Abstract
Objective
Cognitive impairments in schizophrenia significantly affect functional outcomes and quality of life. This meta-analysis evaluates the effectiveness of transcranial direct current stimulation (tDCS) as an intervention for cognitive deficits in individuals with schizophrenia.
Methods
From May 20 to June 15, 2024, a systematic search of PubMed, Medline, Embase, and the Cochrane central register of controlled trials was conducted. After applying eligibility criteria, 13 randomized sham-controlled trials were included, involving 261 participants in the tDCS group and 247 in the sham group. Standardized mean difference (SMD) was computed to measure the effect size of cognitive outcomes. Statistical analyses were performed using a random-effects model to account for heterogeneity.
Results
The pooled analysis yielded an SMD of 0.09 (95 % CI: −0.17 to 0.35), indicating a non-significant difference between tDCS and sham on cognitive outcomes. Moderate heterogeneity (I2 = 44 %) was observed, attributed to variations in tDCS protocols, participant demographics, and cognitive assessment tools. Although certain studies showed improvements in specific domains like working memory, the overall impact of tDCS on cognitive symptoms was not statistically significant.
Conclusions
This meta-analysis underscores the lack of significant evidence for tDCS in improving cognitive deficits in schizophrenia. The findings highlight the urgent need for standardizing tDCS protocols and employing domain-specific cognitive assessments. This standardization, along with the collection of more domain-specific data, is crucial for future research and the improvement of current methodologies.
Keywords: Transcranial direct current stimulation, Cognitive symptoms, Schizophrenia, Meta-analysis, Randomized controlled trials, Domain-specific improvements
1. Introduction
Schizophrenia affects 1 % of the global population and is characterized by positive symptoms, including hallucinations, delusions, negative symptoms, and cognitive deficits (Owen et al., 2016). The cognitive deficits impact memory, attention, and executive functioning (McGrath et al., 2008; Owen et al., 2016; Saha et al., 2005). While antipsychotics are helpful for positive symptoms, they often fail to improve cognitive impairments and may even worsen them due to their anticholinergic burden (Kahn et al., 2015).
Cognitive deficits in schizophrenia, particularly in working memory, are linked to neurodegenerative changes and developmental abnormalities in the brain, significantly impacting patients' functionality (Lett et al., 2014; Li et al., 2020; Nuechterlein et al., 2011; Parlar and Heinrichs, 2021). These deficits are primarily rooted in dysfunctions within the dorsolateral prefrontal cortex (DLPFC), a critical area for memory processes, which interacts with other brain regions like the basal ganglia and hippocampus (Barch and Ceaser, 2012; Edin et al., 2009; Faget-Agius et al., 2013; Fan et al., 2017; Quidé et al., 2013). Conventional cognitive training methods, such as cognitive remediation therapy, have shown some effectiveness (Hargreaves et al., 2015; Tan et al., 2016), pointing to the potential benefits of enhancing neuroplasticity to improve cognitive outcomes (Lett et al., 2014).
Transcranial direct current stimulation (tDCS) emerges as a promising non-invasive intervention. tDCS modulates neuronal activity by applying a low-intensity direct current by scalp electrodes, typically ranging from 1 to 2 mA. These currents influence neuronal membrane potentials, increasing or decreasing cortical excitability depending on the anode (excitatory) or cathode (inhibitory) placement over specific brain areas. Technical aspects such as electrode configuration, stimulation duration typically between 20 and 30 min, and frequency of sessions are tailored based on clinical objectives, with the typical site of anode placement being left DLPFC (Meinzer et al., 2013; Nitsche and Paulus, 2001).
Recent studies indicate that tDCS can significantly improve cognitive functioning in individuals with schizophrenia, with effects persisting beyond the treatment period (Martin et al., 2013; Orlov et al., 2017). This suggests that combining tDCS could enhance cortical neuroplasticity and cognitive rehabilitation outcomes. However, existing literature, including a meta-analysis by Lingfang Yu et al. (2020), shows that while there is a trend towards cognitive improvement, the results are not uniformly statistically significant (Yu et al., 2020). However, this meta-analysis was conducted four years ago, highlighting a knowledge gap and underscoring the need for an updated review that integrates more recent research to ascertain the effectiveness of tDCS in addressing cognitive symptoms of schizophrenia.
2. Methods
This systematic review and meta-analysis evaluated the efficacy of tDCS on cognitive symptoms in schizophrenia. We adhered to the PRISMA guidelines.
2.1. Search strategy
We comprehensively searched PubMed, Medline, Embase, and the Cochrane Central Register of Controlled Trials (CENTRAL). The search was initiated on May 20, 2024 and conducted using a combination of MeSH terms and keywords, including “Transcranial Electric Stimulation,” “Neurobehavioral Symptoms,” and “Schizophrenia.” Our search algorithm is as follows: (“transcranial electrical stimulation” [Title/Abstract] OR “transcranial direct current stimulation” [Title/Abstract] OR “transcranial direct current stimulation” [MeSH Terms]) AND (“cognitive symptoms” [Title/Abstract] OR “neurobehavioral manifestations” [MeSH Terms]) AND (“schizophrenia” [Title/Abstract] OR “schizophrenia” [MeSH Terms]). Each database was last searched on June 15, 2024, ensuring that the most recent studies were included.
2.2. Inclusion and exclusion criteria
Randomized, sham-controlled trials involving adult patients (aged 18 years or older) diagnosed with schizophrenia and with at least five sessions of tDCS. These studies needed to assess the effects of tDCS on cognitive symptoms and employ validated scales or neuropsychological testing for outcome measurements. All included studies were required to be published in peer-reviewed journals and written in English to ensure the reliability and accuracy of data extraction. Studies were excluded if they were not published in English, were observational or qualitative, lacked a sham control, or were unpublished.
2.3. Screening and selection
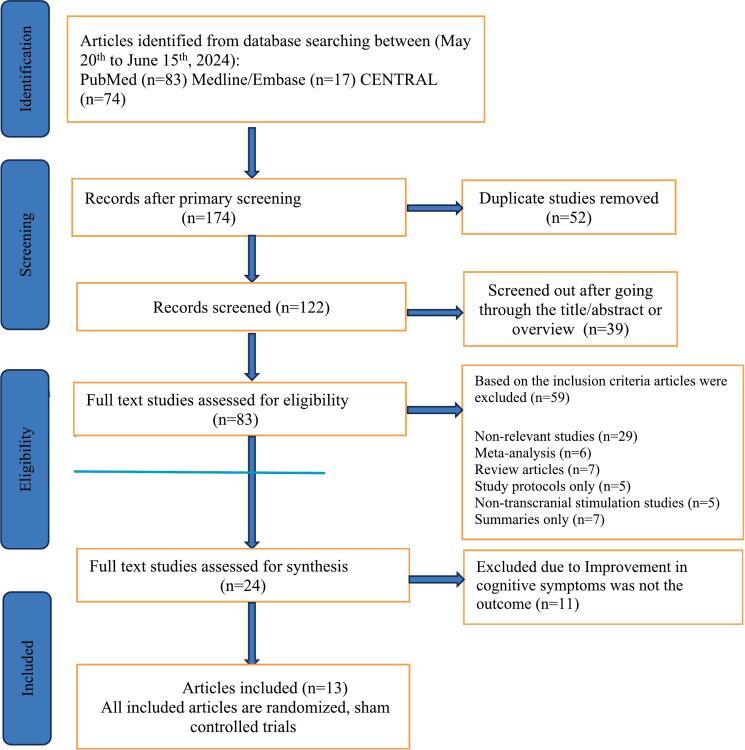
Two independent reviewers, authors SS and AR, initially screened all retrieved studies by titles and abstracts to filter out irrelevant reports. These authors also conducted subsequent detailed assessments of the complete texts to determine adherence to inclusion criteria, and any disagreements during the screening phases were resolved through discussion or with a third author AU to ensure consensus. The selection process, including the number of studies screened, assessed, included, and excluded, is summarized in Fig. 1.
Fig. 1.
Selection of studies to be included in the systematic review.
2.4. Selection outcome
From an initial pool of 174 search records retrieved through our search strategy, we identified 83 articles via PubMed, 17 through Medline and Embase, and 74 through CENTRAL. After a rigorous screening process, we narrowed this down to 122 relevant records. We stored all abstracts and citations in Zotero, a citation management library, to organize and facilitate the review process. Each record was independently screened against our eligibility criteria, focusing on titles and abstracts to ensure relevance; duplicates were promptly removed. Following this initial screening, full texts of the remaining studies were downloaded and reviewed for detailed assessment, as illustrated in Fig. 1.
2.5. Data extraction process
Authors SS and AR independently performed data extraction, utilizing a standardized data extraction form to ensure consistency and minimize bias. This process included gathering detailed bibliographic information, study characteristics, participant demographics, specific attributes of the tDCS interventions, and outcomes related to cognitive functions. To verify the accuracy and reliability of the extracted data, the third author, AU, conducted an independent review of the data.
For studies reporting cognitive outcomes across multiple domains using different measurement scales, we recognized that calculating an overall mean and standard deviation was not feasible due to the instruments' heterogeneity. We extracted available effect sizes directly from the studies or calculated them using reported statistics. The results were also summarized qualitatively to capture the essence of the findings.
2.6. Summary measures
Outcome measures were quantitatively reported as changes in cognitive status scores. The cognitive domains assessed included attention, executive functions, immediate memory, delayed memory, and social cognition. These outcomes were detailed through mean changes in cognitive test scores following the intervention. Variability and precision were captured using standard deviations, standard errors, and statistical significance (p-values). Our analysis included studies reporting standardized mean differences (SMDs) with their 95 % confidence intervals (CIs) and studies providing raw data—means, standard deviations, and sample sizes—from which SMDs could be calculated. Several studies assessed cognitive function using multiple scales without a unified scoring system, making it impractical to calculate overall mean scores. We relied on the reported effect sizes or alternative statistical information for these studies to extract SMDs. Statistical analyses were performed using R software (version 2024.04.2 + 764), utilizing the meta and metafor packages for computations and visualization.
A random-effects model was employed to account for potential between-study heterogeneity. We used the DerSimonian and Laird method with Restricted Maximum Likelihood (REML) estimation to calculate the between-study variance. The metagen function from the meta package facilitated the meta-analysis by incorporating the SMDs and their corresponding SEs. Each study was weighted inversely proportional to its variance, ensuring that studies with smaller SEs significantly influenced the overall effect size. We assessed statistical heterogeneity using I 2statistic and τ2. We created a forest plot to summarize the findings visually. Individual study estimates appeared as red squares, scaled by their meta-analysis weight, and the overall effect size was shown as a blue diamond. Negative SMD values suggested tDCS superiority over sham treatment; positive values indicated the opposite.
2.7. Bias risk assessment
We conducted a risk of bias assessment for each included study using the Robvis 2 software. This tool facilitated a systematic evaluation across critical domains such as random sequence generation and allocation concealment (selection bias), incomplete outcome data (attrition/dropout bias), blinding of participants and personnel (performance bias), and reporting biases. The assessment process involved independently reviewing each study against these criteria to identify potential sources of bias. We summarized the findings using traffic light plots and summary plots generated by the software.
2.8. Publication bias assessment
We did not perform a formal publication bias assessment in this meta-analysis because the limited number of included studies (thirteen) reduces the reliability of such assessments, making statistical tools like funnel plots potentially misleading. Additionally, significant clinical and methodological heterogeneity among the studies violates the assumptions underlying these methods. Our comprehensive literature search across multiple databases aimed to minimize the risk of missing relevant studies, and the emerging nature of tDCS research in schizophrenia suggests a lower likelihood of unpublished negative results. Therefore, conducting a formal publication bias assessment could yield inaccurate conclusions, so we focused on providing a transparent and balanced interpretation of the available evidence without it.
3. Results
3.1. Study selection
A systematic search of PubMed, MEDLINE, Embase, and the CENTRAL databases were performed from May 20 to June 15, 2024. This search yielded 174 records, out of which 52 duplicate studies were removed. The remaining 122 unique records underwent a title and abstract review, which excluded 39 studies based on irrelevance to core criteria. From the remaining 83 full-text studies, a further 59 were excluded based on specific criteria, including studies without sham controls, those not focusing on cognitive outcomes as primary endpoints, and publications in non-English languages. This rigorous selection led to 13 randomized sham-controlled trials meeting all inclusion criteria and forming the basis of the meta-analysis (Fig. 1).
3.2. Study characteristics
The meta-analysis includes 13 randomized sham-controlled trials, collectively involving 261 participants in the tDCS arm and 247 in the sham group. Participant demographics and tDCS parameters are summarized in detail (see Table 1)
Table 1.
Detailed study characteristics and participant demographics of included tDCS trials.
| Author, year | Outcome assessment measures | Duration of intervention | Location of study | tDCS protocol (active treatment arm) | Anode placement site | No. of participant | Age (years) | Clinical profile of participants |
|---|---|---|---|---|---|---|---|---|
| Zhou et al., 2023 (Zhou et al., 2023) | Cambridge Neuropsychological Test Automated Battery | 5 weeks | China | 15 sessions of 2 mA continuous, direct current on weekdays of the 1st, 3rd, and 5th week | Left Prefrontal Cortex | Active:21 Sham:17 | 18–70 (chronic schizophrenia with TD) | Chronic schizophrenia (1-year cumulative antipsychotic use) with Tardive Dyskinesia. |
| Lisoni et al., 2022 (Lisoni et al., 2022) | Brief Assessment of Cognition in Schizophrenia | 3 weeks | Italy | 15 sessions of 2 mA continuous, direct current on weekdays |
Left Dorsolateral Prefrontal Cortex | Active: 25 Sham: 25 |
≥ 18 | Schizophrenia stabilized after at least one month of antipsychotic treatment. |
| Bulubas et al., 2021 (Bulubas et al., 2021) | 1. Penn-CNB 2. Penn Conditional Exclusion Test 3. Penn Letter N-Back test (PLNB) 4. Penn Word Memory Test (PWMT), 5. Penn Face Memory Test (PFMT), 6. Short Visual Object Learning Test (volt) 7. Emotion identification (EMI) |
5 days | Brazil | 10 sessions of 20 min 2 mA continuous, direct current two times per day over the course of one week. Monday to Friday, with inter-session intervals between 180 and 210 min |
Left Prefrontal Cortex | Active:48Sham:42 | 18–55 | Schizophrenia with prominent negative symptoms and stable positive and negative symptoms, with a stable antipsychotic dose for 4 weeks at enrollment. . |
| Chang et al., 2020 (Chang et al., 2020) | 1. Mini-Mental State Examination 2. The Trail Making Test 3. Wisconsin Card Sorting Test 4. Tower of London-Drexel University Test (TOLDXtm 2nd edition) |
5 days | Taiwan | 10 sessions of 20 min 2 mA continuous direct current twice daily, 5 consecutive weekdays with inter-session intervals >2 h | Bilateral Dorsolateral Prefrontal Cortex | Active:30 Sham: 30 |
20–65 | Schizophrenia or schizoaffective disorder, illness duration ≥1 year, stable on antipsychotics but symptomatic for ≥4 weeks (CGI-S ≤ 4). |
| Smith et al., 2020 (Smith et al., 2020) | 1. Repeatable Battery for the Assessment of Neuropsychological Status 2. MATRICS™ Consensus Cognitive Battery (MCCB™) |
10–17 days | China | 10 sessions of 20 min 2 mA continuous direct current stimulation. | Left Dorsolateral Prefrontal Cortex | Active:24 Sham: 25 |
19–60 | Schizophrenia stabilized on medications with no recent acute exacerbation of symptoms, but still with significant cognitive deficit. |
| Weikert et al., 2019 (Weickert et al., 2019) | 1. Weschler Test of Adult Reading (WTAR) 2. Wechsler Adult Intelligence Scale 3rd Edition (WAIS-III) 3. MATRICS™ Consensus Cognitive Battery (MCCB™) |
4 weeks | USA | 20 sessions of 20 min 2 mA continuous direct current stimulation. | Right Dorsolateral Prefrontal Cortex | Active:6 Sham:6 |
18–50 | Patients with schizophrenia or schizo-affective disorder on antipsychotics for at least one year. |
| Jeon et al., 2018 (Jeon et al., 2018) | MATRICS™ Consensus Cognitive Battery (MCCB™) | 10 days | Rep of Korea | 30 mins of active 2 mA tDCS | Left & Dorsolateral Prefrontal Cortex | Active:28 Sham:28 |
18–65 | Patients with schizophrenia clinically stable for at least 3 months. |
| Gomes et al., 2018 (Gomes et al., 2018) | MATRICS™ Consensus Cognitive Battery (MCCB™) | 3 months (10 days intervention and then one assessment at 3 months) |
Brazil | 10 sessions of 20 min 2 mA continuous direct current stimulation. two consecutive weeks (Monday to Friday) |
Left Dorsolateral Prefrontal Cortex | Active:12 Sham:12 |
18–65 | Patients with schizophrenia stable on medication for at least 6 weeks, no co-morbid substance use disorder. |
| Smith et al., 2015 (Smith et al., 2015) | MATRICS™ Consensus Cognitive Battery (MCCB™) | 10–12 days | USA | 5 sessions of 20 min 2 mA continuous direct current stimulation. |
Left Dorsolateral Prefrontal Cortex | Active:19 Sham:18 |
≥18 years | Patients with schizophrenia or schizo-affective disorder and regular cigarette smokers. |
| Lindenmayer et al. 2019 (Lindenmayer et al., 2019) | MATRICS™ Consensus Cognitive Battery (MCCB™) | 4 weeks | USA | 40 sessions of 2 mA for 20 min session, twice daily for four weeks | Left Dorsolateral Prefrontal Cortex | Active:15 Sham:13 | 18–65 | Patients with long-standing treatment resistant schizophrenia and persistent auditory hallucination. |
| Mellin et al. 2018 (Mellin et al., 2018) |
1. Cognitive function (BACS) 2. Brief assessment of cognition in schizophrenia (BACS) |
5 days | USA | 10 sessions of 2 mA for 20 min session, twice daily | Left Dorsolateral Prefrontal Cortex | Active:7 Sham:7 | Mean age 29.57 (10.97) for tDCS And 38.86 (10.01) for sham |
Clinically stable patients with schizophrenia. |
| Koops et al. ., 2018 (Koops et al., 2018) | Stroop test and trail making test | 5 days | Netherland | 10 sessions of 2 mA for 20 min session, twice daily. | Left Dorsolateral Prefrontal Cortex | Active: 28 Sham:26 |
≥18 | Participants had daily auditory hallucinations, were resistant to two antipsychotics, and were on a stable dose for at least two weeks before study entry. |
| Palm et al. 2016 (Palm et al., 2016) | 1.Self-Ordered Pointing Task (SOPT) for WM, 2. Trail-Making Test (TMT-A) for processing speed, and TMT-B for executive functioning. |
5 days | Germany | 10 sessions of 2 mA for 20 min session, twice daily | Left Dorsolateral Prefrontal Cortex | Active: 10 Sham:10 |
18–65 | Patients with predominant negative symptoms a PANSS score > 70, and stable antipsychotic treatment for over 4 weeks. |
3.3. Demographic and clinical profile
Most participants were males, aged between 18 and 65 years, diagnosed with schizophrenia and stabilized on antipsychotic regimens. Exclusion criteria typically covered active comorbidities that might impact cognitive assessments, such as substance use disorders or major neurological conditions. One study included participants with tardive dyskinesia (Zhou et al., 2023).
3.4. Intervention protocols
tDCS settings varied between studies. For electrode placement, DLPFC was a standard target for anodal stimulation in most studies. Stimulation intensities ranged from 1 to 2 mA, with sessions lasting from 20 to 30 min. Some studies applied daily sessions across multiple weeks; eg up to 40 sessions in Lindenmayer et al., 2019 (Lindenmayer et al., 2019), while others used more intensive schedules (e.g., twice daily for five days).
3.5. Outcome measures
Cognitive assessments included validated tools like the MATRICS Consensus Cognitive Battery (MCCB), the Cambridge Neuropsychological Test Automated Battery (CANTAB), the Brief Assessment of cognition in schizophrenia (BACS), the trail-making test, and the Stroop test, among others.
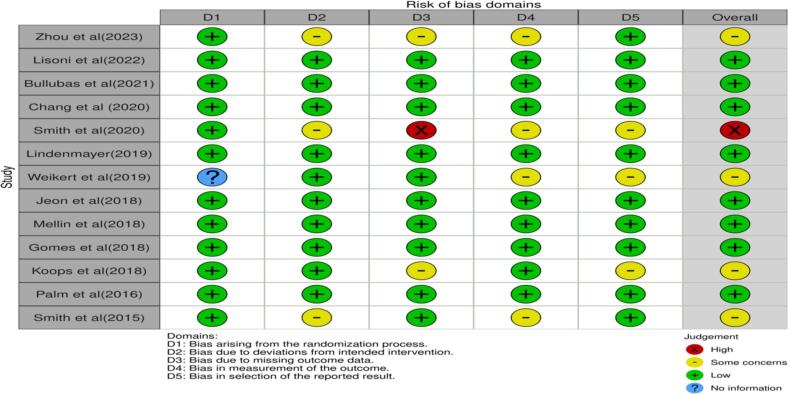
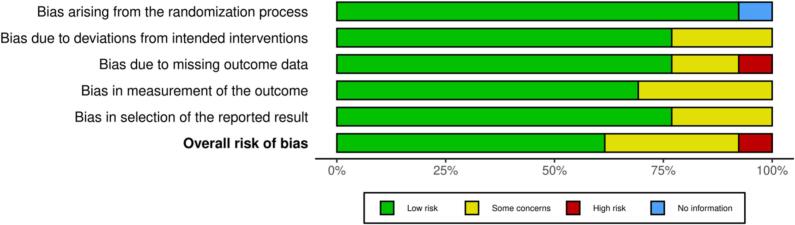
3.6. Risk of bias within studies
Traffic light plot and summary plots were generated through Robvis 2 risk assessment software and are shown as (Fig. 2, Fig. 3).
-
•
Selection bias: All 13 studies were randomized trials and generally exhibited a low risk of selection bias (Domain 1) (Bulubas et al., 2021; Chang et al., 2020; Gomes et al., 2018; Jeon et al., 2018; Koops et al., 2018; Lindenmayer et al., 2019; Lisoni et al., 2022; Mellin et al., 2018; Palm et al., 2016; Smith et al., 2015; Smith et al., 2020; Weickert et al., 2019; Zhou et al., 2023). However, Weikert et al. (Weickert et al., 2019) did not provide details on random sequence generation or allocation concealment raising concerns. Zhou et al. (Zhou et al., 2023), Smith et al. (Smith et al., 2020), and Smith et al. (Smith et al., 2015) had some bias concerns in allocation concealment (Domain 2) because tDCS administrators were aware of group assignments.
-
•
Attrition/dropout bias: Most studies had low attrition and used intention-to-treat analyses to account for losses. However, Smith et al. (Smith et al., 2020) had only 17 out of 24 active tDCS participants and 19 out of 25 sham participants completing the four-week follow-up, without clearly addressing how this was managed, leading to a high risk of attrition bias (Domain 3). Zhou et al. (Zhou et al., 2023) did not mention loss to follow-up, and Koops et al. (Koops et al., 2018) noted most dropouts were from the sham group, potentially influencing results due to treatment inefficacy.
-
•
Performance bias and detection bias:-While all studies were sham-controlled randomized trials with blinding, ensuring low performance bias risk (Bulubas et al., 2021; Chang et al., 2020; Gomes et al., 2018; Jeon et al., 2018; Koops et al., 2018; Lindenmayer et al., 2019; Lisoni et al., 2022; Mellin et al., 2018; Palm et al., 2016; Smith et al., 2015; Smith et al., 2020; Weickert et al., 2019; Zhou et al., 2023), some concerns arose. In Zhou et al. (Zhou et al., 2023), tDCS operators knew patient groupings. Weikert et al. (Weickert et al., 2019) did not clarify whether psychiatrists or psychologists involved in administering tDCS and implementing blinding also participated in outcome assessments, leaving room for potential bias. Smith et al. (Smith et al., 2020) acknowledged partial blinding success.
-
•
Reporting biased results: Smith et al. (Smith et al., 2020) did not specify how they addressed attrition or inclusion of those participants in the final analysis, potentially introducing bias in their findings. Despite expectations that longer illness duration might lessen treatment impact, the active tDCS group with slightly longer illness duration showed greater cognitive improvement than the sham group, possibly influenced by small sample size. Koops et al. (Koops et al., 2018) acknowledged that most dropouts were from the sham group, which could have affected results due to perceived treatment inefficacy.
-
•
Overall risk of bias: We assessed each study's bias risk in the “Risk of Bias” table within the Characteristics of Included Studies section (Table 1). Considering the identified biases, we concluded that, despite some concerns in specific studies, the overall risk of bias affecting our findings is low (Fig. 2, Fig. 3).
Fig. 2.
Risk of bias assessment traffic light plot for the studies included in the review.
Fig. 3.
Risk of bias assessment summary plot.
3.7. Results of individual studies primary outcome
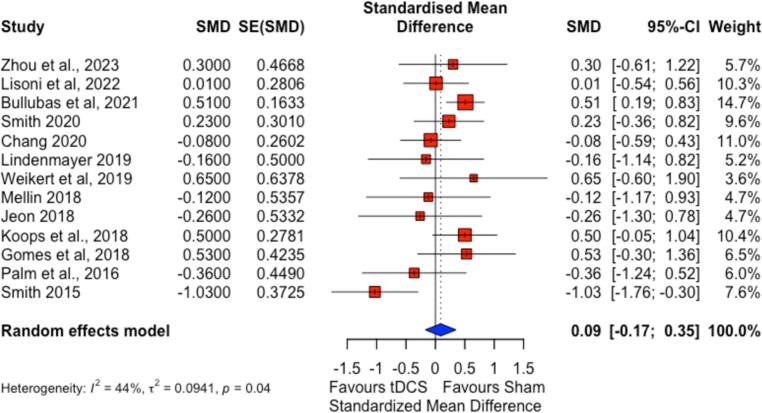
The main outcome measure was the standardized mean difference (SMD) in cognitive performance between tDCS and sham groups. Individual study findings highlighted notable variability. Smith et al. (Smith et al., 2015) demonstrated a significant improvement in cognitive scores for the tDCS group with an SMD of −1.03 (95 % CI: −1.76 to −0.30, p < 0.01). However, Bulubas et al. (Bulubas et al., 2021) found an unexpected benefit in the sham group, with an SMD of 0.53 (95 % CI: 0.19 to 0.83). While other reported no statistically significant cognitive improvements, with confidence intervals spanning zero (Table 2, Table 3).
Table 2.
Summary of study interventions and cognitive outcomes in schizophrenia.
| References | Measure used for outcome assessment. | Result |
|---|---|---|
| Zhou et al., 2023 (Zhou et al., 2023) | Cambridge Neuropsychological Test Automated Battery | No significant improvement |
| Lisoni et al., 2022 (Lisoni et al., 2022) | Brief Assessment of Cognition in schizophrenia | Significant improvement in digit sequencing |
| Bulubas et al., 2021 (Bulubas et al., 2021) | 1. Penn-CNB 2. Penn Conditional Exclusion Test 3. Penn Letter N-Back test (PLNB) 4. Penn Word Memory Test (PWMT), 5. Penn Face Memory Test (PFMT), 6. Short Visual Object Learning Test (volt) 7. Emotion identification (EMI) |
Executive functioning and delayed memory improvement in the sham arm. |
| Chang et al., 2020 (Chang et al., 2020) | 1.Mini-Mental State Examination 2. The Trail Making Test 3. Wisconsin Card Sorting Test 4. Tower of London-Drexel University Test (TOLDXtm 2nd edition) |
No Significant improvement in cognitive symptoms |
| Smith et al., 2020 (Smith et al., 2020) | 1. Repeatable Battery for the Assessment of Neuropsychological Status 2. MATRICS™ Consensus Cognitive Battery (MCCB™) |
There was no immediate change; however, after two weeks, there was a significant improvement in the processing speed and MATRIC composite score. |
| Weikert et al., 2019 (Weickert et al., 2019) | 1. Weschler Test of Adult Reading (WTAR) 2. Wechsler Adult Intelligence Scale 3rd Edition (WAIS-III) 3. MATRICS™ Consensus Cognitive Battery (MCCB™) |
Beneficial effects on cognition in schizophrenia that transfer to other prefrontal dependent cognitive domains. |
| Jeon et al., 2018 (Jeon et al., 2018) | MATRICS™ Consensus Cognitive Battery (MCCB™) | Improvement in MCCB working memory and overall score. |
| Gomes et al., 2018 (Gomes et al., 2018) | MATRICS™ Consensus Cognitive Battery (MCCB™) | No significant improvement |
| Smith et al., 2015 (Smith et al., 2015) | MATRICS™ Consensus Cognitive Battery (MCCB™) | Significant improvement in composite score and working memory |
| Lindenmayer et al. 2019 (Lindenmayer et al., 2019) | MATRICS™ Consensus Cognitive Battery (MCCB™) | Significant improvement in the working memory domain and no change in the composite score. |
| Mellin et al. 2018 (Mellin et al., 2018) | 1. Cognitive function (BACS) 2. Brief assessment of cognition in schizophrenia |
There was no significant difference among the three groups. However, effect size analysis indicated that tDCS had the most critical effect size, with a baseline vs. post cd of 1.5. |
| Koops et al., 2018 (Koops et al., 2018) | Stroop test and trail making test | No significant benefit was measured by the Stroop test. Significant benefit as measured by TMT B after one week. |
| Palm et al. 2016 (Palm et al., 2016) | 1. Self-Ordered Pointing Task (SOPT) for WM, 2.Trail-Making Test (TMT-A) for processing speed, and TMT-B for executive functioning. |
The participants generally improved in reaction time for the cognitive tasks (TMT-A and TMT-B); these improvements were not influenced by their group, nor did they differ when considering the interaction of time and group factors. The working memory task (SOPT) showed no improvements or changes across any conditions. |
Table 3.
Summary of mean outcomes and effect sizes comparing active and Sham tDCS on cognitive symptoms in schizophrenia across studies.
| Study | Active tDCS (Mean ± SD) | Active N | Sham (Mean ± SD) | Sham N | SMD | 95 % CI (Lower) | 95 % CI (Upper) |
|---|---|---|---|---|---|---|---|
| Zhou et al., 2023 (Zhou et al., 2023) | −10.4 ± 48.6 | 21 | −22.4 ± 44.3 | 17 | 0.30 | −0.61 | 1.22 |
| Lisoni et al., 2022 (Lisoni et al., 2022) | 0.27 ± 1.08 | 25 | 0.26 ± 0.67 | 25 | 0.01 | −0.54 | 0.56 |
| Bulubas et al., 2021 (Bulubas et al., 2021) | ⁎ | 48 | ⁎ | 42 | 0.51 | 0.91 | 0.83 |
| Smith et al., 2020 (Smith et al., 2020) | −2.72 ± 0.91 | 24 | −2.94 ± 0.97 | 21 | 0.23 | −0.36 | 0.82 |
| Chang et al., 2020 (Chang et al., 2020) | −0.53 ± 3.12 | 30 | −0.3 ± 2.57 | 30 | −0.08 | −0.59 | 0.43 |
| Lindenmayer et al., 2019 (Lindenmayer et al., 2019) | −3.85 ± 12.2 | 8 | −2.33 ± 3.04 | 8 | −0.16 | −1.14 | 0.82 |
| Weikert et al., 2019 (Weickert et al., 2019) | ⁎ | 6 | ⁎ | 6 | 0.65 | −0.60 | 1.90 |
| Mellin et al., 2018 (Mellin et al., 2018) | −3.79 ± 10.04 | 7 | −2.57 ± 9.31 | 7 | −0.12 | −1.17 | 0.93 |
| Jeon et al., 2018 (Jeon et al., 2018) | −4.98 ± 6.36 | 25 | −3.33 ± 6.07 | 27 | −0.26 | −0.81 | 0.28 |
| Koops et al., 2018 (Koops et al., 2018) | ⁎ | 28 | ⁎ | 26 | 0.5 | −0.05 | 1.04 |
| Gomes et al., 2018 (Gomes et al., 2018) | ⁎ | 12 | ⁎ | 12 | 0.53 | −0.30 | 1.36 |
| Palm et al., 2016 (Palm et al., 2016) | ⁎ | 10 | ⁎ | 10 | −0.36 | −1.24 | 0.52 |
| Smith et al., 2015 (Smith et al., 2015) | −3.47 ± 4.09 | 17 | 0.85 ± 4.08 | 16 | −1.03 | −1.76 | −0.30 |
Mean and standard deviation are not provided for studies that used diverse cognitive assessments or measured different cognitive domains that cannot be directly compared or combined into a single composite score.
3.8. Synthesis of results
Employing a random-effects model to account for between-study variability, the pooled SMD suggested no significant effect of tDCS on cognitive function (SMD = 0.09; 95 % CI: −0.17 to 0.35), indicating minimal difference between tDCS and sham groups. Moderate heterogeneity was noted (I2 = 44 %, τ2 = 0.094, p = 0.04). This heterogeneity likely stemmed from variations in participant demographics, tDCS protocol specifics, and the cognitive assessment tools used. This meta-analysis does not support the efficacy of tDCS over sham treatment for cognitive symptoms in schizophrenia. While certain individual studies showed promise, the overall effect was small and non-significant, with moderate heterogeneity (Fig. 4).
Fig. 4.
Forest plot of the meta-analysis comparing tDCS to sham treatment on cognition in patients with schizophrenia.
3.9. Exploration of heterogeneity
Sources of heterogeneity were explored in detail, considering. Differences in age, cognitive baseline, and illness severity may influence tDCS response, with some studies targeting specific subgroups such as those with prominent negative symptoms (Bulubas et al., 2021). Variations in session frequency, electrode placement, and stimulation duration were observed across studies, impacting consistency. Studies varied in their use of cognitive assessment tools, making direct comparison challenging.
3.10. Sensitivity analysis
Sensitivity analyses were conducted to assess the robustness of the findings:
-
•
Exclusion of High Attrition Studies: Removing Smith et al. (Smith et al., 2020) slightly altered the pooled SMD (SMD = 0.07; 95 % CI: −0.22 to 0.36), but the overall non-significance remained unchanged.
-
•
Outlier Removal: Excluding studies with exceptionally high or low effect sizes did not substantially change the results, confirming the non-significant effect of tDCS on cognitive outcomes in the pooled data.
4. Discussion
This meta-analysis aimed to evaluate the efficacy of tDCS in enhancing cognitive function among individuals with schizophrenia by synthesizing data from 13 double-blind, sham-controlled randomized controlled trials. Despite employing rigorous methodological criteria to mitigate design inconsistencies highlighted in previous reviews (Kostova et al., 2020; Yu et al., 2020) our findings indicate that tDCS does not have a significant overall effect on cognitive symptoms in schizophrenia. The pooled standardized mean difference (SMD) was 0.09 (95 % CI: −0.17 to 0.35), suggesting non-significant difference between tDCS and sham group.
While some individual studies reported significant cognitive improvements with tDCS, particularly in working memory and executive function domains (Smith et al., 2020; Weickert et al., 2019), others found no significant benefits (Chang et al., 2020; Lindenmayer et al., 2019; Zhou et al., 2023). Interestingly, Bulubas et al. (Bulubas et al., 2021) observed an unexpected improvement in the sham group. This variability may be due to the influence of specific tDCS protocol factors—such as stimulation intensity, electrode placement, and session frequency—on therapeutic outcomes. Studies employing both minimal (five sessions) and extensive (forty sessions) tDCS interventions reported improvements in working memory suggesting that session number alone may not determine efficacy (Lindenmayer et al., 2019; Smith et al., 2015).
A significant limitation of this meta-analysis is the heterogeneity among the included studies, compounded by relatively small sample sizes. Moderate heterogeneity was observed (I2 = 44 %), likely stemming from variations in participant demographics—such as age, illness duration, symptom severity, and medication status—which substantially influence each patient's responsiveness to tDCS. For example, both Zhou et al. (Zhou et al., 2023) and Bulubas et al. (Bulubas et al., 2021) enrolled patients with chronic schizophrenia, yet reported different outcomes, possibly due to differences in clinical profiles, such as the presence of tardive dyskinesia in Zhou et al. (Zhou et al., 2023). Small sample sizes reduce statistical power, potentially masking subtle yet clinically relevant effects of tDCS on cognitive domains, thereby limiting the generalizability of the findings.
Cognitive deficits in schizophrenia stem from a complex interplay of genomic, neurobiological, and neuroanatomic factors. Imaging studies reveal that these deficits are linked to structural brain changes, including reduced cortical thickness, increased ventricular volume, and alterations in regions such as the cerebellum, basal ganglia, and dorsolateral prefrontal cortex (Jacobson et al., 2012). These changes may disrupt cortico-cerebellar-thalamic-cortical circuits and reduce prefrontal cortex metabolism, contributing to cognitive impairment. While earlier theories focused on neurodevelopmental disorders, current understanding suggests that cognitive deficits may result from neurodevelopmental abnormalities, disrupted neuronal maturation, and altered neuroplasticity (Paulus, 2011).
Biochemical imbalances also play a role, with evidence pointing to disruptions in inflammatory cytokines, hormones like cortisol and prolactin, neurotrophic factors such as BDNF, and neurotransmitters like GABA and glutamate (Stagg and Nitsche, 2011). Despite extensive research, specific cognitive markers in schizophrenia remain elusive. However, neuroimaging findings such as fractional anisotropy of the corpus callosum and changes in the salience and default mode networks show potential in predicting cognitive performance (Nitsche et al., 2003). tDCS offers a promising approach that modulates neuronal activity. Anodal tDCS increases neuronal excitability, while cathodal tDCS decreases it, affecting neurotransmitter levels (Anticevic et al., 2015; Grent-’t-Jong et al., 2018). The effectiveness of tDCS may vary with the illness stage, emphasizing the need for personalized protocols to account for individual differences in brain anatomy and excitability (Lewis and González-Burgos, 2012; Stagg et al., 2018).
Cognitive functioning in schizophrenia is a complex neurological construct composed of various subcomponents, each potentially governed by distinct neurocircuitries (Millan et al., 2012). Combining these domains into a single composite score may obscure domain-specific effects, hindering a clear understanding of how tDCS impacts specific cognitive functions. For instance, the dorsolateral prefrontal cortex is primarily involved in working memory, whereas the amygdala and prefrontal cortex are associated with social cognition (D'Esposito and Postle, 2015). The lack of uniformity in outcome measurements across studies further complicates direct comparisons and may contribute to inconsistent findings. While six studies used the MATRICS Consensus Cognitive Battery (MCCB), results remained mixed, possibly due to variations in other study variables [Table 1, Table 2].
An important consideration is the potential neurobiological impact of sham tDCS itself. Recent studies suggest that sham protocols, which often include brief periods of active stimulation, may inadvertently exert neurobiological effects beyond non-specific placebo responses (Fonteneau et al., 2019). For example, a 40-s ramp-up/down phase and a 30-s active stimulation at 2 mA—as used in several sham protocols—deliver a substantial amount of current to the brain, potentially leading to functional changes even at low intensities through mechanisms like stochastic resonance. Evidence from healthy participants indicates that even minimal direct current stimulation (e.g., 1.6 s) can enhance verbal memory (Javadi and Walsh, 2012). This could confound results, as improvements may be partially attributed to sham stimulation, masking the true efficacy of active tDCS.
The studies reviewed employed varying numbers of tDCS sessions, ranging from five to forty, with both extremes reporting improvements in working memory (Lindenmayer et al., 2019; Smith et al., 2015). This disparity in session numbers may contribute to the heterogeneity observed in results. Additionally, most studies targeted the dorsolateral prefrontal cortex for anodal stimulation, yet the complexity of cognitive processes—likely involving interconnected neural pathways—suggests that exploring alternative stimulation sites could be beneficial.
The diversity in cognitive outcome measures further complicates the interpretation of tDCS effects. The lack of uniformity in assessment tools makes it challenging to compare results directly or draw broad conclusions about efficacy. Psychological testing instruments may not fully capture nuanced cognitive changes induced by tDCS, highlighting the need for more sensitive measures.
The primary limitations of this meta-analysis include heterogeneity among studies, small sample sizes, and variability in tDCS protocols and outcome measures. Several studies designated cognitive improvement as a secondary outcome, potentially resulting in underpowered analyses for detecting specific changes. Additionally, the variability in patient profiles—including differences in illness severity, duration, and comorbid conditions—complicates comparisons and suggests that tDCS efficacy may depend on specific patient subgroups.
Future research should prioritize the standardization of stimulation protocols, including uniform parameters like intensity, duration, and electrode placement. Additionally, adopting standardized cognitive assessments such as the MCCB enables consistent evaluation across cognitive domains. Investigating these domains individually may reveal selective improvements and clarify differential impacts, potentially obscured by composite scoring. Integrating neuroimaging and neurophysiological data could also allow for the tailoring of tDCS to individual neurobiological profiles, enhancing efficacy. Further, refining sham protocols, expanding to large-scale, multicenter trials, and conducting longitudinal studies would solidify the understanding of tDCS's long-term effects and its overall effectiveness in cognitive rehabilitation for schizophrenia.
5. Conclusion
This meta-analysis highlights both the potential and limitations of tDCS as an intervention for cognitive deficits in schizophrenia. While some studies demonstrate promising results, the overall evidence does not support a significant benefit of tDCS over sham treatment. Addressing the identified limitations through standardized methodologies and personalized approaches may enhance the utility of tDCS in clinical practice. By focusing on domain-specific improvements and tailoring interventions to individual neurobiological profiles, future research can move towards more effective, personalized treatments that significantly improve cognitive outcomes and quality of life for individuals with schizophrenia.
Declaration of Generative AI and AI-assisted technologies in the writing process
While preparing this work, the author AR used Chat GTP to correct the manuscript's grammar. After using this tool/service, all the authors reviewed and edited the content as needed and take(s) full responsibility for the content of the published article.
CRediT authorship contribution statement
Sadia Rehman Safwi: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Abid Rizvi: Writing – original draft, Formal analysis, Data curation, Conceptualization. Mohammad Amir Usmani: Visualization, Supervision, Investigation. Karrar Husain: Writing – review & editing, Visualization, Validation. Kanwarjeet Brar: Writing – review & editing, Writing – original draft, Supervision. Deep Yadava: Writing – review & editing, Supervision, Methodology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
No funding was received for this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scog.2024.100335.
Contributor Information
Abid Rizvi, Email: abid.rizvi@hsc.wvu.edu.
Deep Yadava, Email: deep.yadava@wv.gov.
Appendix A. Supplementary data
The following are the supplementary data related to this article.
Summary of study interventions and detailed cognitive outcomes in reviewed studies on schizophrenia.
Supplementary material
References
- Anticevic A., Yang G., Savic A., Murray J.D., Cole M.W., Repovs G., Yang S., Wang X.-J. Mediodorsal and visual thalamic connectivity differ in schizophrenia and bipolar disorder with and without psychosis history. Schizophr. Bull. 2015;41(6):1335–1344. doi: 10.1093/schbul/sbv074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D.M., Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn. Sci. 2012;16:27–34. doi: 10.1016/j.tics.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulubas L., Goerigk S., Gomes J.S., Brem A.-K., Carvalho J.B., Pinto B.S., Elkis H., Gattaz W.F., Padberg F., Brunoni A.R., Valiengo L. Cognitive outcomes after tDCS in schizophrenia patients with prominent negative symptoms: results from the placebo-controlled STARTS trial. Schizophr. Res. 2021;235:44–51. doi: 10.1016/j.schres.2021.07.008. [DOI] [PubMed] [Google Scholar]
- Chang C.-C., Kao Y.-C., Chao C.-Y., Tzeng N.-S. Examining bi-anodal transcranial direct current stimulation (tDCS) over bilateral dorsolateral prefrontal cortex coupled with bilateral extracephalic references as a treatment for negative symptoms in non-acute schizophrenia patients: A randomized, double-blind, sham-controlled trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2020;96 doi: 10.1016/j.pnpbp.2019.109715. [DOI] [PubMed] [Google Scholar]
- D'Esposito M., Postle B.R. The cognitive neuroscience of working memory. Annu. Rev. Psychol. 2015;66:115–142. doi: 10.1146/annurev-psych-010814-015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edin F., Klingberg T., Johansson P., McNab F., Tegnér J., Compte A. Mechanism for top-down control of working memory capacity. Proc. Natl. Acad. Sci. USA. 2009;106:6802–6807. doi: 10.1073/pnas.0901894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faget-Agius C., Boyer L., Lançon C., Richieri R., Fassino E., Soulier E., Guedj E., Mundler O., Khalfa S., Cermolacce M. Structural and functional reorganization of working memory system during the first decade in schizophrenia. A cross-sectional study. Schizophr. Res. 2013;151:48–60. doi: 10.1016/j.schres.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Fan F., Zou Y., Tan Y., Hong L.E., Tan S. Computerized cognitive remediation therapy affects resting-state brain activity and cognition in schizophrenia. Sci. Rep. 2017;7:4758. doi: 10.1038/s41598-017-05179-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonteneau C., Mondino M., Arns M., Baeken C., Bikson M., Brunoni A.R., Burke M.J., Neuvonen T., Padberg F., Poulet E., Ruffini G., Santarnecchi E., Schellhorn K., Wexler A., Palm U. Sham tDCS: A hidden source of variability? Reflections for further blinded, controlled trials. Brain Stimul. 2019;12(3):668–673. doi: 10.1016/j.brs.2018.12.977. [DOI] [PubMed] [Google Scholar]
- Gomes J.S., Trevizol A.P., Ducos D.V., Gadelha A., Ortiz B.B., Fonseca A.O., Akiba H.T., Azevedo C.C., Guimaraes L.S.P., Shiozawa P., Cordeiro Q., Lacerda A., Dias A.M. Effects of transcranial direct current stimulation on working memory and negative symptoms in schizophrenia: a phase II randomized sham-controlled trial. Schizophr. Res. Cogn. 2018;12:20–28. doi: 10.1016/j.scog.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grent-’t-Jong T., Rivolta D., Gross J., Gajwani R., Lawrie S.M., Schwannauer M., Schyns P.G., Gumley A.I., Uhlhaas P.J. Acute ketamine dysregulates task-related gamma-band oscillations in schizophrenia: relationship to prefrontal gray matter. J. Neurosci. 2018;38(21):4919–4928. doi: 10.1523/jneurosci.0767-18.2018. [DOI] [Google Scholar]
- Hargreaves A., Dillon R., Anderson-Schmidt H., Corvin A., Fitzmaurice B., Castorina M., Schwaiger S., Davoren M., Cotter D., McDonald C., Donohoe G. Computerised working-memory focused cognitive remediation therapy for psychosis—a preliminary study. Schizophr. Res. 2015;169:135–140. doi: 10.1016/j.schres.2015.09.005. [DOI] [PubMed] [Google Scholar]
- Jacobson L., Koslowsky M., Lavidor M. TDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp. Brain Res. 2012;216(1):1–10. doi: 10.1007/s00221-011-2891-9. [DOI] [PubMed] [Google Scholar]
- Javadi A.H., Walsh V. Transcranial direct current stimulation (tDCS) of the left dorsolateral prefrontal cortex modulates declarative memory. Brain Stimul. 2012;5(3):231–241. doi: 10.1016/j.brs.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Jeon D.-W., Jung D.-U., Kim S.-J., Shim J.-C., Moon J.-J., Seo Y.-S., Jung S.-S., Seo B.-J., Kim J.-E., Oh M., Kim Y.-N. Adjunct transcranial direct current stimulation improves cognitive function in patients with schizophrenia: a double-blind 12-week study. Schizophr. Res. 2018;197:378–385. doi: 10.1016/j.schres.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Kahn R.S., Sommer I.E., Murray R.M., Meyer-Lindenberg A., Weinberger D.R., Cannon T.D., O'Donovan M., Correll C.U., Kane J.M., van Os J., Insel T.R., Malaspina D. Schizophrenia. Nat. Rev. Dis. Primers. 2015;1:15067. doi: 10.1038/nrdp.2015.67. [DOI] [PubMed] [Google Scholar]
- Koops S., Blom J.D., Bouachmir O., Slot M.I., Neggers B., Sommer I.E. Treating auditory hallucinations with transcranial direct current stimulation in a double-blind, randomized trial. Schizophr. Res. 2018;201:329–336. doi: 10.1016/j.schres.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Kostova R., Cecere R., Thut G., Uhlhaas P.J. Targeting cognition in schizophrenia through transcranial direct current stimulation: a systematic review and perspective. Schizophr. Res. 2020;220:300–310. doi: 10.1016/j.schres.2020.03.002. [DOI] [PubMed] [Google Scholar]
- Lett T.A., Voineskos A.N., Kennedy J.L., Levine B., Daskalakis Z.J. Treating working memory deficits in schizophrenia: a review of the neurobiology. Biol. Psychiatry. 2014;75:361–370. doi: 10.1016/j.biopsych.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Lewis D.A., González-Burgos G. Neuroplasticity of excitatory and inhibitory cortical circuits in schizophrenia. J. Neurosci. 2012;32(43):14827–14837. doi: 10.1523/jneurosci.3735-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zhou F.-C., Zhang L., Ng C.H., Ungvari G.S., Li J., Xiang Y.-T. Comparison of cognitive dysfunction between schizophrenia and bipolar disorder patients: a meta-analysis of comparative studies. J. Affect. Disord. 2020;274:652–661. doi: 10.1016/j.jad.2020.05.075. [DOI] [PubMed] [Google Scholar]
- Lindenmayer J.P., Kulsa M.K.C., Sultana T., Kaur A., Yang R., Ljuri I., Parker B., Khan A. Transcranial direct-current stimulation in ultra-treatment-resistant schizophrenia. Brain Stimul. 2019;12(1):54–61. doi: 10.1016/j.brs.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Lisoni J., Baldacci G., Nibbio G., Zucchetti A., Butti Lemmi Gigli E., Savorelli A., Facchi M., Miotto P., Deste G., Barlati S., Vita A. Effects of bilateral, bipolar-nonbalanced, frontal transcranial direct current stimulation (tDCS) on negative symptoms and neurocognition in a sample of patients living with schizophrenia: results of a randomized double-blind sham-controlled trial. J. Psychiatr. Res. 2022;155:430–442. doi: 10.1016/j.jpsychires.2022.02.020. [DOI] [PubMed] [Google Scholar]
- Martin D.M., Liu R., Alonzo A., Green M., Player M.J., Sachdev P., Loo C.K. Can transcranial direct current stimulation enhance outcomes from cognitive training? A randomized controlled trial in healthy participants. Int. J. Neuropsychopharmacol. 2013;16:1927–1936. doi: 10.1017/S1461145713000539. [DOI] [PubMed] [Google Scholar]
- McGrath J., Saha S., Chant D., Welham J. Schizophrenia: a concise overview of incidence, prevalence, and mortality. Epidemiol. Rev. 2008;30:67–76. doi: 10.1093/epirev/mxn001. [DOI] [PubMed] [Google Scholar]
- Meinzer M., Lindenberg R., Antonenko D., Flaisch T., Flöel A. Anodal transcranial direct current stimulation temporarily reverses age-associated cognitive decline and functional brain activity changes. J. Neurosci. 2013;33:12470–12478. doi: 10.1523/jneurosci.5743-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellin J.M., Alagapan S., Lustenberger C., Lugo C.E., Alexander M.L., Gilmore J.H., Jarskog L.F., Fröhlich F. Randomized trial of transcranial alternating current stimulation for treatment of auditory hallucinations in schizophrenia. Eur. Psychiatry. 2018;51:25–33. doi: 10.1016/j.eurpsy.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan M.J., Agid Y., Brüne M., Bullmore E.T., Carter C.S., Clayton N.S., Connor R., Fletcher P.C., Flint J., Geyer M.A., Goodwin G.M., Grace A.A., Heidbreder C., Jardri R., Joye K., Kanba S., Kawata M., Keefe R.S.E., Kelly P.A., Lieberman J.A., Lodge D.J., Marsden C.A., Meyer-Lindenberg A., McAllister K.H., Murck H., Rolls E., Saletu B., Spedding M., Sweeney J.A., Thomsen M., Tricklebank M. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat. Rev. Drug Discov. 2012;11(2):141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Nitsche M.A., Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Nitsche M.A., Schauenburg A., Lang N., Liebetanz D., Exner C., Paulus W., Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J. Cogn. Neurosci. 2003;15(4):619–626. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- Nuechterlein K.H., Subotnik K.L., Green M.F., Ventura J., Asarnow R.F., Gitlin M.J., Yee C.M., Gretchen-Doorly D., Mintz J., Wallace C.J., Liberman R.P. Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophr. Bull. 2011;37(Suppl. 2):S33–S40. doi: 10.1093/schbul/sbr084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlov N.D., O'Daly O., Tracy D.K., Daniju Y., Hodsoll J., Valdearenas L., Downar J., Joyce D., Shergill S.S. Stimulating thought: a functional MRI study of transcranial direct current stimulation in schizophrenia. Brain. 2017;140:2490–2497. doi: 10.1093/brain/awx189. [DOI] [PubMed] [Google Scholar]
- Owen M.J., Sawa A., Mortensen P.B. Schizophrenia. Lancet. 2016;388(10039):86–97. doi: 10.1016/S0140-6736(15)01121-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm U., Keeser D., Hasan A., Kupka M.J., Blautzik J., Sarubin N., Kaymakanova F., Unger I., Falkai P., Meindl T., Ertl-Wagner B., Padberg F. Prefrontal transcranial direct current stimulation for treatment of schizophrenia with predominant negative symptoms: a double-blind, sham-controlled proof-of-concept study. Schizophr. Bull. 2016;42(5):1253–1261. doi: 10.1093/schbul/sbw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlar M.E., Heinrichs R.W. Cognitive decline and impairment in schizophrenia spectrum disorders reconsidered. Schizophr. Res. 2021;228:626–632. doi: 10.1016/j.schres.2020.12.012. [DOI] [PubMed] [Google Scholar]
- Paulus W. Transcranial electrical stimulation (tES – tDCS; tRNS, tACS) methods. Neuropsychol. Rehabil. 2011;21(5):602–617. doi: 10.1080/09602011.2011.557292. [DOI] [PubMed] [Google Scholar]
- Quidé Y., Morris R.W., Shepherd A.M., Rowland J.E., Green M.J. Task-related frontostriatal functional connectivity during working memory performance in schizophrenia. Schizophr. Res. 2013;150:468–475. doi: 10.1016/j.schres.2013.08.030. [DOI] [PubMed] [Google Scholar]
- Saha S., Chant D., Welham J., McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5) doi: 10.1371/journal.pmed.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.C., Boules S., Mattiuz S., Youssef M., Tobe R.H., Sershen H., Lajtha A., Nolan K., Amiaz R., Davis J.M. Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: a randomized controlled study. Schizophr. Res. 2015;168(1–2):260–266. doi: 10.1016/j.schres.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Smith R.C., Li W., Wang Y., Jiang J., Wang J., Szabo V., Faull R., Jin H., Davis J.M., Li C. Effects of transcranial direct current stimulation on cognition and symptoms in Chinese patients with schizophrenia. Psychiatry Res. 2020;284 doi: 10.1016/j.psychres.2019.112617. [DOI] [PubMed] [Google Scholar]
- Stagg C.J., Nitsche M.A. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17(1):37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Stagg C.J., Antal A., Nitsche M.A. Physiology of transcranial direct current stimulation. J. Physiol. 2018;596(11):29–58. doi: 10.1113/JP275406. [DOI] [PubMed] [Google Scholar]
- Tan S., Zou Y., Wykes T., Reeder C., Zhu X., Yang F., Guo Y., Chen J., Luo X., Li X., Li C., Tan Y., Li H., Zhang X. Group cognitive remediation therapy for chronic schizophrenia: a randomized controlled trial. Neurosci. Lett. 2016;626:106–111. doi: 10.1016/j.neulet.2016.05.042. [DOI] [PubMed] [Google Scholar]
- Weickert T.W., Salimuddin H., Lenroot R.K., Bruggemann J., Loo C., Vercammen A., Kindler J., Weickert C.S. Preliminary findings of four-week, task-based anodal prefrontal cortex transcranial direct current stimulation transferring to other cognitive improvements in schizophrenia. Psychiatry Res. 2019;280 doi: 10.1016/j.psychres.2019.112487. [DOI] [PubMed] [Google Scholar]
- Yu L., Fang X., Chen Y., Wang Y., Wang D., Zhang C. Efficacy of transcranial direct current stimulation in ameliorating negative symptoms and cognitive impairments in schizophrenia: A systematic review and meta-analysis. Schizophr. Res. 2020;224:2–10. doi: 10.1016/j.schres.2020.10.006. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Xia X., Zhao X., Tang W., Lin W., Cai J. Efficacy and safety of transcranial direct current stimulation (tDCS) on cognitive function in chronic schizophrenia with tardive dyskinesia (TD): a randomized, double-blind, sham-controlled, clinical trial. BMC Psychiatry. 2023;23:623. doi: 10.1186/s12888-023-05112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of study interventions and detailed cognitive outcomes in reviewed studies on schizophrenia.
Supplementary material