Abstract
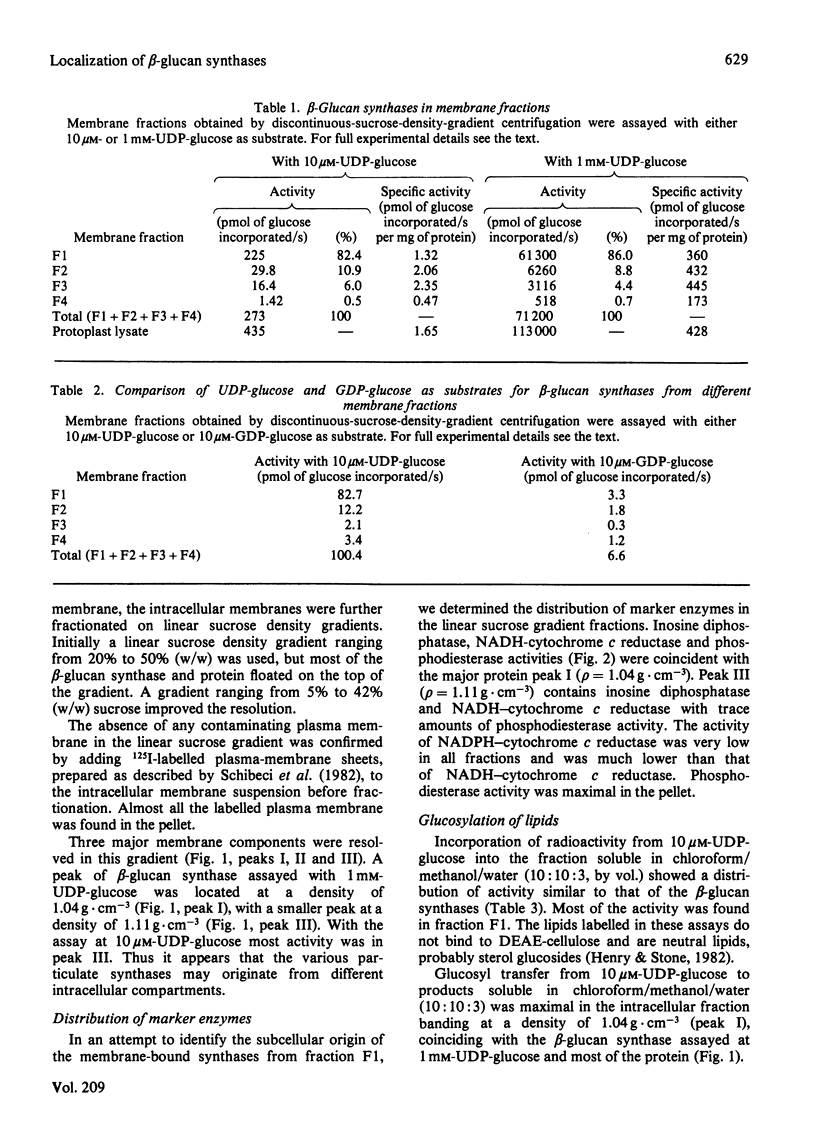
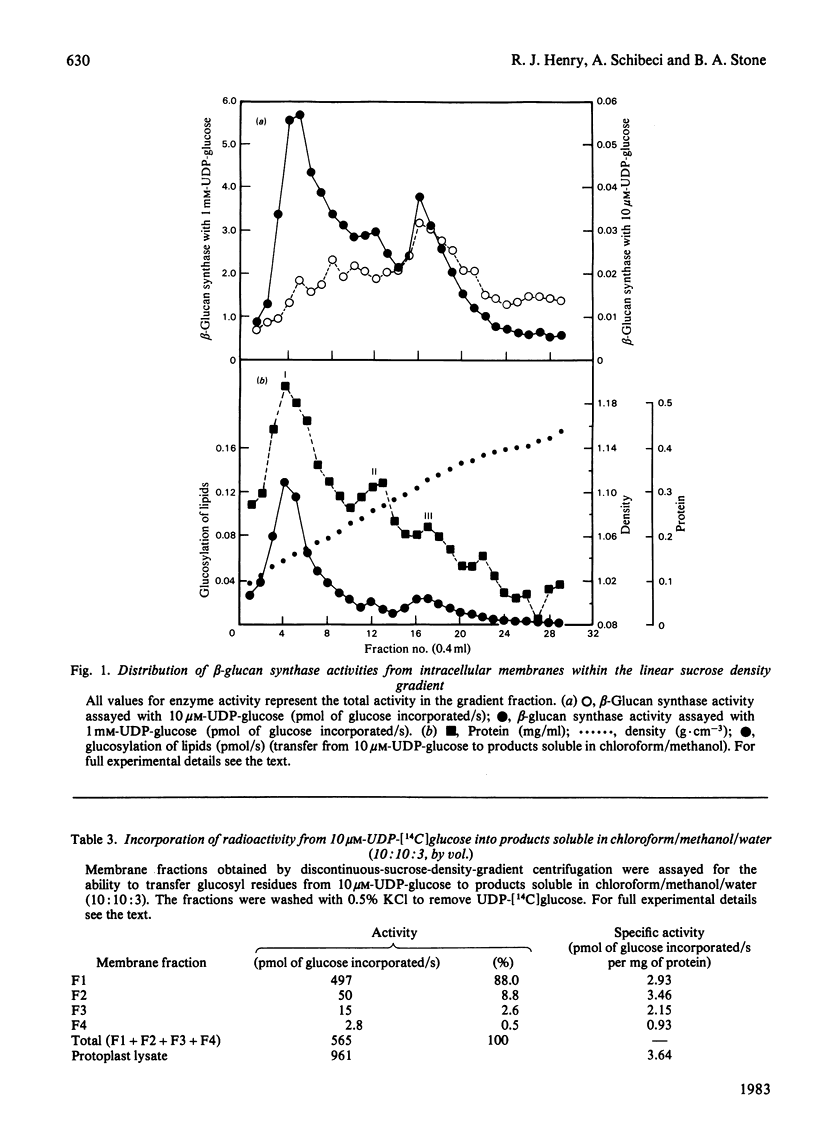
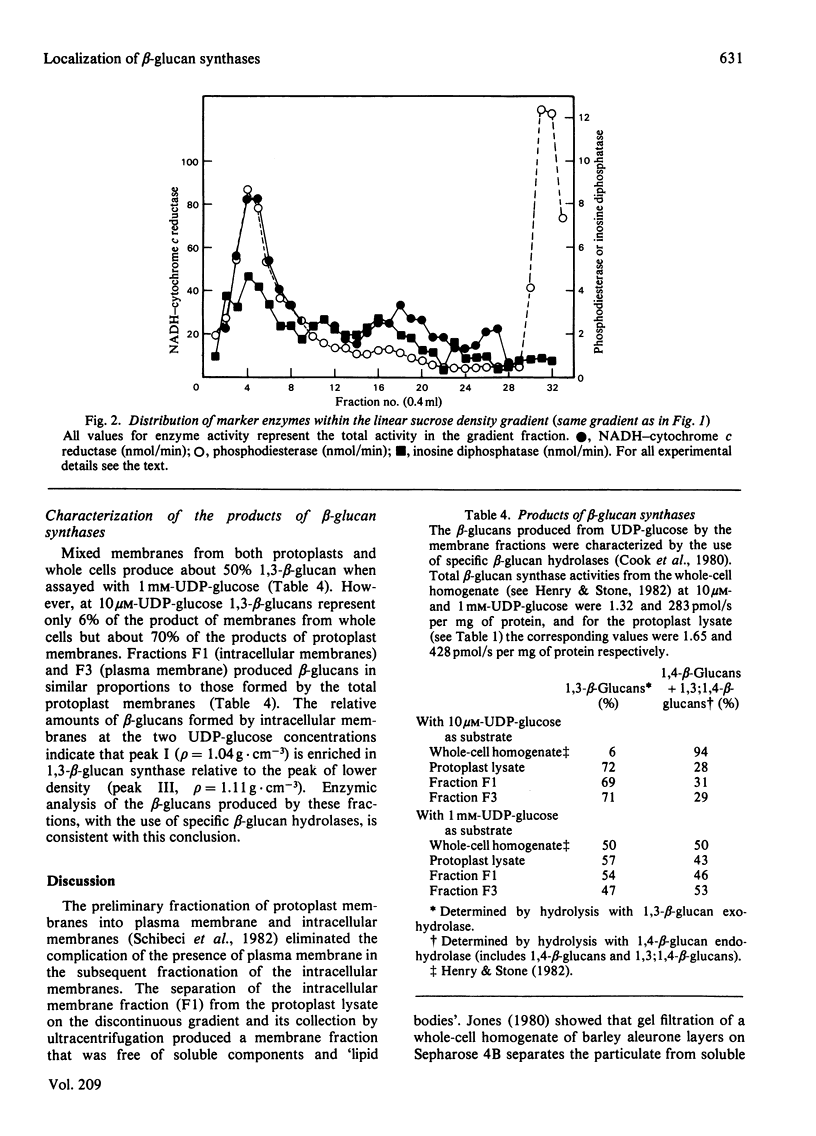
The distribution of beta-glucan synthases between plasma membranes and intracellular membranes of suspension-cultured Italian-ryegrass (Lolium multiflorum Lam.) endosperm cells was examined. Highly purified plasma membranes prepared from protoplasts were only slightly enriched in beta-glucan synthases assayed at 10 microM- and 1 mM-UDP-glucose. Most beta-glucan synthase was associated with intracellular membranes. These membranes were fractionated on a linear sucrose density gradient and were resolved into different membrane fractions containing beta-glucan synthases. Beta-Glucan synthases assayed at 10 microM-UDP-glucose were found in a fraction banding at a density of 1.11 g . cm-3, but most of the beta-glucan synthase assayed at 1 mM-DDP-glucose was at a density of 1.04 g . cm-3.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. L., Ray P. M. Labeling of the Plasma Membrane of Pea Cells by a Surface-localized Glucan Synthetase. Plant Physiol. 1978 May;61(5):723–730. doi: 10.1104/pp.61.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick C. M., Northcote D. H. Glucosylation of phosphorylpolyisoprenol and sterol at the plasma membrane of soya-bean (Glycine max) protoplasts. Biochem J. 1980 Feb 15;186(2):411–421. doi: 10.1042/bj1860411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry R. J., Stone B. A. Factors Influencing beta-Glucan Synthesis by Particulate Enzymes from Suspension-Cultured Lolium multiflorum Endosperm Cells. Plant Physiol. 1982 Mar;69(3):632–636. doi: 10.1104/pp.69.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop-Harrison J., Heslop-Harrison Y. Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Technol. 1970 May;45(3):115–120. doi: 10.3109/10520297009085351. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Pearce R. S., Withers L. A., Willison J. H. Bodies of wall-like material ("wall-bodies") produced intracellularly by cultured isolated protoplasts and plasmolysed cells of higher plants. Protoplasma. 1974;82(3):223–236. doi: 10.1007/BF01276309. [DOI] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E. The golgi apparatus: two organelles in tandem. Science. 1981 Sep 11;213(4513):1212–1219. doi: 10.1126/science.7268428. [DOI] [PubMed] [Google Scholar]
- Schibeci A., Fincher G. B., Stone B. A., Wardrop A. B. Isolation of plasma membrane from protoplasts of Lolium multiflorum (ryegrass) endosperm cells. Biochem J. 1982 Sep 1;205(3):511–519. doi: 10.1042/bj2050511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. M., Stone B. A. Beta-glucan synthesis by cell-free extracts from Lolium multiflorum endosperm. Biochim Biophys Acta. 1973 Jun 20;313(1):72–94. doi: 10.1016/0304-4165(73)90189-x. [DOI] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Van Der Woude W. J., Lembi C. A., Morré D. J. beta-Glucan Synthetases of Plasma Membrane and Golgi Apparatus from Onion Stem. Plant Physiol. 1974 Sep;54(3):333–340. doi: 10.1104/pp.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]


