Opinion Statement
Gastrointestinal stromal tumor (GIST) is characterized by well-defined oncogenes. Despite the significant improvement in treatment outcomes with adjuvant imatinib therapy for patients, drug resistance remains a major challenge for GIST therapy. This review focuses on the mechanisms contributing to drug resistance phenotype in GIST, such as primary imatinib-resistant mutants, secondary mutations, non-covalent binding of TKI to its target, tumor heterogeneity, re-activation of pro-survival/proliferation pathways through non-KIT/PDGFRA kinases, and loss of therapeutic targets in wild-type GIST. Corresponding suggestions are proposed to overcome drug-resistance phenotype of GIST. This review also summarizes the suitability of currently approved TKIs on different KIT/PDGFRA mutations and updates related clinical trials. Recent potent drugs and emerging strategies against advanced GISTs in clinical trials are presented. Additionally, metabolic intervention offers a new avenue for clinical management in GIST. A landscape of metabolism in GIST and metabolic changes under imatinib treatment are summarized based on currently published data. The OXPHOS pathway is a promising therapeutic target in combination with TKI against sensitive KIT/PDGFRA mutants. Comprehensive understanding of the above resistance mechanisms, experimental drugs/strategies and metabolic changes is critical to implement the proper therapy strategy and improve the clinical therapy outcomes for GIST.
Keywords: GIST, Tyrosine kinase inhibitor, Drug resistance, Metabolism, Imatinib
Introduction
Gastrointestinal stromal tumor (GIST) is a type of sarcoma that originates from precursor cell of the interstitial cells of Cajal (ICC) [1]. Before the identification of specific oncogenic mutations in GIST, surgical resection was the primary treatment option. The five-year survival rate for localized GIST was approximately 54%, while cases with incomplete resections or unresectable tumors had a median survival of about 22 months [2]. The identification of KIT and PDGFRA mutations defines GISTs as a unique cancer type [3, 4]. Approximately 85% of GIST cases harbor gain-of-function mutations (GOF) in KIT and PDGFRA genes. These mutations have become critical therapeutic targets, leading to the development of tyrosine kinase inhibitors (TKIs) such as imatinib, sunitinib, regorafenib, ripretinib, and avapritinib. Combining surgery with TKI therapy has increased the five-year survival rate to 92% for localized GISTs and 50% for metastatic cases [5]. Despite the success of TKI therapy, challenges such as drug resistance and disease progression remain persistent. The mechanisms of drug resistance are caused by multiple reasons in the context of GISTs. Based on available data, we discuss the current drug resistance mechanisms and their underlying molecular basis. Strategies to overcome the above resistance were proposed and discussed. Clinical trials related to the current approved TKIs and new emerging therapeutic drugs are presented. Metabolism changes are also summarized and analyzed in order to find possible therapeutic targets to benefit the therapy of GISTs. Through this review, we hope to offer fresh insights that could benefit future therapy strategies, drug development, management of GISTs in clinical, leading to better therapeutic outcomes in patients with GIST.
Molecular Pathology of GIST
KIT/PDGFRA Mutant GISTs
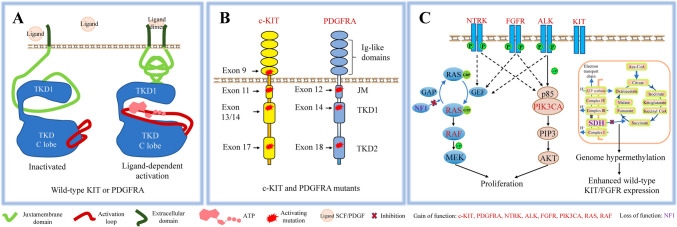
GOF mutations in KIT were observed in a major proportion (about 80%) in patients with GIST, and play crucial roles in GIST tumorigenesis and progression [6]. The most common KIT mutations occur in exon 11, affecting the cytosolic juxtamembrane domain (Fig. 1B). Mutation types include point(s) mutation, insertions and peptide deletions that disrupt the binding of the inhibitory JM to the tyrosine kinase domain (TKD) [7]. Exon 11 mutations render tumors sensitive to imatinib. GIST cell lines (GIST 430KIT:Δ560−576 and GIST T1KIT:Δ560−578) are sensitive to imatinib (IC50: 30–60 nM) [8], as does the mast cell line HMC1.1KIT:V560G (IC50: 10.7 nM) [9]. Clinically, adjuvant imatinib therapy prolongs the five-year survival of GIST patients harboring exon 11 mutations [10–13]. Exon 9 mutations in the extracellular domain are the second most common KIT mutations. These alterations mimic the cytosolic conformational rearrangement of wild-type KIT induced by stem cell factor (SCF) (Fig. 1A). These mutations confer resistance to imatinib, with both in vitro studies and clinical data supporting this observation. Cell lines transformed by KITdup502−503 are resistant to imatinib [9], and patients with these mutations also exhibit resistance to imatinib in clinical setting [12]. Less common mutations in exon 13/14 (located in TKD N lobe, TKD1) and 17 (located in TKD C lobe, TKD2), enhance ATP binding and/or increase kinase activation velocity, resulting in varying degrees of resistance to imatinib [14, 15]. GIST 882KIT:K642E cells show moderate resistance to imatinib (IC50: 173 nM) [8]. Other mutations, as in cell lines (P815KIT:D814Y, Kasumi-1KIT:N822K, Ba/F3KIT:D816V), show moderate to high resistance to imatinib [8, 9]. These mutations, although rare in primary GIST, often arise as secondary mutations following imatinib treatment, and significantly aggravate the phenomenon of drug resistance (Fig. 1B).
Fig. 1.
Molecular pathology of GIST. A. Schemes of inactivated and activated wild-type oncoprotein (KIT/PDGFRA). B. Mutation pattern of KIT/PDGFRA mutants. C. Mutations in wild-type GISTs.
GOF mutations in PDGFRA, while less frequent (about 5%) than KIT mutations, have a similar mutation pattern like KIT mutants in GISTs. The most common PDGFRA mutations occur in the TKD C lobe (exon 18), resulting in enhanced ATP affinity and/or catalytic velocity [16] (Fig. 1B). Mutations in exon 18 exhibit various sensitivity to imatinib. Ba/F3 or CHO cells with PDGFRA mutations (D842V, RD841-842KI) are resistant to imatinib, while CHO cells with PDGFRA mutation (D842Y, D846Y, Y849C, Del DIMH842-845, Del DIMH843-846, Del I843 and HDSN845-848P) are sensitive to imatinib (IC50 < 200 nM). In clinical trials, patients with PDGFRAD842V are resistant to adjuvant imatinib therapy [9, 17, 18]. The second most frequent mutations locate in the cytosolic JM domain (exon 12). Primary GIST cells with PDGFRA mutations (V561D, SPDHE566-571R and insertion ER561-562) are sensitive to imatinib treatment in vitro (IC50 < 200 nM) [17]. The third most frequent mutations locate in the TKD N lobe (exon 14). Cells with PDGFRAN659K mutation are sensitive to imatinib [17]. Mutations in exon 14 often emerge as secondary mutations, which significantly enhance drug resistance in clinical setting [19].
Wild-Type GISTs
Approximately 10–15% of GIST cases have no mutations in the KIT/PDGFRA genes and are classified as wild-type (WT) GISTs. These cases often involve gene alterations in other proteins, such as succinate dehydrogenase (SDH), neurofibromin 1 (NF1), RAS, BRAF, and PIK3CA, all contributing to tumorigenesis [20, 21] (Fig. 1C).
SDH-deficient GISTs, accounting for less than 9% of all cases, represent the third most common GIST subtype [21, 22]. SDH couples the oxidation of succinate to fumarate in the citric acid cycle with the reduction of ubiquinone to ubiquinol in the electron transport chain. Accumulation of succinate caused by loss-of-function (LOF) SDH mutation inhibits several alpha-ketoglutarate dioxygenases, inducing the pseudohypoxia pathway and causing epigenetic changes that lead to reprogramming of cell metabolism [23]. In GISTs with SDH deficiency, significant hypermethylation has been observed compared to kinase-mutant GISTs. This hypermethylation pattern is a unifying feature of SDH-deficient GISTs, and methyl-divergence process is not random [24]. Hypermethylation of two insulators that separate FGFR and KIT from upstream super-enhancers enhances FGFR and KIT expression, which contributes to GIST progression [25].
GISTs With Other RTKs and Cytosolic Kinases Mutations
Activating mutations in receptor tyrosine kinases (RTKs) and their downstream signaling pathways account for approximately 5% of GIST cases. FGFR, NTRK, and ALK translocations can act as oncogenic drivers in these subtypes [26, 27]. Additionally, GOF mutations in BRAF, RAS, or PIK3CA affect key molecules in RTK signaling cascades, contributing to GIST pathogenesis through constant activation of pathways like the MAPK or PI3K pathways [28].
NF1-Deficient GISTs
Some GIST cases can also result from mutations in the NF1 gene. The NF1 gene encodes neurofibromin 1, which stimulates the GTPase activity of RAS and thus negatively regulates RAS/MAPK signaling pathway. LOF mutations in NF1 lead to deregulated activation of the RAS/MAPK pathway, contributing to the tumorigenesis of GIST [20].
Molecular genetic testing is useful in identifying the underlying genetic mutations in GISTs and assists therapeutic decisions. This information guides prognosis, aids in predicting tumor response to therapy, and understands mechanisms of resistance, allowing for the development of personalized treatment strategies, especially for non-KIT/PDGFRA mutant GISTs.
Resistance Mechanisms to Approved Adjuvant Therapy
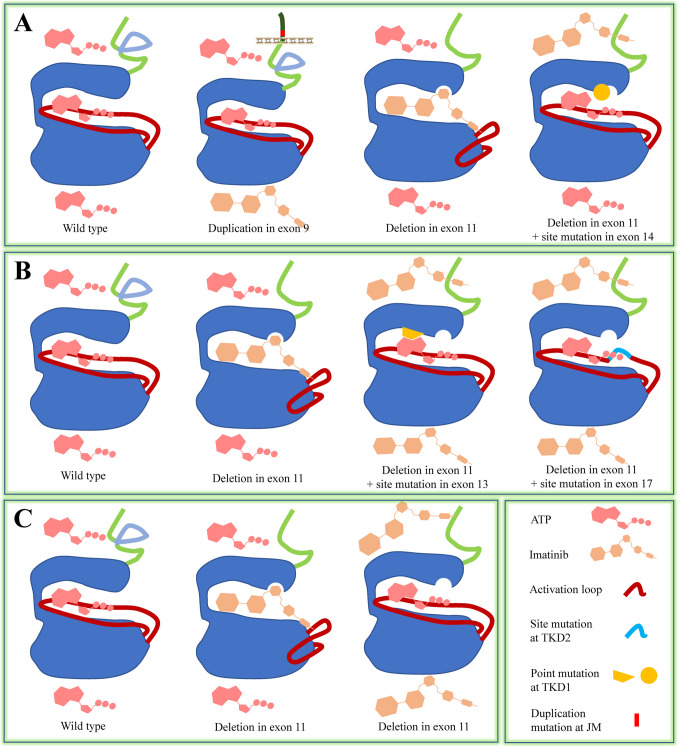
KIT functions as a receptor for an extracellular ligand (SCF) and as an enzyme (kinase) that catalyzes the transfer of a phosphate group from ATP to tyrosine residues on KIT and downstream proteins. Mutations in the extracellular and cytosolic JM domains lead to the TKD being in an activation state, thus exempting the KIT mutants from the control of SCF. Other mutations in TKD enhance its tendency to remain in the activation state and/or increase its catalytic velocity [14, 16, 29, 30]. These mutations give KIT different characteristics. Firstly, the vivid spatial conformation variations in the catalytic center of different KIT mutants create different degrees of steric hindrance, which affects the entry of KIT TKIs [30, 31] (Fig. 2A). As a result, KIT mutants exhibit varying resistance to TKIs inhibition. Secondly, spatial conformation in the catalytic center of KIT/PDGFRA mutants can lead to increased affinity for ATP (Km[ATP]) and/or enhanced catalytic velocity (Kcat) [14–16] (Fig. 2B). Since the enzyme (kinase) activity of KIT/PDGFRA involves transferring phosphate group from ATP to other tyrosine residues, TKIs and ATP compete to occupy the ATP-binding site in the catalytic center [32] (Fig. 2B). For mutants with increased ATP affinity, ATP more readily enters the catalytic center and is utilized by the kinase than TKIs. Therefore, higher concentrations of TKIs are needed to achieve the same inhibitory effect toward mutants with increased ATP affinity than toward mutants without increased ATP affinity, thus demonstrating a resistance phenotype.
Fig. 2.
Drug resistance mechanisms in GIST cells with KIT/PDGFRA mutants, KIT as a model. A. Steric hindrance. Duplication in exon 9 induces TKD in an activation conformation, which prohibits entry of imatinib [15]. Deletion in exon 11 creates a TKD conformation, which permits imatinib entry. Double mutations in exon 11 (deletion) and exon 14 (T670I) create a TKD conformation which prohibit imatinib entry [30]. B. Enhanced affinity for ATP or enhanced catalytic velocity. Deletion in exon 11 creates a TKD conformation, which permits imatinib entry. Double mutation in exon 11 (deletion) and exon 13/14 (V654A, T670I) create a TKD conformation which could have enhanced affinity for ATP [15]. Double mutation in exon 11 (deletion) and exon 17 (activation loop) create a TKD conformation which could have enhanced catalytic velocity and affinity to ATP [15]. C. Leaky activation. Reversible binding of imatinib to catalytic center permits leaky entry of ATP and activation of KIT.
Approved TKIs for GIST treatment are categorized into two groups: type I and type II kinase inhibitors. Type I TKIs occupy the conserved ATP-binding site and extend into different proximal regions. However, they usually have low selectivity due to the conserved nature of ATP-binding site among different types of kinases [32, 33]. Sunitinib and avapritinib fall into this category. Type II TKIs occupy both the ATP-binding site and an allosteric site, which increases their selectivity. Imatinib, regorafenib, and ripretinib are type II TKIs. Currently, all TKIs bind to KIT/PDGFRA mutants in a noncovalent manner, which allows cytosolic ATP (5–10 mM) to enter the ATP-binding site and be utilized by the catalytic center to phosphorylate itself and downstream substrates (Fig. 2C). This "leaky" activation contributes to the survival, if not the proliferation, of GIST cells under TKI treatment. Covalent-binding TKIs could potentially address the limitations associated with non-covalent TKIs by forming an irreversible chemical bond with the catalytic center through their electrophilic groups and nucleophilic amino acids (such as cysteine, serine, and threonine) surrounding the catalytic center [34]. However, this strategy has not been investigated for GIST, unlike in the cases of BTK, EGFR and HER2 where covalent TKIs are approved for therapy [35–37]. Covalent-binding TKIs generally have higher potency than reversible inhibitors, as they provide prolonged inhibition of signaling pathways. Their potential to form a covalent bond with the non-target wild-type kinase may result in increased toxic effects compared to reversible TKIs [38]. However, this potential toxicity can be mitigated through chemical group modification on the TKIs [39, 40].
In clinical settings, multiple KIT/PDGFRA mutants with varying sensitivity to individual TKI (tumor heterogeneity) after imatinib treatment exist in the tumor tissue and thus contribute to resistance of TKI therapy [41]. Other drug resistance mechanisms for KIT/PDGFRA-mutant GISTs have also been identified. The FGF/FGFR axis has been shown to reactivate the MAPK/ERK pathway which is previously inhibited by imatinib, and supports the survival of GIST cells [42]. Upregulated expression of MET receptor is observed in imatinib-resistant GIST H209 cells (exon 11 + 17 mutations) and contributes resistance to imatinib [43]. The underlying resistance mechanism is that growth factor-activated RTKs (FGFR, MET) share the MAPK/ERK pathway with KIT/PDGFRA, and activation of these RTKs by ligands supports survival and proliferation of GIST cells in the context of TKI treatment. Another resistance mechanism involves cellular quiescence. Quiescent cells evade drug-induced cytotoxicity by arresting cell cycle at the G0 phase, rendering them less susceptible to TKIs. The DREAM complex has been implicated in maintaining quiescence in residual GIST T1 cells under imatinib treatment, contributing to drug resistance [44]. For non-KIT/PDGFRA-mutant GISTs, these cancer cells are naturally resistant to adjuvant therapy, because oncogenic pathways in these subtypes of GISTs are not the targets of approved TKI drugs.
These findings underscore the multifaceted nature of drug resistance in GISTs, involving both genetic mutations and adaptive cellular pathways. Understanding these drug-resistance mechanisms is crucial for developing more effective treatment methods.
Approved TKIs for Primary and Advanced GISTs
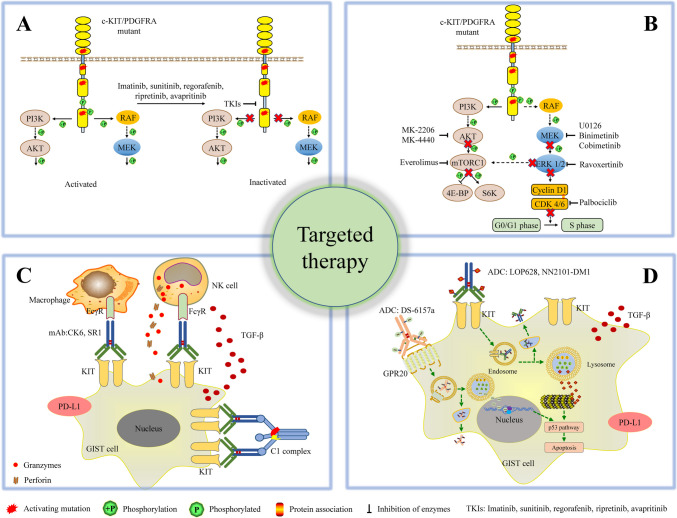
Imatinib, a drug specifically designed to target the BCR-ABL oncoprotein in chronic myeloid leukemia, has also proven effective for primary GISTs [45] (Fig. 3A). The most common exon 11 KIT mutations in GIST, found in about 65% of cases, are highly sensitive to imatinib. In analysis of prospectively collected data of patients with advanced GIST with known mutational status (KIT/PDGFRA) and treated with imatinib (344 patients), median follow-up was 5.2 years, median progression-free survival (mPFS) was 40.6 months (95% CI: 32.8–48.5), median overall survival (mOS) was 82.4 months (95% CI: 67.7–97.1) [46]. For gastric GIST, female gender (HR 0.60; 95% CI 0.37–0.97) and KIT exon 11 point mutations (HR 0.22; 0.70–0.73) correlated with longer PFS, female gender and no PDGFRA exon 18 D842V (HR 0.32; 0.14–0.71) were factors associated with OS; for non-gastric GIST, KIT exon 11 mutation (557–558 deletions, point mutations, other KIT exon 11 alternations) (HR 0.40; 0.23–0.70) and no PDGFRA exon 18 D842V mutation (HR 0.11; 0.04–0.34) correlated with longer PFS, KIT exon 11 other deletions/indels/duplications (HR 0.57; 0.36–0.88) were factors associated with OS [46]. In parallel, a clinical trial in South Korea identified several prognostic factors for poor PFS, including male gender (HR = 3.4, p = 0.01), the presence of extra-hepatic metastasis (HR = 4.3, p < 0.01), and a primary genotype other than the KIT exon 11 mutation (HR = 7.3, p < 0.01) [47]. In a phase III clinical trial on patients with KIT-positive GIST removed at surgery, recurrence-free survival (RFS) was longer in the 36-month imatinib group compared with the 12-month imatinib group (5-year RFS, 65.6% vs 47.9%) [48]. Another clinical trial evaluated 6 years vs. 3 years of adjuvant imatinib in patients with localized GIST at high risk of relapse (136 enrolled, NCT02260505). The results demonstrated that extending adjuvant imatinib treatment from the recommended 3 years duration to 6 years significantly reduced risk of recurrence, providing further evidence for the benefit of extended therapy duration [49].
Fig. 3.
Targeted therapy in GIST. A. Current approved adjuvant therapies for GIST. B. Emerging TKIs targeting MAPK/ERK, PI3K/AKT, mTORC1, and the cell cycle. C. Therapeutic antibodies targeting KIT. D. Antibody–drug conjugates for GIST therapy.
Sunitinib is used as a second-line therapy when imatinib is ineffective. It is particularly useful for mutations involving the cytosolic JM domain (exon 11) and/or the ATP-binding site (exon 13/14) [8, 50]. In a phase III clinical trial evaluating safety and efficacy of sunitinib (50 mg/day, 4 weeks on and 2 weeks off) on patients with histologically proven GIST after imatinib treatment failure due to resistance or intolerance, median follow-up was 41.7 months (95% CI: 40.3–43.8), final mOS analysis for sunitinib (n = 243) versus placebo (n = 118) (without cross-over of placebo-treated patients to open-label sunitinib) was 72.7 weeks (95% CI, 61.3–83.0) versus 39.0 weeks (95% CI, 28.0–54.1). The median time to progression (mTTP) was 26.6 weeks in the sunitinib group (95% CI, 16.0–32.1) versus 6.4 weeks in the placebo group (95% CI: 4.4–10.0) [51].
Regorafenib, considered a third-line therapy, has not shown superior efficacy compared to sunitinib for mutations involving JM domain (exon 11) and/or ATP-binding site (exon 13/14) but demonstrates potency against mutations involving extracellular domain (exon 9) and/or activation loop (exon 17) [8, 50]. In a phase III clinical trial (NCT01271712, 199 patients analyzed) evaluating efficacy and safety of regorafenib for patients with metastatic or unresectable GIST and failure of at least previous imatinib and sunitinib, mPFS was 4.8 months for regorafenib (n = 133) and 0.9 months for placebo (n = 66), providing evidence that this highly refractory GIST population can benefit from regorafenib therapy [52]. Another clinical trial analyzing the efficacy of regorafenib on different genotypes in patients with advanced GIST refractory to imatinib and/or sunitinib (62 patients enrolled) found that, in the subpopulation with secondary mutations, exon 17/18 mutations exhibited longer mPFS (7.3 vs. 1.9 months, p = 0.001) and longer mOS (20.3 vs. 7.7 months, p = 0.059) than non-activation loop mutations [53].
Ripretinib, a fourth-line therapy, has shown broad efficacy against most mutant types of KIT/PDGFRA, with moderate inhibition of JM domain and ATP-binding site mutations [9]. In a phase III trial (NCT03353753) on 129 patients with advanced GIST who received prior treatment with at least imatinib, sunitinib, and regorafenib, ripretinib as a fourth-line therapy was generally well tolerated and improved mPFS over placebo (6.3 vs. 1 months) [54]. However, in a phase III clinical trial (NCT03673501), ripretinib demonstrated a mPFS of 8.0 months, which was comparable but not superior to sunitinib (8.3 months) as a second-line treatment for advanced GIST [55]. Final overall survival (OS) analysis from the same trial (NCT03673501) showed that similar efficacy of mOS existed in the ripretinib group (35.5 months) and the sunitinib groups (31.5 months) for all patients. Importantly, ripretinib demonstrated a better safety profile compared to sunitinib, making it a promising alternative as a second-line therapy drug for long-term management of advanced GIST [56].
Avapritinib is specifically used for patients with PDGFRA exon 18 D842V mutation [57]. In a clinical trial (NCT02508532), among patients with advanced GIST who had unresectable PDGFRA D842V or other mutant GIST who progressed on imatinib and ≥ 1 other TKI, for 4L + population (primarily KIT, median 4 prior TKI) patients (n = 111), avapritinib achieved an objective response rate (ORR) of 22%, with 52 patients achieving stable disease (SD) and a median duration of response (mDOR) of 10.2 months (95% CI: 7.2-∞); for patients with PDGFRA exon 18 mutation who had undergone one prior TKI treatment (n = 43), avapritinib showed an impressive ORR of 86%, with mDOR not yet reached (95% CI: 11.3-∞) [58]. Another clinical trial evaluated the long-term efficacy, tolerability and overall survival in patients with unresectable or metastatic PDGFRA D842V-mutant GIST treated with avapritinib (38 patients enrolled), results demonstrated that 300–400 mg/day achieved an ORR of 95%, with a complete response (CR) of 13% (5/38 patients) and partial response (PR) of 82% (31/38 patients), confirming the efficacy of avapritinib in targeting PDGFRA D842V-mutant GIST (regardless of prior therapy) [59].
Despite these approved TKIs to address the insensitivity of KIT/PDGFRA mutants, tumor heterogeneity with multiple mutants in patient samples presents as an additional challenge [41]. Combination of TKIs could be a possible strategy to tackle this problem. A phase IB clinical trial (NCT02164240) (n = 13) was conducted to evaluate the safety and effectiveness of rapid alternation of sunitinib and regorafenib on metastatic and/or unresectable GIST with prior failure to at least imatinib, sunitinib, and regorafenib (fourth-line and beyond), SD was observed in 4 patients, mPFS was 1.9 months (95% CI, 1.4–3.6 months), mOS was 10.8 months (95% CI, 5.9-∞). The study also found early decrease and prolonged suppression of circulating tumor DNA (ctDNA) (KIT primary and/or secondary mutations) until clone reemergence at disease progression [60].
Emerging Kinase Inhibitors Targeting RTKs for Advanced GISTs
At the molecular level, inhibition of relevant membrane receptor tyrosine kinases (RTKs) such as VEGFR, KIT, PDGFR, FGFR, MET, FLT3, and AXL offers the potential to reverse the resistance phenotype in advanced GIST by inhibiting activation and re-activation of MAPK/ERK and PI3K/AKT pathways. Several multi-targeted TKIs are undergoing clinical trials.
Anlotinib (an inhibitor of KIT, VEGFR, PDGFR, and FGFR) showed intermediate inhibitory efficacy against GIST cells with KITV654 mutation in vitro (IC50 > 500 nM). In a phase II clinical trial (64 patients enrolled) (NCT04106024) evaluating the activity of anlotinib (12 mg/kg/day) on GIST patients who failed the first-line imatinib treatment, 7/64 patients (10.9%) had partial response, 39/64 patients (60.9%) patients had stable disease, mPFS was 8.0 months (95% CI, 4.7–11.3 months) and mOS was not reached. They reported no statistical significance compared with the PFS of sunitinib at the same period for patients after imatinib failure [61].
Nilotinib inhibits the activation of ABL1/BCR-ABL1, KIT, PDGFRs, and the discoidin domain receptor. In a phase III clinical trial on patients with confirmed unresectable or metastatic GIST and had not received previous systemic therapy for GIST or had a recurrence of GIST 6 months or more after stopping imatinib treatment (647 patients enrolled), 2-year PFS of nilotinib group (324 patients, 400 mg/day) (51.6%) showed no superior efficacy than imatinib group (320 patients, 400 mg/day) (59.2%) as first-line therapy for advanced GISTs [62].
THE-630, a promising pan-mutant KIT inhibitor designated as an orphan drug for GISTs, showed potent activity against all classes of KIT mutations (exon 11 deletion, exon 9 insertion, exon13/14 mutation, exon18 mutation, secondary mutations including exon 9 + 13 and exon 9 + 18) in vitro (IC50 < 50 nM) and inhibitory efficacy in mouse models [63]. In a phase I/II clinical trial (NCT05160168) on advanced GIST (unresectable or metastatic) previously treated with imatinib and ≥ 1 additional TKI, THE-630 achieved stable disease at doses of 9 mg/day (3/3 patients) and 12 mg/day (3/3 patients) with reduced KIT-mutant allele fractions in ctDNA [64].
NB003 targets KIT/PDGFRA with primary and secondary mutations. In a phase I clinical trial (NCT04936178) in patients with advanced GIST who progressed on or intolerant to imatinib and other standard of care, NB003 showed a manageable safety profile, the ORR was 26.2% (11/42 patients) and KIT-mutant allele fractions in ctDNA (10/11 patients) were reduced [65].
Olverembatinib is a multitargeted TKI with promising preclinical activity against GISTs. In a phase I study (NCT03594422) on patients with TKI-resistant, metastatic, SDH-deficient GISTs, 5/20 patients experienced PR and clinical benefit rate (CR + PR + SD > 4 cycles) was 93.8% for patients receiving > 4 cycles of olverembatinib, implying antitumor activity of olverembatinib in patients with TKI-resistant SDH deficient GIST [66].
Emerging Inhibitors Targeting Cytosolic Kinases for Advanced GISTs
Approved TKIs for GISTs inhibit the “initiator” of oncogenic signaling cascades. However, from a practical viewpoint, inhibiting the initiator alone is often inadequate for the long-term suppression of GIST cells both in vitro and in vivo. The leaky activation of signaling pathways due to non-covalent binding of TKIs or re-activation of other RTKs is sufficient for the survival of cancer cells [67] (Fig. 2C). TKIs targeting the PI3K/AKT pathway, MAPK/ERK pathway, mTORC1 pathway, and cell cycle pathway have been reported in GISTs [68–72] (Fig. 3B). These inhibitors have demonstrated efficacy in preclinical experiments, either alone or in combination with imatinib. In a phase II clinical trial evaluating safety and efficacy of everolimus (a PI3K/mTOR inhibitor) in combination with imatinib on advanced GIST patients, for patients with imatinib failure alone (stratum1, 23 patients for analysis), 4/23 (17%) were progression free at 4 months; for patients with failure of imatinib and other TKI or cyclin-dependent kinase inhibitors (stratum2, 35 patients for analysis), 13/35 (37%) were progression free at 4 months. Both stratums met the predefined criteria for PFS [73]. In a phase IB trial (60 patients enrolled, NCT01468688) on patients who failed prior therapy with imatinib and sunitinib, no PR or CR was observed in the combination group (imatinib and buparlisib (PI3K inhibitor)), SD was observed in 19 patients (54.3%) [74].
Combining these inhibitors with approved TKIs provide a possible strategy to overcome the drug-resistance phenotype, as they further block the leaked and reactivated pro-survival signals from oncoproteins or RTKs under non-covalent TKI adjuvant therapy. When selecting targets, upstream kinases or components in the oncogenic signaling cascade should be prioritized. Downstream signaling pathways are intertwined and complex, and inhibition of downstream kinases can lead to the reactivation of upstream kinases. For example, ERK inhibition can result in feedback reactivation of the MAPK pathway via RAS/CRAF [75, 76].
Other Drugs in Clinical Trials for Advanced GISTs
Pimitespib inhibits HSP90 by competitively blocking HSP90 enzymatic activity and disrupts the formation of the correct conformation of nascent KIT protein. Pimitespib shows efficacy against imatinib-sensitive and -insensitive GIST cells with KIT mutations, both in vitro and in vivo [77]. In a phase III trial (CHAPTER-GIST-301) involving patients with progressive disease (PD) or intolerance after treatment with imatinib, sunitinib, and regorafenib, patients treated with pimitespib (160 mg/day) had a mPFS of 2.8 months (95% CI: 1.6–2.9) (n = 46) versus 1.4 months (95% CI: 0.9–1.8 months) in placebo group (n = 27) [78], showing efficacy in patients refractory to multiple TKIs. A phase I clinical study (NCT05245968) is underway to evaluate the safety and preliminary efficacy of different treatment regimens in patients with imatinib-refractory GIST in the second-line setting: PIMI (pimitespib 120 mg on 5 days on/2 days off) with imatinib (400 mg/day), PIMI monotherapy and standard therapy sunitinib (50 mg, 4 weeks on/2 weeks off) [79].
Selinexor inhibits exportin1-mediated nuclear export of RNA, and demonstrates efficacy on imatinib-sensitive and -insensitive GIST cells both in vitro and in vivo [80]. In a phase IB/II study (NCT04138381) involving imatinib-resistant GIST patients, the toxicity of selinexor was manageable, clinical benefit rate (CR + PR + SD) ≥ 16 weeks was 42% (95% CI: 0.181–0.685) in cohort A (n = 12, imatinib 400 mg/day, weekly selinexor) and 28% (95% CI: 0.213–0.354) in cohort B (n = 18, selinexor 60 mg twice weekly) [81].
Guadecitabine (DNA methyltransferase inhibitor) has been tested in clinical trial for SDH-deficient GIST patients to decrease hypermethylation, but it did not achieve the target response rate of 30% of patients [82]. Further research is needed to verify this therapeutic strategy.
Antibody-Based Drugs for Advanced GISTs
In GISTs, the majority of cases contain wild-type or mutated KIT/PDGFRA, which serves as an ideal target for antibody-based drugs. Therapeutic antibodies block the binding of ligands to their cognate receptors and induce antibody/complement-dependent cell cytotoxicity (ADCC/CDC) (Fig. 3C). Blocking the complex formation of SCF/KIT and PDGF/PDGFRA has limited efficacy, as approximately 85% of GIST patients have KIT or PDGFRA mutants, which is independent of ligands. Regarding ADCC/CDC, these cytotoxicity mechanisms rely on the function of leukocytes (NK cells, macrophages, and γδ T cells) and the complement system [83, 84]. However, tumor microenvironment in GIST also contains immunosuppressive cytokines/factors, and GIST cells express immunosuppressive ligands on their membranes, both of which inhibit the cytotoxic function of immune cells [85, 86]. A humanized anti-KIT antibody, CK6, demonstrated little inhibitory effect in GIST mouse models [87]. SR1, another anti-KIT antibody, inhibited GIST 882 cells (IC50: 70 nM) but had much less efficacy against imatinib-resistant GIST 48 and GIST 430 cells (IC50 > 5 µM). Although SR1 inhibited the proliferation of GIST cells in mouse models [88], the GIST tumors located in the peritoneal cavity and the antibody was injected intraperitoneally. This may explain the better in vivo efficacy of SR1 compared to CK6 [87]. Clinical trials (NCT02452424, NCT01316263) evaluating antibody targeting (CSF1R + PD-1, PDGFRAα) in patients with advanced GIST were terminated due to limited efficacy or poor accrual. Overall, therapeutic antibody therapy has limited efficacy in preclinical and clinical settings.
Antibody drug conjugate (ADC) is another drug candidate for primary and advanced GISTs. Antibodies confer specificity for highly toxic chemotherapeutic drugs, such as mertasine and camptothecin (Fig. 3D). ADCs targeting KIT (LOP628 and NN2101-DM1) have demonstrated strong inhibitory effects at sub-nanomolar concentrations against both imatinib-sensitive and -insensitive GIST cells (GIST T1 and GIST 430/654) both in vitro and in vivo [89, 90]. DS-6157a, an ADC targeting GPR20, exhibited GPR20-expression-dependent antitumor activity in GIST xenograft models, but its preclinical potency was less than that of ADCs targeting KIT [91]. In a clinical trial evaluating the efficacy of DS-6157a on advanced GIST (34 patients available for analysis, NCT04276415), the maximum tolerated dose (MTD) was 6.4 mg/kg, 18 patients (53%) experienced stable disease, mPFS across all dose levels was 3.6 months, no objective responses existed in patients with KIT-mutant GIST. Tumor shrinkage was observed in all 4 patients with wild-type KIT GIST treated at different doses. Although this trial was terminated, it provides potential of ADC for the treatment of GIST with wild-type KIT [92]. CAR T therapy is another powerful method to combat cancer. Currently, only SCF has been reported as a targeting domain for CAR T therapy in GISTs, showing efficacy in animal models [93].
Involvement of Metabolic Interventions for Therapy of GIST
Oncoproteins regulate key enzymes in metabolic pathways, providing energy and materials essential for cell proliferation through mechanisms involving AKT kinase and the transcription factor c-MYC [94–96]. In cancer cells, crucial metabolic processes include the uptake and utilization of amino acids and glucose, as well as the biosynthesis of carbohydrates, polysaccharides, proteins, lipids, and nucleic acids. Targeting critical enzymes in these metabolic pathways—such as those involved in glucose metabolism, glutamine metabolism, fatty acid synthesis, and nucleotide synthesis—holds promise [97]. Changes in the metabolism of GIST cells were assessed based on the presence or absence of TKIs treatment and their sensitivity to TKI therapy.
Metabolic Changes in GISTs Without Imatinib Treatment
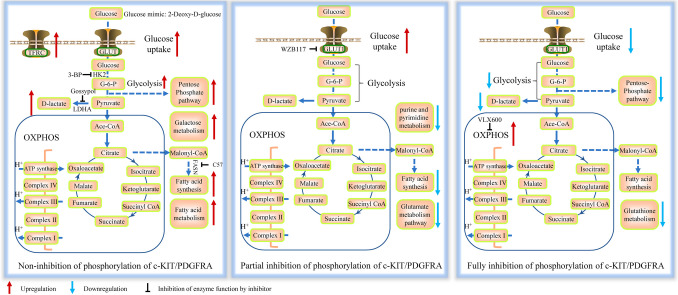
Glycolysis is a key feature of altered metabolism in cancer and presents as a potential therapeutic target. In a study of GIST patient samples, glucose uptake mediated by glucose transporter 1 (GLUT1) and glycolysis activity involving hexokinase-1 (HK1), lactate dehydrogenase A (LDHA), and pyruvate kinase isozyme M2 (PKM2) were found to be upregulated in high-risk tumors compared to low-risk tumors [98]. In GIST T1 (IC50 10.7 nM) and GIST 882 (IC50 17.8 nM) cells, treatment with imatinib for 2 years resulted in T1R (IC50 46 µM) and 882R (IC50 55 µM) cells without secondary mutations, which show elevated glycolysis, increased lactate production (ECAR), and a lower oxygen consumption rate (OCR) [67]. Another study reported that 882R (IC50 5 µM) cells exhibited higher glucose uptake, increased expression of LDHA and oxidative phosphorylation (OXPHOS) protein compared with GIST 882 cells. HK inhibitor (3-bromopyruvate) and LDHA inhibitor gossypol effectively inhibited glycolysis, reduced cell viability and induced apoptosis [99]. Glucose mimic 2-deoxyglucose (2DG) inhibits HK activity, thereby disrupting glycolysis. Although 2DG (IC50 0.5–2.5 µM) inhibited the proliferation of imatinib-resistant GIST cells in vitro, it had limited inhibitory effects in vivo, possibly due to insufficient drug concentration [100]. In addition to glycolysis, other glucose-related metabolic pathways have also been reported in GIST. A preliminary study using transcriptome and metabolome analysis, comparing GIST tissue with adjacent normal tissue revealed that galactose metabolism was involved in GIST malignant progression via aldose reductase [101]. The pentose phosphate pathway (PPP) generates NADPH and ribulose 5-phosphate from glucose, with ribulose 5-phosphate being the precursor for nucleotide synthesis. 6-Phosphogluconate dehydrogenase (PGD) catalyzes the conversion of 6-phosphogluconate to ribulose 5-phosphate, and was upregulated in imatinib-resistant GIST cells [67] (Fig. 4A).
Fig. 4.
Metabolism changes in GISTs. A. Metabolic changes in GISTs without imatinib treatment. B. Metabolic changes in GISTs under insufficient concentrations of imatinib. C. Metabolic changes in GISTs with sufficient concentrations of imatinib.
Lipids play essential roles in tumor cells as they are building blocks of cell membranes, an energy source, and participate in cell signaling [102]. Metabolite analysis of blood samples from GIST patients revealed significant changes in lipid and lipid-like metabolites, including ceramide, sphingosine, and sphingosine-1-phosphate (S1P), compared to untreated samples. Sphingosine kinase-1 (SPK1), a key enzyme in S1P synthesis, has been implicated in imatinib resistance in GIST cells according to KEGG analysis. DMS (SPK1 inhibitor) improved the efficacy of imatinib in GIST T1 cells [103]. In imatinib-resistant GIST 48R cells with secondary mutations in KIT (V560D + D820A), overexpression of fatty acid synthase (FASN) plays a critical role in promoting growth and migration, as well as sustaining resistance to imatinib. C75 (FASN inhibitor) led to changes in proliferation and apoptosis, but this inhibition also resulted in ERK hyperactivation [104]. Glutathione peroxidase 4 (GPX4) is an enzyme that converts lipid hydroperoxides into non-toxic lipids and alcohols. Higher levels of GPX4 and GSH were observed in imatinib-resistant GIST T1R cells (exon 11 deletion + D820Y) compared with imatinib-sensitive controls. The high level of GPX4 could contribute to the resistance of GIST T1R to RSL3 (GPX4 inhibitor) [105].
Transferrin receptor (TFRC) is a cell surface receptor essential for cellular iron uptake. KEGG analysis indicates that TFRC-related genes were closely associated with metabolic pathways such as pyrimidine metabolism, purine metabolism, OXPHOS, and glycolysis/gluconeogenesis. Data mining from the Gene Expression Omnibus (GEO) database revealed that TFRC expression was upregulated in high-risk GIST patients and was indicative of a poor prognosis for recurrence [106]. TFRC may be considered as a potential therapeutic target for GIST.
Metabolic Changes in GIST Under Insufficient Concentrations of Imatinib
In this category, with drug concentrations around the IC50 for GIST cells, hyperactivated KIT-mediated signaling pathways are only partially inhibited. Consequently, cell proliferation, metabolic pathways and disease progression are less affected due to insufficient drug concentrations. For instance, the blood concentration of imatinib is typically 1–2 µM [107], while the IC50 of imatinib for KIT mutants (double mutations in exon 11 and 17) was 1.2 µM [8]. As a result, 400 mg/day of imatinib has shown no effect on disease control for GIST patients with secondary mutations in clinical trials [108].
An untargeted metabolomic analysis of GIST xenograft samples treated with imatinib revealed that imatinib-resistant samples (KITdup502_503) exhibited the fewest number of statistically significant metabolites compared to imatinib-sensitive GIST samples (KITV560D and KITDEL558_565) [109], confirming that imatinib has a reduced effect on imatinib-resistant cells. Comparison of imatinib-resistant xenograft GIST samples (KITdup502_503) in the presence or absence of imatinib treatment showed that the most downregulated metabolite-related pathways were purine and pyrimidine metabolism and unsaturated fatty acid biosynthesis [102]. The glutamate metabolism pathway was also affected by imatinib treatment [102]. Glutaminase in the glutamate metabolism pathway converts glutamine to glutamate, and CB-839 (glutaminase inhibitor) had been tested on solid tumors, including GIST (NCT02071862). In imatinib-resistant GIST T1R (IC50: 5 µM) and 882R (IC50: 5 µM) cells, no increase of OXPHOS proteins was observed following imatinib treatment (1 µM for 48 h) [99]. In imatinib-resistant (IC50: 127 nM) GIST T1R cells (with secondary mutation in PDGFRA, c.1701A > G), GLUT1 in the glycolysis pathway was upregulated under imatinib (13 nM). WZB117 (GLUT1 inhibitor) inhibited the imatinib-resistant phenotype of GIST cells in combination with imatinib [110] (Fig. 4B). Under these conditions, co-targeting the metabolism pathway along with imatinib may have limited effectiveness. Therefore, a rational increase in drug dosage or switching to other approved TKIs could be beneficial.
Metabolic Changes in GISTs Under Sufficient Concentrations of Imatinib
When KIT/PDGFRA is completely inhibited at drug concentrations significantly above the IC50, most GIST cells undergo apoptosis. However, a small portion of GIST cells enter a cell-cycle-arrested state, exhibiting quiescent-like, senescence-like, and stem-cell-like characteristics that make them resistant to cell death, and capable of repopulation [44]. Targeting critical metabolism pathways in these surviving cells could provide a synergistic effect with TKI treatment and improve clinical outcomes.
Imatinib treatment can decrease glucose uptake by reducing membrane GLUT4 [95]. Glucose consumption and lactate production in imatinib-sensitive GIST T1 and 882 cells decreased under imatinib treatment (20 nM, 12 h), indicating a reduction in glycolysis activity [111]. In another study, imatinib (13 nM, 72 h) reduced the expression levels of glycolysis pathway-related molecules, such as GLUT1, HK2, PKM2, and LDH, in GIST T1 (imatinib IC50: 6.5 nM) cells [110]. Glycolysis, pentose phosphate pathway, and glutathione metabolism were also reported to be downregulated in imatinib-treated GIST T1 cells. Additionally, imatinib combined with RSL3 exhibited promising efficacy in imatinib-sensitive mutants [105]. Of note, imatinib (1 µM for 48 h) increased the expression levels of OXPHOS proteins (CII-SDHB, CIII-UQCRC2, CV-ATP5A) in imatinib-sensitive GIST T1 and 882 cells [99] (Fig. 4C). VLX600 (OXPHOS inhibitor) significantly enhanced the efficacy of imatinib in eliminating GIST cells [111]. Therefore, OXPHOS could be a promising therapeutic target for imatinib-sensitive GIST patients in combination with adjuvant therapy.
Perspective
Developing novel TKIs capable of addressing steric hindrance and the catalytic velocity of oncoproteins offers one solution to overcome drug resistance, such as THE-630 drug [8]. Since GIST cells rely heavily on ATP by mutated kinase, strategies that reduce ATP concentrations could enhance TKI efficacy. Notably, diabetes has been identified as a poor prognostic factor in GIST patients, likely due to the elevated blood glucose levels that fuel cancer cells [112, 113]. Targeting glucose metabolism pathways has shown potential to deprive cancer cells of the energy necessary for proliferation. Early in vitro studies have demonstrated the promise of this approach, and dietary interventions to restrict blood glucose concentration may further reduce ATP levels, increasing sensitivity to TKIs [114]. Tumor heterogeneity presents another hurdle for TKI therapy. After molecular genetic testing on tumor biopsy, careful selection and combination of approved TKIs with appropriate dosing schemes could help effectively target multiple secondary mutations in KIT/PDGFRA [50]. Furthermore, combination of adjuvant therapy with TKIs that target downstream signaling pathways, such as PI3K/AKT, MAPK/ERK, or cell cycle regulators, may enhance the therapeutic effect and offer a more comprehensive blockade of hyperactivated pathways. Although the potential toxicity of combination therapies is a concern, lower concentration of drugs in combination could provide better inhibition and lower toxicity than a single drug. Additionally, the reversibility of binding exhibited by most currently approved TKIs contributes to the persistence of GIST cells. The reversible nature of currently approved TKIs allows for the leaky activation of oncoproteins, contributing to GIST cell survival and resistance. The development of the type II covalent TKIs, which form irreversible bonds with their targets, could greatly enhance the inhibition efficacy and reduce drug resistance. Another innovative strategy involves antibody–drug conjugates (ADCs), which could be a promising method for targeting both wild-type and mutated KIT/PDGFRA, as mutations in these proteins rarely occur in the ligand-binding sites. However, clinical trials need to conduct to find which subtype(s) of GIST patients may benefit most from ADC therapy. CAR T-cell therapy also demonstrated potential in the treatment of GIST, although the tumor microenvironment, particularly immunosuppressive ligands or inhibitory factors expressed by the tumor, remains a challenge. Addressing these factors is crucial to introducing CAR T therapy in GIST. For wild-type GISTs, ADC (DS-6157a) and kinase inhibitor (Olverembatinib) provide potential to treat these TKI-resistant GISTs.
Targeting metabolic pathways has shown effects in vitro but has had little impact in mouse models, likely due to insufficient inhibition of the oncoproteins. Metabolic inhibition should be combined with TKIs to enhance therapy for GIST. By decreasing energy production, reducing carbon intermediates necessary for biosynthesis, and lowering ATP levels, metabolic inhibition may sensitize GIST cells to TKIs, particularly in patients with TKI-sensitive mutations. As the landscape of GIST treatment continues to evolve, an integrated, multifaceted approach involving novel TKIs, combination therapy and metabolic inhibition, may overcome drug resistance, yield significant therapeutic outcomes for patients with GIST.
Key References
- Huang, W., et al., A novel fusion between CDC42BPB and ALK in a patient with quadruple wild-type gastrointestinal stromal tumor. Mol Genet Genomic Med, 2022. 10(5): p. e1881.
- This reference is of importance because clearly demonstrates that ALK mutation is one of the oncogenes that contribute to the drug resistance of wild-type GIST in the clinical setting. Clearly demonstrates that the non-KIT/PDGFRA mutants are one of the drug resistance mechenisms.
- Chan, A., et al., Final Efficacy Results of Neratinib in HER2-positive Hormone Receptor-positive Early-stage Breast Cancer From the Phase III ExteNET Trial. Clinical Breast Cancer, 2021. 21(1): p. 80-91.e7.
- This reference is of importance because clearly presents an example of covalent-binding TKI to better inhibit the oncogenic pathways in RTK-mutated cancers than the non-covalent binding TKIs in clinical setting.
- Wolska-Washer, A. and T. Robak, Acalabrutinib: a bruton tyrosine kinase inhibitor for the treatment of chronic lymphocytic leukemia. Expert Review of Hematology, 2022. 15(3): p. 183-194.
- This reference is of importance because clearly presents an example of covalent-binding TKI to better inhibit the oncogenic pathways in cytosolic-kinase-mutated cancers than the non-covalent binding TKI in clinical setting.
- Sobczuk, P., et al., Impact of mutational status on long-term treatment outcomes in patients with advanced gastrointestinal stromal tumors (GIST) treated in the first line with imatinib. Annals of Oncology, 2022. 33: p. S1238.
- This referecne is of outstanding importance because this clinical trial comfirms that exon 11 mutation and no PDGFRA exon 18 D842V are correlated with longer median progression free survival when patients are treated by imatinib.
- Blay, J.Y., et al., A randomized study of 6 versus 3 years of adjuvant imatinib in patients with localized GIST at high risk of relapse. Annals of Oncology, 2024; 10.1016/j.annonc.2024.08.2343.
- This reference is of outstanding importance because it clearly suggests that 6 years of imatinib treatment is better than 3 years of imatinib treatment in reducing recurrence of GIST. Previous clinical trial suggests that 3 years of imatinib treatment is better than 1 year of imatinib treatment. This reference supports the longer time of imatinib treatment is effective and safe.
- Chen, S., et al., Regorafenib third-lined therapy in advanced GISTs: A single center analysis based on different genotypes. Journal of Clinical Oncology, 2022. 40(16_suppl): p. 11537-11537.
- This reference is of outstanding importance because it clearly reviews the clinical efficacy of regorafenib, and supports that exon 17/18 secondary mutation correlates with longer mPFS than non-activation loop mutants. The result from this reference also correlates with in vitro data.
- Bauer, S., et al., Ripretinib Versus Sunitinib in Patients With Advanced Gastrointestinal Stromal Tumor After Treatment With Imatinib (INTRIGUE): A Randomized, Open-Label, Phase III Trial. Journal of Clinical Oncology, 2022. 40(34): p. 3918-3928.
- This reference is of outstanding importance because it reports that ripretinib has the similar effcicay with sunitinib as a second line therapy drug for GIST patients after imatinib failure. Ripretinib has better safety profile than sunitinib.
- Zalcberg, J.R., et al., Overall survival and long-term safety with ripretinib vs sunitinib in patients with advanced gastrointestinal stromal tumor previously treated with imatinib: Final analyses from INTRIGUE. Journal of Clinical Oncology, 2024. 42(3_suppl): p. 748-748.
- This reference is of outstanding importance because it reports that ripretinib has the similar effcicay with sunitinib as a second line therapy drug for GIST patients after imatinib failure. Ripretinib has better safety profile than sunitinib.
- Zhou, Y., et al., Activity of Anlotinib in the Second-Line Therapy of Metastatic Gastrointestinal Stromal Tumors: A Prospective, Multicenter, In Vitro Study. The Oncologist, 2023. 28(4): p. e191-e197.
- This reference is of outstanding importance because it reports that anlotinib is a promising drug for GIST patients after imatinib failure. This reference also implies that anlotinib may have similar efficacy to sunitinib. Providing evidence that the development of new TKIs to overcome primary and secondary mutants is possible.
- George, S., et al., Initial results from the phase (ph) 1 portion of a ph 1/2 study of THE-630 in patients (pts) with advanced gastrointestinal stromal tumor (GIST). Journal of Clinical Oncology, 2023. 41(16_suppl): p. e23508-e23508.
- This reference is of outstanding importance because it reports that THE-630 is a promising drug for GIST patients after imatinib failure and ≥1 TKI treatment. Providing evidence that the development of new TKI to overcome primary and secondary mutants is possible.
- Li, J., et al., Phase 1 study of NB003, a broad-spectrum KIT/PDGFRα inhibitor, in patients with advanced gastrointestinal stromal tumors (GIST). Journal of Clinical Oncology, 2024. 42(16_suppl): p. 11518-11518.
- This reference is of importance because it reports that NB003 is a promising drug for GIST patients after imatinib failure. Providing evidence that the development of new TKIs to overcome primary and secondary mutants is possible.
- Qiu, H., et al., Promising antitumor activity of olverembatinib (HQP1351) in patients (pts) with tyrosine kinase inhibitor- (TKI-) resistant succinate dehydrogenase- (SDH-) deficient gastrointestinal stromal tumor (GIST). Journal of Clinical Oncology, 2022. 40(16_suppl): p. 11513-11513.
- This reference is of importance because it reports that olverembatinib is a promising drug for SDH-deficient GIST patients. Providing evidence that the development of new TKI to treat this subtype of wild-type GIST patients is possible.
- Kurokawa, Y., et al., Pimitespib in patients with advanced gastrointestinal stromal tumor (CHAPTER-GIST-301): a randomized, double-blind, placebo-controlled phase III trial. Annals of Oncology, 2022. 33(9): p. 959-967.
- This reference is of importance because it reports that pimitespib is a promising drug for advanced GIST patients. The target of pimitespib is not the traditional KIT/PDGFRA mutants, it targets HSP90. Providing evidence that development of new drug targeting non-authentic targets could help overcome drug resistance in advanced GIST patients.
- Serrano, C., et al., 1489MO A phase Ib/II study of selinexor as single agent and in combination with imatinib in patients with advanced gastrointestinal stromal tumor (GIST): SeliGIST/GEIS-41 trial. Annals of Oncology, 2022. 33: p. S1228.
- This reference is of importance because it reports that selinexor is a promising drug for advanced GIST patients. Unlike traditional KIT/PDGFRA mutant targets, selinexor targets the XPO1-mediated nuclear export of RNA. Providing evidence that development of new drug targeting non-authentic targets is possible to overcome drug resistance in advanced GIST patients.
- Ligon, J.A., et al., A Phase II Trial of Guadecitabine in Children and Adults with SDH-Deficient GIST, Pheochromocytoma, Paraganglioma, and HLRCC-Associated Renal Cell Carcinoma. Clinical Cancer Research, 2023. 29(2): p. 341-348.
- This reference is of importance because it reports that guadecitabine does not achive target response rate in SDH-deficient GIST patients. However, it provides preliminary result of targeting the hypermethylation process in SDH-deficient GIST.
- George, S., et al., A Phase I, Multicenter, Open-Label, First-in-Human Study of DS-6157a in Patients with Advanced Gastrointestinal Stromal Tumor. Clin Cancer Res, 2023. 29(18): p. 3659-3667.
- This reference is of importance because it reports that antibody drug conjugate (DS-6157a) is a possible drug for wild-type GIST patients which are resistance to TKI therapy. Providing evidence that antibody-drug conjugate is a possible drug format to overcome drug resistance for advanced GIST patients.
- Liao, Y., et al., The association between fasting blood glucose and prognosis in gastrointestinal stromal tumor patients after curable resection. Updates in Surgery, 2023. 75(5): p. 1219-1226.
- This reference is of importance because it reports that lower blood glucose concentration is beneficial for improving the therapy outcome. Signifying that linking the TKI therapy with inhibiting metabolism is possible to improve drug efficacy, overcome drug resistance.
Acknowledgements
This work was supported by Shenzhen Science and Technology Innovation Commission (Grant No. JCYJ20220530145014033).
Authors Contribution
Chunxiao He: Writing – original draft; Writing – review & editing. Zilong Wang: Writing – review & editing. Jiaying Yu: Writing – review & editing. Shuang Mao: Writing – review & editing. Xi Xiang: Writing – review & editing.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights
This article does not contain studies with human or animal subjects performed by any of the authors.
Competing Interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chunxiao He and Zilong Wang contributed equally to this work.
Contributor Information
Chunxiao He, Email: hechx26@mail.sysu.edu.cn.
Xi Xiang, Email: xiangx25@mail.sysu.edu.cn.
References and Recommended Reading
- 1.Fletcher CDM, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33(5):459–65. [DOI] [PubMed] [Google Scholar]
- 2.DeMatteo RP, et al. Two hundred gastrointestinal stromal tumors: Recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heinrich MC, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299(5607):708–10. [DOI] [PubMed] [Google Scholar]
- 4.Hirota S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577–80. [DOI] [PubMed] [Google Scholar]
- 5.Zhu H, et al. Update of epidemiology, survival and initial treatment in patients with gastrointestinal stromal tumour in the USA: A retrospective study based on SEER database. BMJ Open. 2023;13(7):e072945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasota J, Miettinen M. Clinical significance of oncogenic KIT and PDGFRA mutations in gastrointestinal stromal tumours. Histopathology. 2008;53(3):245–66. [DOI] [PubMed] [Google Scholar]
- 7.Wardelmann E, et al. c-kit mutations in gastrointestinal stromal tumors occur preferentially in the spindle rather than in the epithelioid cell variant. Mod Pathol. 2002;15(2):125–36. [DOI] [PubMed] [Google Scholar]
- 8.Garner AP, et al. Ponatinib inhibits polyclonal drug-resistant KIT oncoproteins and shows therapeutic potential in heavily pretreated gastrointestinal stromal tumor (GIST) patients. Clin Cancer Res. 2014;20(22):5745–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith BD, et al. Ripretinib (DCC-2618) is a switch control kinase inhibitor of a broad spectrum of oncogenic and drug-resistant KIT and PDGFRA variants. Cancer Cell. 2019;35(5):738-751.e9. [DOI] [PubMed] [Google Scholar]
- 10.Oppelt PJ, Hirbe AC, Van Tine BA. Gastrointestinal stromal tumors (GISTs): Point mutations matter in management, a review. J Gastrointest Oncol. 2017;8(3):466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinrich MC, et al. Correlation of kinase genotype and clinical outcome in the North American intergroup Phase III trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by cancer and leukemia group b and southwest oncology group. J Clin Oncol : Official J Am Soc Clin Oncol. 2008;26(33):5360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corless CL, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol : Official J Am Soc Clin Oncol. 2014;32(15):1563–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinrich MC, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003;21(23):4342–9. [DOI] [PubMed] [Google Scholar]
- 14.Yun C-H, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci. 2008;105(6):2070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gajiwala KS, et al. KIT kinase mutants show unique mechanisms of drug resistance to imatinib and sunitinib in gastrointestinal stromal tumor patients. Proc Natl Acad Sci. 2009;106(5):1542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang L, et al. Structural and biochemical studies of the PDGFRA kinase domain. Biochem Biophys Res Commun. 2016;477(4):667–72. [DOI] [PubMed] [Google Scholar]
- 17.Corless CL, et al. PDGFRA mutations in gastrointestinal stromal tumors: Frequency, spectrum and in vitro sensitivity to imatinib. J Clin Oncol. 2005;23(23):5357–64. [DOI] [PubMed] [Google Scholar]
- 18.Yoo C, et al. Efficacy of imatinib in patients with platelet-derived growth factor receptor alpha-mutated gastrointestinal stromal tumors. Cancer Res Treat. 2016;48(2):546–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grunewald S, et al. Resistance to avapritinib in PDGFRA-driven GIST is caused by secondary mutations in the PDGFRA kinase domain. Cancer Discov. 2021;11(1):108–25. [DOI] [PubMed] [Google Scholar]
- 20.Klug LR, et al. New treatment strategies for advanced-stage gastrointestinal stromal tumours. Nat Rev Clin Oncol. 2022;19(5):328–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li B, et al. Advances in immunology and immunotherapy for mesenchymal gastrointestinal cancers. Mol Cancer. 2023;22(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janeway KA, et al. Defects in succinate dehydrogenase in gastrointestinal stromal tumors lacking KIT and PDGFRA mutations. Proc Natl Acad Sci U S A. 2011;108(1):314–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eijkelenkamp K, et al. Clinical implications of the oncometabolite succinate in mutation carriers. Clin Genet. 2020;97(1):39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Killian JK, et al. Succinate dehydrogenase mutation underlies global epigenomic divergence in gastrointestinal stromal tumor. Cancer Discov. 2013;3(6):648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flavahan WA, et al. Altered chromosomal topology drives oncogenic programs in SDH-deficient GISTs. Nature. 2019;575(7781):229-+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi E, et al. FGFR1 and NTRK3 actionable alterations in “Wild-Type” gastrointestinal stromal tumors. J Transl Med. 2016;14(1):339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang W, et al. A novel fusion between CDC42BPB and ALK in a patient with quadruple wild-type gastrointestinal stromal tumor. Mol Genet Genomic Med. 2022;10(5):e1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haefliger S, et al. Molecular profile of gastrointestinal stromal tumors in sixty-eight patients from a single Swiss institution. Pathobiology. 2020;87(3):171–8. [DOI] [PubMed] [Google Scholar]
- 29.Mol CD, et al. Structure of a c-Kit product complex reveals the basis for kinase transactivation *. J Biol Chem. 2003;278(34):31461–4. [DOI] [PubMed] [Google Scholar]
- 30.Mol CD, et al. Structural basis for the autoinhibition and STI-571 inhibition of c-Kit tyrosine kinase*. J Biol Chem. 2004;279(30):31655–63. [DOI] [PubMed] [Google Scholar]
- 31.Treiber DK, Shah NP. Ins and outs of kinase DFG motifs. Chem Biol. 2013;20(6):745–6. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Z, Bourne PE. Overview of current type I/II kinase inhibitors. In: Shapiro P, editor. Next generation kinase inhibitors: moving beyond the ATP binding/catalytic sites. Cham: Springer International Publishing; 2020. p. 13–28. [Google Scholar]
- 33.Liu Y, Gray NS. Rational design of inhibitors that bind to inactive kinase conformations. Nat Chem Biol. 2006;2(7):358–64. [DOI] [PubMed] [Google Scholar]
- 34.Zheng L, et al. Development of covalent inhibitors: Principle, design, and application in cancer. MedComm - Oncol. 2023;2(4):e56. [Google Scholar]
- 35.Beyett TS, et al. Molecular basis for cooperative binding and synergy of ATP-site and allosteric EGFR inhibitors. Nat Commun. 2022;13(1):2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Honigberg LA, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci. 2010;107(29):13075–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan A, et al. Final efficacy results of neratinib in HER2-positive hormone receptor-positive early-stage breast cancer from the Phase III ExteNET trial. Clin Breast Cancer. 2021;21(1):80-91.e7. [DOI] [PubMed] [Google Scholar]
- 38.Li D, et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene. 2008;27(34):4702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Butterworth S, et al. The structure-guided discovery of osimertinib: the first US FDA approved mutant selective inhibitor of EGFR T790M. Medchemcomm. 2017;8(5):820–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolska-Washer A, Robak T. Acalabrutinib: A bruton tyrosine kinase inhibitor for the treatment of chronic lymphocytic leukemia. Expert Rev Hematol. 2022;15(3):183–94. [DOI] [PubMed] [Google Scholar]
- 41.Liegl B, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol: J Pathol Soc Great Britain and Ireland. 2008;216(1):64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li F, et al. FGFR-mediated reactivation of MAPK signaling attenuates antitumor effects of imatinib in gastrointestinal stromal tumors. Cancer Discov. 2015;5(4):438–51. [DOI] [PubMed] [Google Scholar]
- 43.Cohen NA, et al. Pharmacological inhibition of KIT activates MET signaling in gastrointestinal stromal tumors. Can Res. 2015;75(10):2061–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boichuk S, et al. The DREAM complex mediates GIST cell quiescence and is a novel therapeutic target to enhance imatinib-induced apoptosis. Can Res. 2013;73(16):5120–9. [DOI] [PubMed] [Google Scholar]
- 45.Demetri GD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347(7):472–80. [DOI] [PubMed] [Google Scholar]
- 46.Sobczuk P, et al. Impact of mutational status on long-term treatment outcomes in patients with advanced gastrointestinal stromal tumors (GIST) treated in the first line with imatinib. Ann Oncol. 2022;33:S1238. [Google Scholar]
- 47.Cho H, et al. Prognostic factors for residual lesion surgery following disease control with standard dose imatinib (IM) treatment in patients (pts) with advanced gastrointestinal stromal tumor (GIST). Annals Oncol. 2018;29:viii582. [Google Scholar]
- 48.Joensuu H, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: A randomized trial. JAMA. 2012;307(12):1265–72. [DOI] [PubMed] [Google Scholar]
- 49.Blay JY, et al. A randomized study of 6 versus 3 years of adjuvant imatinib in patients with localized GIST at high risk of relapse. Annals of Oncology. 2024. 10.1016/j.annonc.2024.08.2343. [DOI] [PubMed] [Google Scholar]
- 50.Serrano C, et al. Complementary activity of tyrosine kinase inhibitors against secondary kit mutations in imatinib-resistant gastrointestinal stromal tumours. Br J Cancer. 2019;120(6):612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demetri GD, et al. Complete longitudinal analyses of the randomized, placebo-controlled, Phase III trial of sunitinib in patients with gastrointestinal stromal tumor following imatinib failure. Clin Cancer Res. 2012;18(11):3170–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demetri GD, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen S, et al. Regorafenib third-lined therapy in advanced GISTs: A single center analysis based on different genotypes. Journal of Clinical Oncology. 2022;40(16_suppl):11537–11537. [Google Scholar]
- 54.Blay J-Y, et al. Ripretinib in patients with advanced gastrointestinal stromal tumours (INVICTUS): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(7):923–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bauer S, et al. Ripretinib versus sunitinib in patients with advanced gastrointestinal stromal tumor after treatment with imatinib (INTRIGUE): A randomized, open-label, Phase III trial. J Clin Oncol. 2022;40(34):3918–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zalcberg JR, et al. Overall survival and long-term safety with ripretinib vs sunitinib in patients with advanced gastrointestinal stromal tumor previously treated with imatinib: Final analyses from INTRIGUE. J Clin Oncol. 2024;42(3_suppl):748–748. [Google Scholar]
- 57.Evans EK, et al. A precision therapy against cancers driven by KIT/PDGFRA mutations. Sci Transl Med. 2017;9(414):eaao1690. [DOI] [PubMed] [Google Scholar]
- 58.Heinrich MC, et al. Clinical activity of avapritinib in ≥ fourth-line (4L+) and PDGFRA Exon 18 gastrointestinal stromal tumors (GIST). J Clin Oncol. 2020;38(4_suppl):826–826. [Google Scholar]
- 59.Kang YK, et al. Long-term efficacy, tolerability and overall survival in patients (pts) with unresectable or metastatic (U/M) PDGFRA D842V-mutant gastrointestinal stromal tumour (GIST) treated with avapritinib: NAVIGATOR phase I trial update. Ann Oncol. 2020;31:S1287–8. [Google Scholar]
- 60.Serrano C, et al. Phase I study of rapid alternation of sunitinib and regorafenib for the treatment of tyrosine kinase inhibitor refractory gastrointestinal stromal tumors. Clin Cancer Res. 2019;25(24):7287–93. [DOI] [PubMed] [Google Scholar]
- 61.Zhou Y, et al. Activity of anlotinib in the second-line therapy of metastatic gastrointestinal stromal tumors: A prospective, multicenter. In Vitro Study Oncol. 2023;28(4):e191–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blay J-Y, et al. Nilotinib versus imatinib as first-line therapy for patients with unresectable or metastatic gastrointestinal stromal tumours (ENESTg1): A randomised phase 3 trial. Lancet Oncol. 2015;16(5):550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rivera VM, et al. Abstract 1292: Preclinical characterization of THE-630, a next-generation inhibitor for KIT-mutant gastrointestinal stromal tumors (GIST). Cancer Res. 2021;81(13_Supplement):1292–1292. [Google Scholar]
- 64.George S, et al. Initial results from the phase (ph) 1 portion of a ph 1/2 study of THE-630 in patients (pts) with advanced gastrointestinal stromal tumor (GIST). J Clin Oncol. 2023;41(16_suppl):e23508–e23508. [Google Scholar]
- 65.Li J, et al. Phase 1 study of NB003, a broad-spectrum KIT/PDGFRα inhibitor, in patients with advanced gastrointestinal stromal tumors (GIST). J Clin Oncol. 2024;42(16_suppl):11518–11518. [Google Scholar]
- 66.Qiu H, et al. Promising antitumor activity of olverembatinib (HQP1351) in patients (pts) with tyrosine kinase inhibitor- (TKI-) resistant succinate dehydrogenase- (SDH-) deficient gastrointestinal stromal tumor (GIST). Journal of Clinical Oncology. 2022;40(16_suppl):11513–11513. [Google Scholar]
- 67.Xu K, et al. HIF-1α regulates cellular metabolism, and Imatinib resistance by targeting phosphogluconate dehydrogenase in gastrointestinal stromal tumors. Cell Death Dis. 2020;11(7):586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zook P, et al. Combination of imatinib mesylate and AKT inhibitor provides synergistic effects in preclinical study of gastrointestinal stromal tumor. Clin Cancer Res. 2017;23(1):171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kozinova M, et al. Combined inhibition of AKT and KIT restores expression of programmed cell death 4 (PDCD4) in gastrointestinal stromal tumor. Cancers. 2021;13(15):3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ran L, et al. Combined inhibition of MAP kinase and KIT signaling synergistically destabilizes ETV1 and suppresses GIST tumor growth. Cancer Discov. 2015;5(3):304–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schaefer IM, et al. Concurrent inhibition of CDK2 adds to the anti-tumour activity of CDK4/6 inhibition in GIST. Br J Cancer. 2022;127(11):2072–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oosterom, A.v, et al. A phase I/II trial of the oral mTOR-inhibitor everolimus (E) and imatinib mesylate (IM) in patients (pts) with gastrointestinal stromal tumor (GIST) refractory to IM: Study update. J Clin Oncol. 2005;23(16_suppl):9033–9033. [Google Scholar]
- 73.Schöffski P, et al. A phase I-II study of everolimus (RAD001) in combination with imatinib in patients with imatinib-resistant gastrointestinal stromal tumors. Ann Oncol. 2010;21(10):1990–8. [DOI] [PubMed] [Google Scholar]
- 74.Gelderblom H, et al. Imatinib in combination with phosphoinositol kinase inhibitor buparlisib in patients with gastrointestinal stromal tumour who failed prior therapy with imatinib and sunitinib: A Phase 1b, multicentre study. Br J Cancer. 2020;122(8):1158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Merchant M, et al. Combined MEK and ERK inhibition overcomes therapy-mediated pathway reactivation in RAS mutant tumors. PLoS ONE. 2017;12(10):e0185862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Corcoran RB, et al. EGFR-mediated reactivation of MAPK signaling contributes to insensitivity of BRAF-mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2012;2(3):227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saito Y, et al. TAS-116 inhibits oncogenic KIT signalling on the Golgi in both imatinib-naïve and imatinib-resistant gastrointestinal stromal tumours. Br J Cancer. 2020;122(5):658–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kurokawa Y, et al. Pimitespib in patients with advanced gastrointestinal stromal tumor (CHAPTER-GIST-301): a randomized, double-blind, placebo-controlled phase III trial. Ann Oncol. 2022;33(9):959–67. [DOI] [PubMed] [Google Scholar]
- 79.Hirano H, et al. CHAPTER-GIST-101: A phase I study of pimitespib combined with imatinib in patients with imatinib-refractory gastrointestinal stromal tumor. J ClinOncol. 2024;42(23_suppl):TPS97–TPS97. [Google Scholar]
- 80.Nakayama R, et al. Preclinical activity of selinexor, an inhibitor of XPO1, in sarcoma. Oncotarget. 2016;7(13):16581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Serrano C, et al. 1489MO a phase Ib/II study of selinexor as single agent and in combination with imatinib in patients with advanced gastrointestinal stromal tumor (GIST): SeliGIST/GEIS-41 trial. Ann Oncol. 2022;33:S1228. [Google Scholar]
- 82.Ligon JA, et al. A phase II trial of guadecitabine in children and adults with SDH-deficient GIST, pheochromocytoma, paraganglioma, and HLRCC-associated renal cell carcinoma. Clin Cancer Res. 2023;29(2):341–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ochoa MC, et al. Antibody-dependent cell cytotoxicity: Immunotherapy strategies enhancing effector NK cells. Immunol Cell Biol. 2017;95(4):347–55. [DOI] [PubMed] [Google Scholar]
- 84.Capietto A-H, Martinet L, Fournié J-J. Stimulated γδ T cells increase the in vivo efficacy of trastuzumab in HER-2+ breast cancer. J Immunol. 2011;187(2):1031–8. [DOI] [PubMed] [Google Scholar]
- 85.Yoon H, et al. TGF-β1-mediated transition of resident fibroblasts to cancer-associated fibroblasts promotes cancer metastasis in gastrointestinal stromal tumor. Oncogenesis. 2021;10(2):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seifert AM, et al. PD-1/PD-L1 blockade enhances T-cell activity and antitumor efficacy of imatinib in gastrointestinal stromal tumors. Clin Cancer Res. 2017;23(2):454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Looy T, et al. Therapeutic efficacy assessment of CK6, a monoclonal KIT antibody, in a panel of gastrointestinal stromal tumor xenograft models. Transl Oncol. 2015;8(2):112–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Edris B, et al. Anti-KIT monoclonal antibody inhibits imatinib-resistant gastrointestinal stromal tumor growth. Proc Natl Acad Sci. 2013;110(9):3501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim JO, et al. A novel anti-c-Kit antibody–drug conjugate to treat wild-type and activating-mutant c-Kit-positive tumors. Mol Oncol. 2022;16(6):1290–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Abrams T, et al. Preclinical antitumor activity of a novel anti–c-KIT antibody–drug conjugate against mutant and wild-type c-KIT–positive solid tumors. Clin Cancer Res. 2018;24(17):4297–308. [DOI] [PubMed] [Google Scholar]
- 91.Iida K, et al. Identification and therapeutic targeting of GPR20, selectively expressed in gastrointestinal stromal tumors, with DS-6157a, a first-in-class antibody–drug conjugate. Cancer Discov. 2021;11(6):1508–23. [DOI] [PubMed] [Google Scholar]
- 92.George S, et al. A phase I, multicenter, open-label, first-in-human study of DS-6157a in patients with advanced gastrointestinal stromal tumor. Clin Cancer Res. 2023;29(18):3659–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Katz SC, et al. Anti-KIT designer T cells for the treatment of gastrointestinal stromal tumor. J Transl Med. 2013;11:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pavlova NN, Zhu J, Thompson CB. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022;34(3):355–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tarn C, et al. Therapeutic effect of imatinib in gastrointestinal stromal tumors: AKT signaling dependent and independent mechanisms. Can Res. 2006;66(10):5477–86. [DOI] [PubMed] [Google Scholar]
- 96.Lourenco C, et al. MYC protein interactors in gene transcription and cancer. Nat Rev Cancer. 2021;21(9):579–91. [DOI] [PubMed] [Google Scholar]
- 97.Stine ZE, et al. Targeting cancer metabolism in the era of precision oncology. Nat Rev Drug Discovery. 2022;21(2):141–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cho MH, et al. Clinicopathologic features and molecular characteristics of glucose metabolism contributing to 18F-fluorodeoxyglucose uptake in gastrointestinal stromal tumors. Plos One. 2015;10(10):e0141413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang WK, et al. Heterogeneity of metabolic vulnerability in imatinib-resistant gastrointestinal stromal tumor. Cells. 2020;9(6):1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mühlenberg T, et al. Inhibition of KIT-glycosylation by 2-deoxyglucose abrogates KIT-signaling and combination with ABT-263 synergistically induces apoptosis in gastrointestinal stromal tumor. Plos One. 2015;10(3):e0120531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yin X, Zhang B. TLE3-AKR1B1 signaling axis participating in the malignant progression of gastrointestinal stromal tumor by regulating galactose metabolism. Ann Oncol. 2023;34:S127–S127. [Google Scholar]
- 102.Macioszek S, et al. Metabolomic and transcriptomic response to imatinib treatment of gastrointestinal stromal tumour in xenograft-bearing mice. Transl Oncol. 2023;30:101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen Y, et al. SPK1/S1P axis confers gastrointestinal stromal tumors (GISTs) resistance of imatinib. Gastric Cancer. 2023;26(1):26–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li C-F, et al. Overexpressed fatty acid synthase in gastrointestinal stromal tumors: Targeting a progression-associated metabolic driver enhances the antitumor effect of imatinib. Clin Cancer Res. 2017;23(16):4908–18. [DOI] [PubMed] [Google Scholar]
- 105.Ishida T, et al. Targeted therapy for drug-tolerant persister cells after imatinib treatment for gastrointestinal stromal tumours. Br J Cancer. 2021;125(11):1511–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhuang C, et al. Overexpressed transferrin receptor implied poor prognosis and relapse in gastrointestinal stromal tumors. Front Oncol. 2023;13:1151687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Q, et al. Association of imatinib plasma concentration and single-nucleotide polymorphisms with adverse drug reactions in patients with gastrointestinal stromal tumors. Mol Cancer Ther. 2018;17(12):2780–7. [DOI] [PubMed] [Google Scholar]
- 108.Serrano C, et al. 2023 GEIS guidelines for gastrointestinal stromal tumors. Ther Adv Med Oncol. 2023;15:17588359231192388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Macioszek S, et al. A multiplatform metabolomics approach for comprehensive analysis of GIST xenografts with various mutations. Analyst. 2023;148(16):3883–91. [DOI] [PubMed] [Google Scholar]
- 110.Shima T, et al. Glucose transporter-1 inhibition overcomes imatinib resistance in gastrointestinal stromal tumor cells. Oncol Rep. 2022;47(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Vitiello GA, et al. Mitochondrial inhibition augments the efficacy of imatinib by resetting the metabolic phenotype of gastrointestinal stromal tumor. Clin Cancer Res. 2018;24(4):972–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liao Y, et al. The association between fasting blood glucose and prognosis in gastrointestinal stromal tumor patients after curable resection. Updat Surg. 2023;75(5):1219–26. [DOI] [PubMed] [Google Scholar]
- 113.Supsamutchai C, et al. A cohort study of prognostic factors associated with recurrence or metastasis of gastrointestinal stromal tumor (GIST) of stomach. Ann Med Surg. 2018;35:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sadeghian M, et al. A review of fasting effects on the response of cancer to chemotherapy. Clin Nutr. 2021;40(4):1669–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.