Abstract
Polymers with high viscosity and good viscosity–temperature property have attracted attention as chemical agents for enhanced oil recovery. The role of polymers in the emulsifying stability of an amphoteric polyacrylamide (TSPAM) with good viscosity–temperature property in different flooding systems was investigated by means of a new emulsion stability model and molecular simulations. The results indicated that TSPAM exhibited superior emulsion stability compared to hydrolyzed polyacrylamide (HPAM). The half-life of emulsions containing TSPAM was 1–7 min longer than that of emulsions containing HPAM. The results of molecular simulation revealed that the HPAM molecules adsorbed at the oil–water interface in a “point adsorption” mode, whereas the TSPAM molecules adsorbed in a “surface adsorption” mode, resulting in higher interfacial adsorption efficiency and more stable interfacial film. The polymer flooding systems containing TSPAM showed a larger interfacial thickness of 1.029 nm and higher emulsification efficiency compared to the polymer flooding systems containing HPAM. The addition of Na2CO3 or surfactants further improved the stability of emulsions in the binary systems containing TSPAM. The stability of emulsions containing all three oil displacement agents was the strongest, with the half-life extended by 3.8–13.6 min. Amphoteric polyacrylamide significantly enhanced the stability of the emulsions. Through the integration of experimental and molecular simulation techniques, the molecular structure of polyacrylamide can be optimized, facilitating the development of more efficient oil recovery formulations for enhanced oil recovery applications.
1. Introduction
The high price of crude oil and future energy demand worldwide have necessitated the need for enhanced oil recovery (EOR) processes. Chemical flooding, especially surfactant–polymer (SP) flooding and alkali–surfactant–polymer (ASP) flooding, has been identified as a cost-effective chemical EOR process because of the synergistic impact and manifold functions of mixed chemicals in chemical EOR.1 Hydrolyzed polyacrylamide (HPAM) is the most widely polymer employed in chemical EOR.2 Due to electrostatic repulsion between the carboxylic groups of HPAM molecules, the aqueous solution of HPAM has high viscosity and improves the water–oil mobility ratio.3 However, the viscosity and elasticity of the aqueous solution of HPAM decrease rapidly with the increase of salt content, especially the solutions with high concentrations of Ca2+ and Mg2+.4 The HPAM undergoes obvious hydrolysis and thermal-oxidative degradation at high temperatures, leading to a significant drop in solution viscosity and a poor oil displacement effect.5 For crude oil reservoirs with high contents of Ca2+ and Mg2+ or high-temperature reservoirs, ASP flooding containing HPAM or sulfonated polyacrylamide (SPAM) experiences the precipitation of a large amount of Ca2+ and Mg2+ or a decrease in solution viscosity at high temperatures.6 The results of the flooding tests showed that the emulsion stability of the flooding system containing polymer plays an important role in the chemical EOR, and the emulsion stability of the flooding system was influenced not only by the viscosity of the solution but also by the chemical structure and the concentration of the polymer.5 Therefore, increasing temperature resistance, salt resistance, and emulsion stability are some important areas of research in polymers for enhanced oil recovery. Over the past several years, researchers have shown great interest in copolymers based on acrylamide (AM) and the modification of conventional polymers for their application in EOR.7 Many compounds were used as monomers to synthesize polymers with good emulsification properties, such as N,N-dimethylallyl amine (DMAA),8 2-ethylhexyl bromide (EHB),8N-phenylacrylamide,9N,N′-((2-hydroxy-4,5-dimethylbenzene-1,3-diyl)-dimethanediyl)bisprop-2-enamide (HDDE),10N-acryloyl morpholine (ACMO),10 isobornyl methacrylate (IBOMA),10 2-acrylamido-2-methylpropanesulfonic acid (AMPS),11 2-(dimethylamino)-ethyl methacrylate (DMAEMA),12 [2-(methacryloyloxy)ethyl]trimethylammonium chloride (MADQUAT),13 (3-acrylamidopropyl)trimethylammonium chloride (APTAC),14N-vinylpyrrolidone (NVP),15 methacryloxyethyl-dimethyl cetyl ammonium chloride (DMDCC),16 and methacryloyl ethyl sulfobetaine.17 Field tests showed that emulsification is a significant mechanism for enhancing oil recovery, and emulsification has the ability to improve both the displacement efficiency and sweep efficiency.18
Different methods have been used to study the emulsification properties of the flooding system. Rui Zhang and co-workers19 investigated the emulsification properties of a comb-shaped polymeric surfactant by emulsification rate experiments and low-field nuclear magnetic analysis and characterized the emulsion stability by the relaxation time area and peak spacing. Han Zhao and co-workers20 studied the emulsifying ability and the emulsion stability of a combination system using an amphiphilic polymer (APC16) and an anionic surfactant (erucyl dimethyl amidopropyl betaine, EDAB) through the bottle test and a Turbiscan laser particle size analyzer. However, the stability kinetics of the emulsion has rarely been studied so far.
In previous works, a novel amphoteric polyacrylamide (TSPAM) with tertiary amine groups and sulfonic acid groups was synthesized and used as a single-polymer flooding agent to enhance oil recovery.11 Results showed that the rheological properties and oil displacement performance of the TSPAM system were superior to those of HPAM (hydrolysis degree = 30%; Mw = 3.5 × 107). The TSPAM solution had a higher viscosity retention rate (53.3%) than HPAM (35.3%) at a total salinity of 20000 mg/L from 30 to 99 °C, and EOR with synthetic copolymer flooding increased by 6.8% at 80 °C compared to that with HPAM flooding. Emulsion stability in the flooding system is critical for EOR technology applications, which was unexplored for TSPAM flooding in prior works. In this work, molecular dynamics simulation was utilized to study oil–water interface properties containing the polymer, and a new emulsion stability model was used to investigate the stability kinetics of simulated emulsions containing different flooding systems. The effect of TSPAM concentration on the stability kinetics of the simulated emulsion was studied to obtain the optimum concentration for forming a stable emulsion in different flooding systems.
2. Experimental Section
2.1. Materials
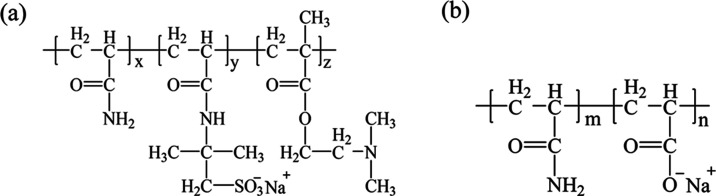
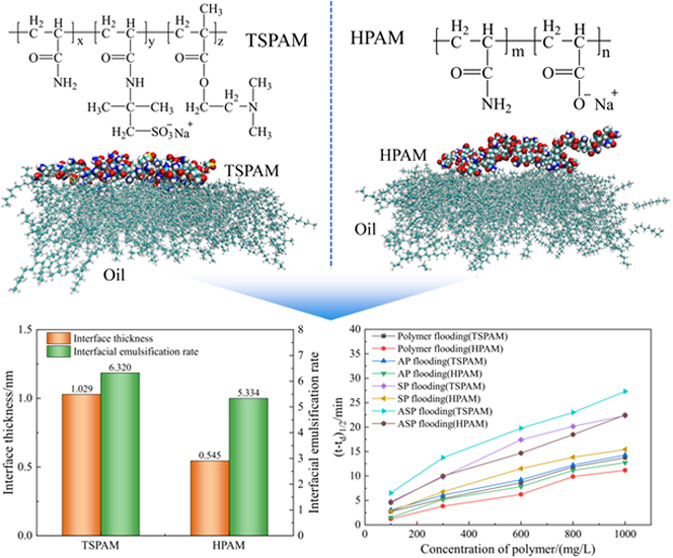
Sodium chloride (NaCl, AR), calcium chloride (CaCl2, AR), magnesium chloride (MgCl2, AR), and sodium carbonate (Na2CO3, AR) were purchased from Tianjin Guangfu Fine Chemical Research Institute. Sodium dodecylbenzene sulfonate (SDBS, 98 wt %) was purchased from Sigma-Aldrich. All chemicals were used without further purification. HPAM (hydrolysis degree = 25%; Mw = 2.5 × 107) was provided by Daqing Refining & Chemical Company. Crude oil with a kinematic viscosity of 20.21 mm2/s at 45 °C and a freezing point of 22.75 °C was provided by Daqing Oil Field (China), and the contents of aromatic hydrocarbons and asphaltenes in the crude oil were 19.24 and 0.42%, respectively. TSPAM (Mw = 9.99 × 106) (Figure 1) was synthesized using AM, AMPS, and DMAEMA as monomers.11
Figure 1.
Chemical structures of the polymers TSPAM (a) and HPAM (b).
2.2. Measurement of the Emulsification Property
The bottle test method was utilized to evaluate the emulsion stability of the simulated emulsion. The simulated emulsion samples were prepared by mixing the crude oil and the solution (Vwater/Voil = 13:7) in a homogenizer.20 The mixture of crude oil and the solution containing various oil displacement agents was homogenized for 10 min at 10,000 r/min to form a simulated emulsion. The test bottles, each filled with 100 mL of the simulated emulsion, were heated to 45 °C. The oil–water separation observed at different time points allowed for the evaluation of emulsion stability. The volume of the water layer was recorded at different times, and the emulsification properties of HPAM and TSPAM were investigated using the new emulsion stability model reported by Faruk Civan.21 The emulsion stability model reported by Faruk Civan expresses the instantaneous demulsification rate of a simulated emulsion as follows22
| 1 |
where X is the demulsification rate of the emulsion at time t, Xf is the maximum demulsification rate of the simulated emulsion, n is the order of reaction (n = 0.4),22 and kd is the demulsification rate constant. The initial condition is defined by X = 0, t = td, where td is the delayed time required for the initial demulsification of the simulated emulsion (also known as half-life). Under isothermal conditions, eq 1 is expressed by the following equation
| 2 |
The half-life of the simulated emulsion, determined by the maximum decomposable emulsion fraction, can be calculated by substituting X = 0.5Xf in eq 2 as follows
| 3 |
2.3. Molecular Dynamics Simulation
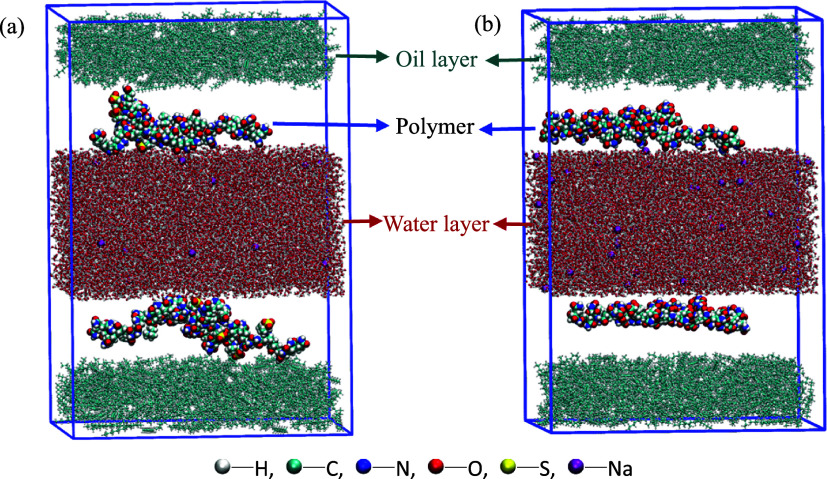
Molecular dynamics simulations of TSPAM and HPAM at the oil/water interface were conducted using the Gromacs 2020 program.23 The AMBER99SB-ILDN force field was utilized for water molecules and Na+ ions, while the GAFF force fields were employed for the remaining molecules. The optimized structures and RESP charges of undecane, TSPAM (36 acrylamide monomers + 1 DMAEMA monomer + 3 AMPS monomers), and HPAM (30 acrylamide monomers + 10 sodium acrylate monomers) molecules were obtained using Gaussian2016, xtb,24 and Multiwfn.25 The Sobtop software26 was utilized to generate the molecular simulation files for undecane, TSPAM, and HPAM molecules. The simulation box (Figure 2) built using the Packmol software27 consisted of two oil layers (10 nm × 10 nm × 2.5 nm), two polymer layers (10 nm × 10 nm × 2.5 nm), and one water layer (10 nm × 10 nm × 5.0 nm). The water layer was in the middle of the two oil layers, and the polymer molecules are located in the interface of oil and water. There were 400 undecane molecules in each oil layer. There were Na+ ions from polymer molecules and 16,000 water molecules in the water layer.28 There were two TSPAM or HPAM molecules in each polymer layer. The simulation was conducted at a temperature of 45 °C and a pressure of 0.1 MPa. The simulated time period was 800 ps with a time step of 2 fs.
Figure 2.
Boxes containing the (a) oil/water/TSPAM system and (b) oil/water/HPAM system.
3. Results and Discussion
3.1. Emulsion Stability of a Single-Polymer Flooding System
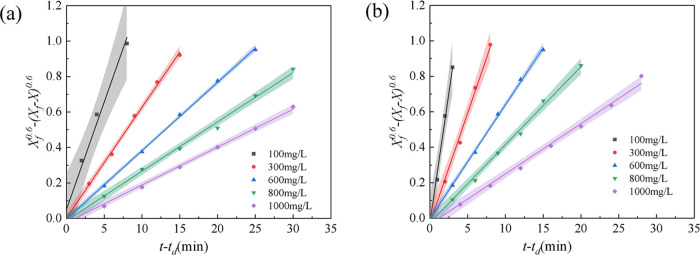
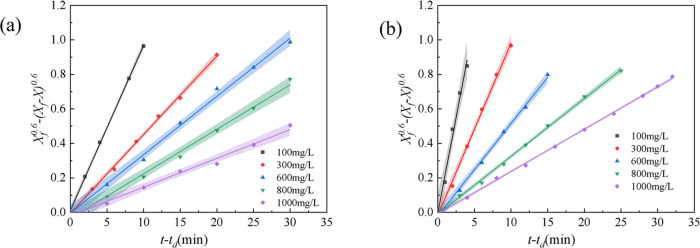
When the emulsification temperature was 45 °C and the total salinity was 32 g/L (the mass ratio of NaCl, CaCl2, and MgCl2 was 60:4:3), the effects of polymer concentration on the emulsification properties in the polymer flooding system were investigated, and the results are shown in Figure 3. With an increase in the polymer concentration in the simulated emulsion, the slope of the kinetic curve decreased, indicating an increase in the stability of the simulated emulsion. The slope of the kinetic curve for the simulated emulsion containing HPAM was higher than that for the simulated emulsion containing TSPAM (Figure 3), indicating that the stability of the simulated emulsion containing TSPAM was superior to that of the simulated emulsion containing HPAM. The possible reason was the interaction among the carboxylic acid anions, sulfonic acid anions, and quaternary ammonium cations in the TSPAM molecules, which reduced the degrees of shrinkage in the copolymer backbone. However, the polymer backbone of HPAM only contained carboxylic acid anions, which resulted in a stronger repulsion between polymer chains.
Figure 3.
Stability kinetics of the simulated emulsion of the polymer flooding system containing TSPAM (a) and HPAM (b).
To better analyze the influence of the polymer concentration on the stability of the simulated emulsion formed by the single-polymer flooding system, the kinetic parameters of the simulated emulsion were obtained by processing the curves in Figure 3(a),(b), and the results are listed in Table 1. Increasing the polymer concentration decreased the kd values and increased the (t – td)1/2 values, indicating the enhanced stability of the simulated emulsion. This was because the polymer molecules had two roles in the simulated emulsion.29 On the one hand, the increase in polymer concentration in the simulated emulsion was favorable to the increase in the apparent viscosity of the system,11 which caused a decrease in the coalescence rate of oil droplets and increased the stability of the simulated emulsion. On the other hand, the polymer chain of the adsorbed polymer molecule at the oil–water interface increased the viscoelasticity and the absolute value of the zeta potential.30 This was favorable for enhancing the stability of the interface film. The repulsive interaction between hydrophilic groups extending outward (Figure 4(a)) inhibited oil droplet coalescence, promoting emulsion stability upon polymer addition.
Table 1. Effect of the Polymer Concentration on the Stability Parameters of the Emulsion Containing the Polymer.
|
kd (min–1) |
(t – td)1/2 (min) |
R2 |
||||
|---|---|---|---|---|---|---|
| concentration (mg/L) | TSPAM | HPAM | TSPAM | HPAM | TSPAM | HPAM |
| 100 | 0.210 | 0.467 | 2.70 | 1.20 | 0.9891 | 0.9948 |
| 300 | 0.104 | 0.200 | 5.37 | 3.82 | 0.9993 | 0.9966 |
| 600 | 0.064 | 0.107 | 8.62 | 6.22 | 0.9998 | 0.9996 |
| 800 | 0.046 | 0.071 | 11.81 | 9.86 | 0.9984 | 0.9968 |
| 1000 | 0.034 | 0.045 | 13.74 | 11.16 | 0.9969 | 0.9952 |
Figure 4.
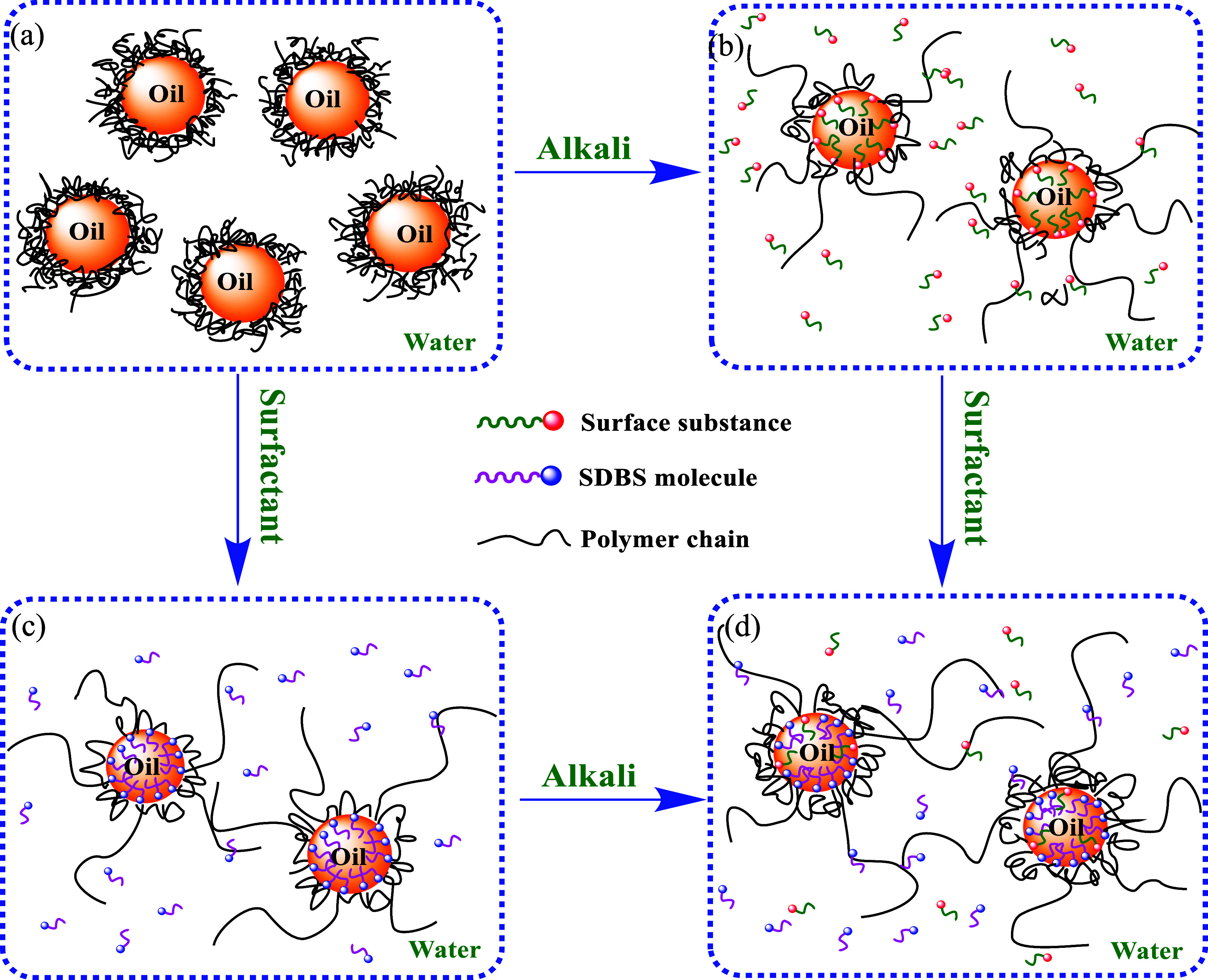
Absorption of the three compounds at the oil–water interface of different flooding systems. (a) Emulsion containing the polymer, (b) emulsion containing the polymer and alkali, (c) emulsion containing the polymer and the surfactant, and (d) emulsion containing the polymer, alkali, and surfactant.
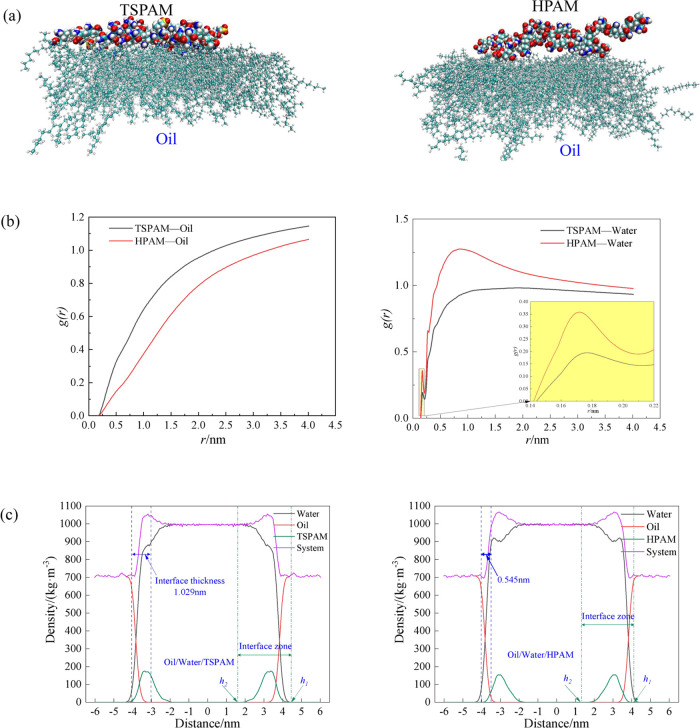
The kd values for the simulated emulsion containing TSPAM were lower than those for the simulated emulsion containing HPAM, indicating the greater stability of the simulated emulsion containing TSPAM at the same polymer concentration. However, the trend of the (t – td)1/2 value was opposite to that of the kd value. These results showed that the stability of the simulated emulsion containing TSPAM was higher than that of the simulated emulsion containing HPAM. To investigate the effects of TSPAM and HPAM at the oil–water interface, molecular dynamics simulations were performed, and the results are depicted in Figure 5. Figure 5(a) shows that HPAM adsorbs at the oil–water interface in a “point adsorption” mode, while TSPAM adsorbs in a “surface adsorption” mode. This difference is attributed to the chemical structure of the polymers. The TSPAM molecule (Figure 1(a)) does not contain a carboxyl group and has more lipophilic groups compared to HPAM (Figure 1(b)), resulting in increased lipophilicity of the TSPAM molecule. The difference in hydrophilicity and lipophilicity affected not only the adsorption state of polymer molecules at the oil–water interface but also the aggregation of oil or water molecules around the polymer molecules. The radial distribution function (RDF)30 characterizes the aggregation of oil or water molecules around the polymer molecules, as illustrated in Figure 5(b). The RDF value between TSPAM and oil was notably higher than that of HPAM. However, the RDF value of the first peak between TSPAM and water was significantly lower than that of HPAM and water. This observation further demonstrated that the interactions between TSPAM and oil molecules were considerably strong, facilitating the distribution of oil molecules at the interface and influencing the interface thickness. Seung Soon Jang and co-workers31 proposed the ″90–90%″ criterion for calculating the interfacial thickness, which is defined as the distance between two positions where the densities of oil and water reached 90% of their respective bulk densities. The oil–water interface thickness for the HPAM and TSPAM systems (Figure 5(c)) was 0.545 and 1.029 nm, respectively. The notable increase in interface thickness suggested an improvement in the miscibility between oil and water molecules. To investigate the impact of TSPAM and HPAM molecules on oil–water emulsification, the interfacial emulsification rate was determined using the following equation.32
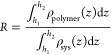 |
4 |
where ρpolymer(z) and ρsys(z) are the density profiles of the polymer (TSPAM or HPAM) and all components along the z axis direction, respectively. This definition indicates that h1 represents the height where polymers begin to emerge at the interface, while h2 indicates the height where polymers completely disappear from the interface. The interfacial emulsification rate of the system can be determined by integrating the density distribution within the interfacial zone depicted in Figure 5(c). The interfacial emulsification rates of the HPAM and TSPAM systems were 5.334 and 6.320, respectively. TSPAM exhibited superior performance in forming oil–water emulsions compared with HPAM.
Figure 5.
Molecular dynamics simulation results of the oil/water/polymer system. (a) Adsorption of polymer molecules on the oil surface, (b) RDF of the polymer and oil or water, and (c) Z-direction density distribution of the oil/water/polymer system.
Compared with HPAM, TSPAM is favorable for enhancing emulsification properties because of its stronger lipophilicity and surface adsorption at the oil–water interface, which led to an increase of the interface thickness and interfacial emulsification rate.
3.2. Emulsion Stability of the Alkali–Polymer Flooding System
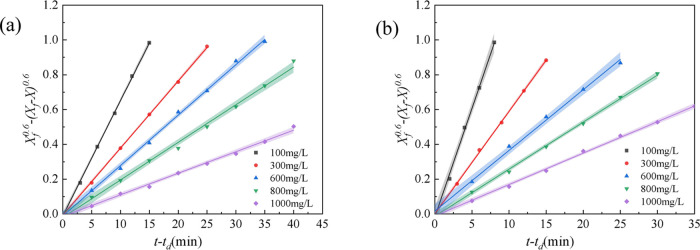
The effect of polymer concentration on the stability of two types of simulated emulsions containing the alkali–polymer (AP) flooding system was investigated at a Na2CO3 concentration of 1.2 wt %. The results are shown in Figure 6 and Table 2. The kd values of the two types of simulated emulsions containing alkali and polymer decreased rapidly with increasing polymer concentration, and the (t – td)1/2 values increased in the range of 100–600 mg/L with increasing polymer concentration. The kd values of the simulated emulsion containing alkali and polymer were lower than those with a single polymer, and the (t – td)1/2 values were higher as well, which showed that the stability of the simulated emulsion containing alkali and polymer was superior to the simulated emulsion containing a single polymer. A possible reason is that alkali played two roles in the simulated emulsion containing alkali and polymer.33 On the one hand, surface-active compounds formed by reacting with alkali and the acidic components in the crude oil adsorbed at the interface (Figure 4(b)) and reduced the interface tension of oil and water. On the other hand, the repulsion between the repeating units of polymer increased due to the interaction between alkali and the ionic groups of polymer molecules, leading to a tighter arrangement at the oil–water interface and enhanced the interface film strength of oil and water.
Figure 6.
Stability kinetics of the simulated emulsion of the AP flooding system containing TSPAM (a) and HPAM (b).
Table 2. Stability Parameters of the Simulated Emulsion Containing the AP Flooding System.
|
kd (min–1) |
(t – td)1/2 (min) |
R2 |
||||
|---|---|---|---|---|---|---|
| concentration (mg/L) | TSPAM | HPAM | TSPAM | HPAM | TSPAM | HPAM |
| 100 | 0.187 | 0.367 | 3.02 | 1.52 | 0.9933 | 0.9951 |
| 300 | 0.076 | 0.113 | 6.09 | 5.17 | 0.9981 | 0.9986 |
| 600 | 0.056 | 0.085 | 9.21 | 7.83 | 0.9947 | 0.9939 |
| 800 | 0.043 | 0.052 | 12.23 | 11.14 | 0.9906 | 0.9983 |
| 1000 | 0.028 | 0.035 | 14.28 | 12.72 | 0.9790 | 0.9963 |
Table 2 shows that the kd values of the simulated emulsion containing HPAM are higher than those of the simulated emulsion containing TSPAM in the presence of alkali, while the (t – td)1/2 values were exactly the opposite, which was similar to that of the simulated emulsion containing the polymer flooding system. The results indicate that the stability of the simulated emulsion containing TSPAM was higher than that of the simulated emulsion containing HPAM in the presence of alkali. Compared with the simulated emulsion containing HPAM, the changes in kd values and (t – td)1/2 values for the simulated emulsion containing TSPAM were smaller before and after the alkali addition. This is due to the sulfonic anionic group of TSPAM, which had good salt and alkali tolerance. However, the anionic group of HPAM was a carboxyl group, and the repulsion among these groups increased in the presence of alkali, which caused an increase in the viscosity and stability of the simulated emulsion system.
3.3. Emulsion Stability of the Surfactant–Polymer Flooding System
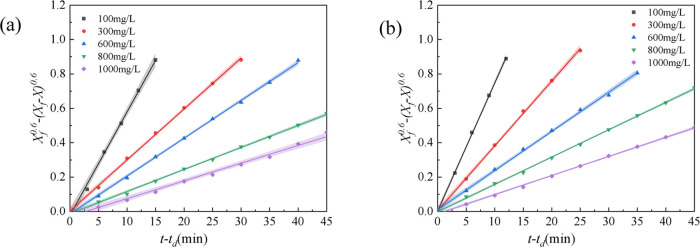
To obtain the optimum concentration of TSPAM in the surfactant–polymer flooding system, the effect of polymer concentration on the stability of the simulated emulsion containing the surfactant–polymer flooding system was investigated when the SDBS concentration was 0.3 wt %. The results are shown in Figure 7 and Table 3. The addition of SDBS in the polymer flooding system decreased the kd values and increased the (t – td)1/2 values, especially the increase of the (t – td)1/2 values was large, indicating that the SDBS molecule in the simulated emulsion formed a stabilized interfacial film by arranging at the oil–water interface with the polymer molecule. The kd values decreased and the (t – td)1/2 values increased with increasing polymer concentration, which was similar to the polymer flooding system. The (t – td)1/2 values of the surfactant–polymer flooding system were significantly higher than those of the single polymer flooding system and the alkali–polymer flooding system at the same polymer concentration. The (t – td)1/2 values increased rapidly with increasing polymer concentration in the range 100–600 mg/L, and the variation of (t – td)1/2 values was slight with a further increase in the polymer concentration. The reason was that the interaction between the polymer molecules and the SDBS molecules adsorbed at the oil–water interface led to an increase in the number of polymer molecules absorbed at the interface, reducing the space between adsorbed molecules and enhancing the stability of the interfacial film.34 The adsorption capacity of the polymer molecules and SDBS molecules at the oil–water interface reached saturation (Figure 4(c)) when the polymer concentration reached a certain level.35 For the surfactant–polymer flooding system, the kd values of the simulated emulsion containing TSPAM were lower than that of the simulated emulsion with HPAM, indicating that TSPAM had a better effect on improving the stability of the simulated emulsion than HPAM in the presence of SDBS.
Figure 7.
Stability kinetics of the simulated emulsion of the SP flooding system containing TSPAM (a) and HPAM (b).
Table 3. Stability Parameters of the Simulated Emulsion Containing the SP Flooding System.
|
kd (min–1) |
(t – td)1/2 (min) |
R2 |
||||
|---|---|---|---|---|---|---|
| concentration (mg/L) | TSPAM | HPAM | TSPAM | HPAM | TSPAM | HPAM |
| 100 | 0.109 | 0.203 | 4.76 | 2.78 | 0.9916 | 0.9986 |
| 300 | 0.065 | 0.098 | 9.79 | 6.77 | 0.9990 | 0.9984 |
| 600 | 0.048 | 0.060 | 17.42 | 11.50 | 0.9925 | 0.9985 |
| 800 | 0.035 | 0.044 | 20.14 | 13.84 | 0.9962 | 0.9992 |
| 1000 | 0.020 | 0.029 | 22.33 | 15.41 | 0.9854 | 0.9981 |
3.4. Emulsion Stability of the Alkali–Surfactant–Polymer Flooding System
The influence of polymer concentration on the stability of the simulated emulsion containing the alkali–surfactant–polymer flooding system was investigated at SDBS 0.3 wt % and alkali 1.2 wt %, as shown in Figure 8 and Table 4. The addition of SDBS and alkali to the polymer flooding system rapidly decreased the kd values and increased the (t – td)1/2 values compared with the polymer flooding system. At a polymer concentration of 600 mg/L, kd values and (t – td)1/2 values for the ASP flooding system containing TSPAM were 0.036 min–1 and 19.77 min, respectively, whereas the kd values and (t – td)1/2 values for the SP flooding system containing TSPAM were 0.048 min–1 and 17.42 min–1, respectively. The results indicated that the addition of alkali and surfactant increased the stability of the simulated emulsion containing polymer and that the three compounds of the ASP flooding system had good synergy. This was because the surfactant molecules formed by the reaction of alkali and crude oil, SDBS molecules, and polymer molecules adsorbed at the oil–water interface to form a stabilized interface film,6 which caused a decrease in the kd values. The kd values decreased rapidly and the (t – td)1/2 values increased rapidly for the ASP flooding system with increasing polymer concentration. This was because there was competitive adsorption among the three compounds in the ASP flooding system at the oil–water interface (Figure 4(d)), and the absorptivity of the polymer at the oil–water interface increased with increasing polymer concentration. The results of Figure 8 and Table 4 also showed that the kd values of the ASP flooding system containing TSPAM were far lower than those of the ASP flooding system containing HPAM, indicating that the flooding system containing TSPAM had higher emulsifying stability in oil and water in the process of oil displacement.
Figure 8.
Stability kinetics of the simulated emulsion of the ASP flooding system containing TSPAM (a) and HPAM (b).
Table 4. Stability Parameters of the Simulated Emulsion in the Presence of Surfactant and Alkali.
|
kd (min–1) |
(t – td)1/2 (min) |
R2 |
||||
|---|---|---|---|---|---|---|
| concentration (mg/L) | TSPAM | HPAM | TSPAM | HPAM | TSPAM | HPAM |
| 100 | 0.097 | 0.124 | 6.53 | 4.56 | 0.9983 | 0.9998 |
| 300 | 0.050 | 0.073 | 13.73 | 9.98 | 0.9997 | 0.9982 |
| 600 | 0.036 | 0.049 | 19.77 | 14.67 | 0.9994 | 0.9994 |
| 800 | 0.021 | 0.036 | 23.00 | 18.46 | 0.9990 | 0.9978 |
| 1000 | 0.016 | 0.022 | 27.29 | 22.47 | 0.9919 | 0.9981 |
4. Conclusions
In this study, the influence of TSPAM on the stability of emulsions was examined by using an emulsification model and molecular simulation technology. By analyzing both macroscopic and molecular levels, the characteristics and effects of TSPAM molecules were identified on emulsion stability across various emulsification systems. Compared with HPAM, a polymer commonly employed in chemical flooding, TSPAM molecules incorporate lipophilic groups, which alter the mode of molecular interface distribution from point adsorption to surface adsorption. This modification enhances the adsorption efficiency of the molecules at the oil–water interface, resulting in an interface thickness of 1.029 nm and an interfacial emulsification rate of 6.320. These findings indicate that amphiphilic polyacrylamide, achieved through the addition of lipophilic monomers, significantly improved the ability to stabilize the oil–water interface. TSPAM, in combination with Na2CO3 and surfactants, exhibits a strong synergistic effect in enhancing the emulsion stability. Emulsions containing Na2CO3 demonstrated an extended half-life by 0.32–0.54 min compared to those with TSPAM alone, while emulsions containing surfactants showed an even greater extension of half-life by 2.06–8.59 min. Notably, emulsions incorporating both Na2CO3 and surfactants exhibited the highest stability, with half-life extended by 3.83–13.55 min. The stability of emulsions containing TSPAM is markedly superior to that of the emulsion containing HPAM, with the half-life of the emulsion extending by 1 to 7 min, indicating its potential as a polymer for enhanced oil recovery. This research provides valuable scientific data to support the application of TSPAM in enhanced oil recovery. The final recovery strategy can be determined by integrating various factors, including reservoir characteristics, viscosity enhancement, stability, and economic considerations.
Acknowledgments
This work was supported by the Postdoctoral Foundation of Heilongjiang Province of China (16190023). We are grateful to the State Key Lab of Inorganic Synthesis and Preparative Chemistry of Jilin University and Analysis and Test Center of Northeast Petroleum University for the characterization work.
The authors declare no competing financial interest.
References
- Zou C.; Zhao P.; Ge J.; Lei Y.; Luo P. β-Cyclodextrin modified anionic and cationic acrylamide polymers for enhancing oil recovery. Carbohydr. Polym. 2012, 87 (1), 607–613. 10.1016/j.carbpol.2011.08.031. [DOI] [PubMed] [Google Scholar]
- Chen H.-x.; Tang H.; Gong X.; Wang J.; Liu Y.; Duan M.; Zhao F. Effect of partially hydrolyzed polyacrylamide on emulsification stability of wastewater produced from polymer flooding. J. Petrol. Sci. Eng. 2015, 133, 431–439. 10.1016/j.petrol.2015.06.031. [DOI] [Google Scholar]
- Yang D.; Sun Y.; Ghadiri M.; Wu H.; Qiao H.; He L.; Luo X.; Lü Y. Effect of Hydrolyzed polyacrylamide used in polymer flooding on droplet–interface electrocoalescence: variation of critical electric field strength of partial coalescence. Sep. Purif. Technol. 2019, 227, 115737 10.1016/j.seppur.2019.115737. [DOI] [Google Scholar]
- Tavakkoli O.; Kamyab H.; Shariati M.; Mustafa Mohamed A.; Junin R. Effect of Nanoparticles on the Performance of Polymer/Surfactant Flooding for Enhanced Oil Recovery: A Review. Fuel 2022, 312, 122867 10.1016/j.fuel.2021.122867. [DOI] [Google Scholar]
- Zhu L.; Chang Z.; Li M.; Wang E. Oxidative degradation of partially hydrolyzed polyacrylamide in aqueous solution I. influence of temperature. Polym. Mater. Sci. Eng. 2000, 16 (1), 113–116. [Google Scholar]
- Miao L.; Li F.; Sun D.; Wu T.; Li Y. Interfacial and electrokinetic properties of asphaltenes and alkali/surfactant/polymer in produced water system. J. Petrol. Sci. Eng. 2015, 133, 18–28. 10.1016/j.petrol.2015.05.018. [DOI] [Google Scholar]
- Ghada M.; Anoud S.; Divya B.; Gupta A.; Ahmad S. Polymeric surfactants: recent advancement in their synthesis, properties, and industrial applications. Macromol. Chem. Phys. 2023, 224 (17), 2300107 10.1002/macp.202300107. [DOI] [Google Scholar]
- Zhang P.; Wang W.; Zhou Y.; Ruan G.; Yu H.; Ji W. Preparation and solution properties of a novel cationic hydrophobically modified polyacrylamide for enhanced oil recovery. J. Macromol. Sci. A 2018, 55 (11–12), 764–769. 10.1080/10601325.2018.1526042. [DOI] [Google Scholar]
- Bhut P. R.; Pal N.; Mandal A. Characterization of Hydrophobically Modified Polyacrylamide in Mixed Polymer-Gemini Surfactant Systems for Enhanced Oil Recovery Application. ACS Omega 2019, 4 (23), 20164–20177. 10.1021/acsomega.9b02279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G.; Yu L.; Jiang X. Synthesis and properties of an acrylamide-based polymer for enhanced oil recovery: A preliminary study. Adv. Polym. Technol. 2018, 37 (8), 2763–2773. 10.1002/adv.21949. [DOI] [Google Scholar]
- Li F.; Zhu W.; Yu D.; Song H.; Wang K. Rheological properties and enhanced oil recovery performance of a novel sulfonate polyacrylamide. J. Macromol. Sci. A 2018, 55 (6), 449–454. 10.1080/10601325.2018.1470462. [DOI] [Google Scholar]
- Li F.; Zhu W.; Song H.; Wang K.; Li W. Study of surfactant-polymer system containing a novel ternary sulfonated polyacrylamide at the oil-water interface properties. J. Dispersion Sci. Technol. 2018, 39 (10), 1524–1531. 10.1080/01932691.2017.1421081. [DOI] [Google Scholar]
- Kujawa P.; Rosiak J. M.; Selb J.; Candau F. Micellar Synthesis and Properties of Hydrophobically Associating Polyampholytes. Macromol. Chem. Phys. 2001, 202 (8), 1384–1397. . [DOI] [Google Scholar]
- Gussenov I. S.; Mukhametgazy N.; Shakhvorostov A.; Kudaibergenov S. Comparative study of oil recovery using amphoteric terpolymer and hydrolyzed polyacrylamide. Polymers 2022, 14 (15), 3095. 10.3390/polym14153095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Che L.; Zheng J.; Huang G.; Wu X.; Chen P.; Zhang L.; Hu Q. Synthesis and thermal degradation property study of N-vinylpyrrolidone and acrylamide copolymer. RSC Adv. 2014, 4 (63), 33269–33278. 10.1039/C4RA05720A. [DOI] [Google Scholar]
- Liu R.; Pu W.; Wang L.; Chen Q.; Li Z.; Li Y.; Li B. Solution properties and phase behavior of a combination flooding system consisting of hydrophobically amphoteric polyacrylamide, alkyl polyglycoside and nalcohol at high salinities. RSC Adv. 2015, 5 (86), 69980–69989. 10.1039/C5RA13865E. [DOI] [Google Scholar]
- Zhang X.; Li G.; Chen Y.; Wang K.; Yang E. The synthesis of associative copolymers with both amphoteric and hydrophobic groups and the effect of the degree of association on the instability of emulsions. Polymers 2021, 13 (22), 4041. 10.3390/polym13224041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H.; Kang W.; Zhang L.; Chen J.; Li Z.; Zhou Q.; Wu H. Experimental study on a fine emulsion flooding system to enhance oil recovery for low permeability reservoirs. J. Petrol. Sci. Eng. 2018, 171, 974–981. 10.1016/j.petrol.2018.08.011. [DOI] [Google Scholar]
- Zhang R.; Huo J.; Peng Z.; Feng Q.; Zhang J.; Wang J. Emulsification properties of comb-shaped trimeric nonionic surfactant for high temperature drilling fluids based on water in oil. Colloid. Surface A 2017, 520, 855–863. 10.1016/j.colsurfa.2017.01.017. [DOI] [Google Scholar]
- Zhao H.; Kang W.; Yang H.; Huang Z.; Zhou B.; Sarsenbekuly B. Emulsification and stabilization mechanism of crude oil emulsion by surfactant synergistic amphiphilic polymer system. Colloid. Surface A 2021, 609, 125726 10.1016/j.colsurfa.2020.125726. [DOI] [Google Scholar]
- Civan F.; Alarcon L. J.; Campbell S. E. Laboratory confirmation of new emulsion stability model. J. Petrol. Sci. Eng. 2004, 43 (1), 25–34. 10.1016/j.petrol.2003.11.002. [DOI] [Google Scholar]
- Nie C.; Han G.; Ni J.; Guan S.; Du H.; Zhang Y.; Wang H. Stability dynamic characteristic of oil-in-water emulsion from alkali–surfactant–polymer flooding. ACS Omega 2021, 6 (29), 19058–19066. 10.1021/acsomega.1c02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen H. J. C.; van der Spoel D.; van Drunen R. GROMACS: A message-passing parallel molecular dynamics implementation. Comput. Phys. Commun. 1995, 91 (1), 43–56. 10.1016/0010-4655(95)00042-E. [DOI] [Google Scholar]
- Bannwarth C.; Caldeweyher E.; Ehlert S.; Hansen A.; Pracht P.; Seibert J.; Spicher S.; Grimme S. Extendedtight-binding quantum chemistry methods. WIREs Comput. Mol. Sci. 2021, 11 (2), e1493 10.1002/wcms.1493. [DOI] [Google Scholar]
- Lu T.; Chen F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33 (5), 580–592. 10.1002/jcc.22885. [DOI] [PubMed] [Google Scholar]
- Lu T.Sobtop, Version 1.0[Dev 3.1]. http://Sobereva.Com/Soft/Sobtop.
- Martínez L.; Andrade R.; Birgin E. G.; Martinez J. M. PACKMOL: a package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2009, 30 (13), 2157–2164. 10.1002/jcc.21224. [DOI] [PubMed] [Google Scholar]
- Wu G.; Zhu Q.; Yuan C.; Wang H.; Li C.; Sun S.; Hu S. Molecular Dynamics Simulation of the Influence of Polyacrylamide on the Stability of Sodium Dodecyl Sulfate Foam. Chem. Eng. Sci. 2017, 166, 313–319. 10.1016/j.ces.2017.03.011. [DOI] [Google Scholar]
- Chen H.-x.; Tang H.; Gong X.; Wang J.; Liu Y.; Duan M.; Zhao F. Effect of partially hydrolyzed polyacrylamide on emulsification stability of wastewater produced from polymer flooding. J. Petrol. Sci. Eng. 2015, 133, 431–439. 10.1016/j.petrol.2015.06.031. [DOI] [Google Scholar]
- Lu C.; Liu W.; Yuan Z.; Wang L.; Zhang Z.; Gao Q.; Ding W. Study on the Behavior of Saturated Cardanol-Based Surfactants at the Crude Oil/Water Interface through Molecular Dynamics Simulations. J. Phys. Chem. B 2023, 127 (41), 8938–8949. 10.1021/acs.jpcb.3c05517. [DOI] [PubMed] [Google Scholar]
- Jang S. S.; Lin S.; Maiti P. K.; Blanco M.; Goddard W. A.; Shuler P.; Tang Y. Molecular Dynamics Study of a Surfactant-Mediated Decane–Water Interface: Effect of Molecular Architecture of Alkyl Benzene Sulfonate. J. Phys. Chem. B 2004, 108 (32), 12130–12140. 10.1021/jp048773n. [DOI] [Google Scholar]
- Ma J.; Song X.; Luo J.; Zhao T.; Yu H.; Peng B.; Zhao S. Molecular Dynamics Simulation Insight into Interfacial Stability and Fluidity Properties of Microemulsions. Langmuir 2019, 35 (42), 13636–13645. 10.1021/acs.langmuir.9b02325. [DOI] [PubMed] [Google Scholar]
- Zheng D.-D.; Zhou Z.; Zhang Q.; Zhang L.; Zhu Y.; Zhang L. Effect of inorganic alkalis on interfacial tensions of novel betaine solutions against crude oil. J. Petrol. Sci. Eng. 2017, 152, 602–610. 10.1016/j.petrol.2017.01.033. [DOI] [Google Scholar]
- Mahdavi S. Z.; Aalaie J.; Miri T.; Razavi S. M. R.; Rahmani M. R. Study of polyacrylamide-surfactant system on the water–oil interface properties and rheological properties for EOR. Arab. J. Chem. 2017, 10 (8), 1136–1146. 10.1016/j.arabjc.2016.05.006. [DOI] [Google Scholar]
- Guzmán E.; Llamas S.; Maestro A.; Fernández-Peña L.; Akanno A.; Miller R.; Ortega F.; Rubio R. G. Polymer–surfactant systems in bulk and at fluid interfaces. Adv. Colloid Interface Sci. 2016, 233, 38–64. 10.1016/j.cis.2015.11.001. [DOI] [PubMed] [Google Scholar]