -chymotrypsin. V. The atomic structure of tosyl- -chymotrypsin at 2 A resolution. J Mol Biol. 1972 Jul 21;68(2):187–240. doi: 10.1016/0022-2836(72)90210-0. [DOI] [PubMed] [Google Scholar]
- Brown K. G., Erfurth S. C., Small E. W., Peticolas W. L. Conformationally dependent low-frequency motions of proteins by laser Raman spectroscopy. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1467–1469. doi: 10.1073/pnas.69.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careri G., Fasella P., Gratton E. Statistical time events in enzymes: a physical assessment. CRC Crit Rev Biochem. 1975 Aug;3(2):141–164. doi: 10.3109/10409237509102555. [DOI] [PubMed] [Google Scholar]
- Fanconi B., Peticolas W. L. Simplified force field calculations of the low-frequency motions of the alpha-helix. Biopolymers. 1971 Nov;10(11):2223–2229. doi: 10.1002/bip.360101115. [DOI] [PubMed] [Google Scholar]
- Fanconi B., Small E. W., Peticolas W. L. Phonon dispersion curves and normal coordinate analysis of -poly-L-alanine. Biopolymers. 1971;10(8):1277–1298. doi: 10.1002/bip.360100804. [DOI] [PubMed] [Google Scholar]
- Ito K., Shimanouchi T. Vibrational frequencies and modes of alpha-helix. Biopolymers. 1970;9(4):383–399. doi: 10.1002/bip.1970.360090402. [DOI] [PubMed] [Google Scholar]
- Karplus M., McCammon J. A. Protein structural fluctuations during a period of 100 ps. Nature. 1979 Feb 15;277(5697):578–578. doi: 10.1038/277578a0. [DOI] [PubMed] [Google Scholar]
- Koshland D. E. Application of a Theory of Enzyme Specificity to Protein Synthesis. Proc Natl Acad Sci U S A. 1958 Feb;44(2):98–104. doi: 10.1073/pnas.44.2.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAULING L., COREY R. B., BRANSON H. R. The structure of proteins; two hydrogen-bonded helical configurations of the polypeptide chain. Proc Natl Acad Sci U S A. 1951 Apr;37(4):205–211. doi: 10.1073/pnas.37.4.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell H. M., Lozansky E. D., Lessen M. Structural and energetic considerations of wave propagation in DNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):11–19. doi: 10.1101/sqb.1979.043.01.004. [DOI] [PubMed] [Google Scholar]


