Abstract
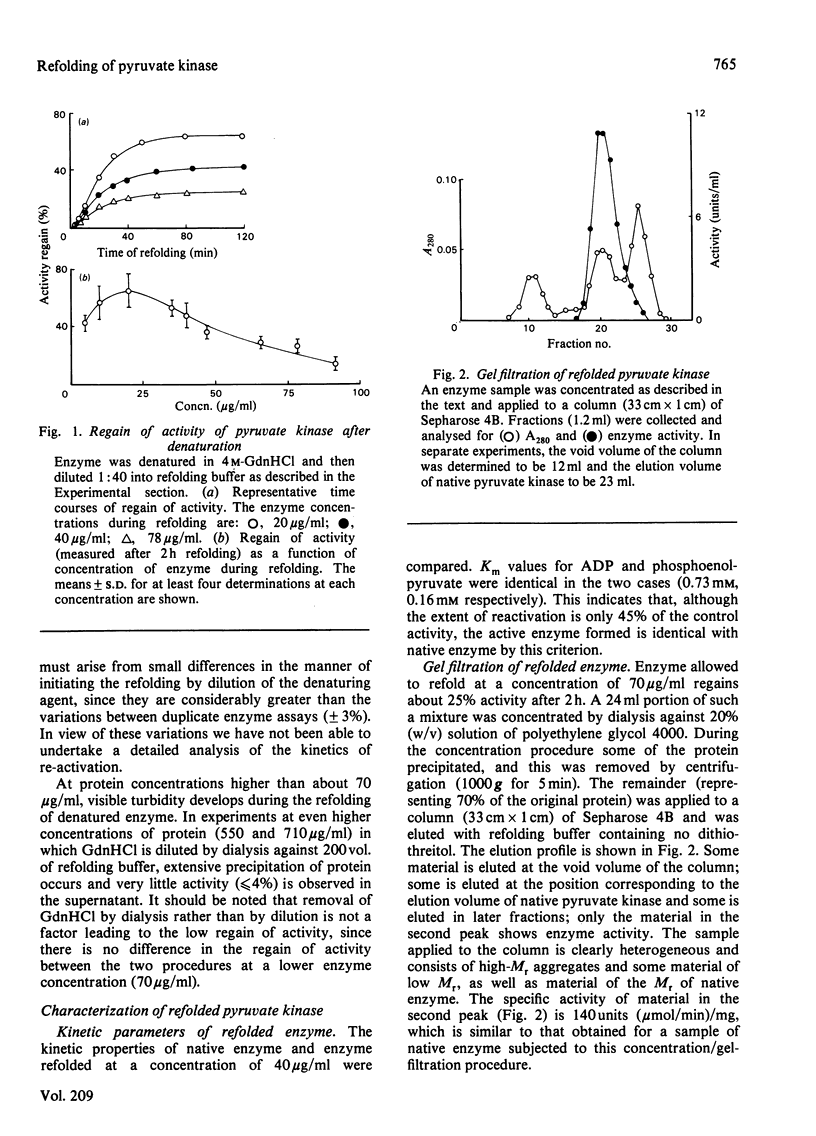
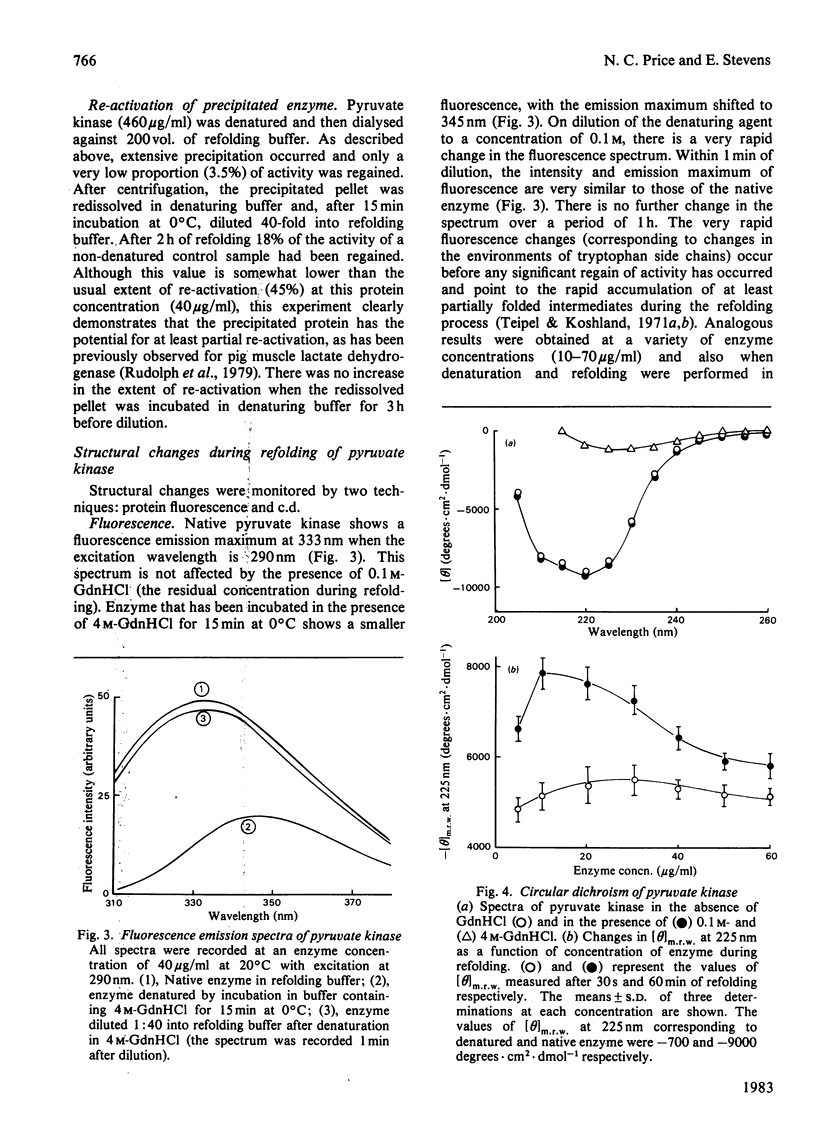
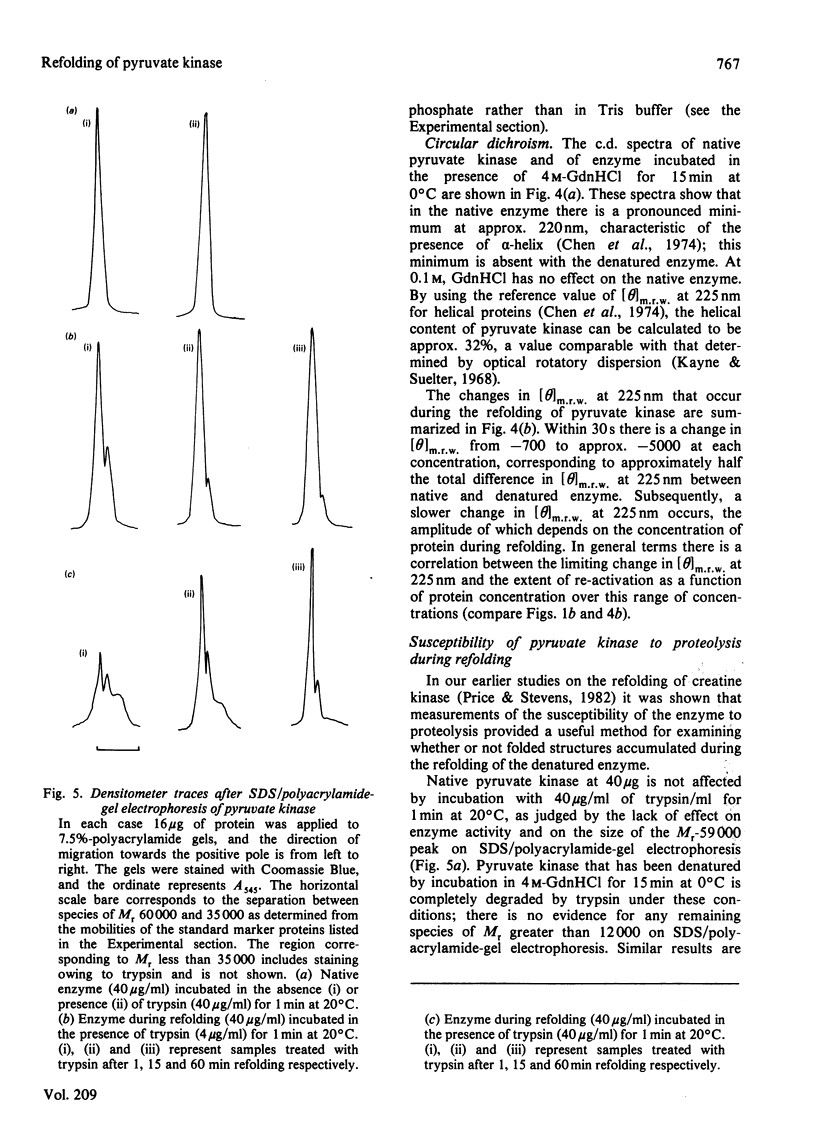
The refolding of rabbit muscle pyruvate kinase after denaturation by guanidine hydrochloride was studied. On dilution of the denaturing agent, enzyme activity is only partially regained. The extent of regain of activity is dependent on protein concentration, showing a marked decrease at higher concentrations. The failure to regain complete activity appears to be related to the formation of inactive aggregates, which can be separated from active enzyme by gel filtration. Insoluble aggregates can be partially re-activated after solubilization in guanidine hydrochloride. Changes in the circular-dichroism and fluorescence spectra during refolding suggest that a partially folded, inactive species is formed rapidly; this differs from native enzyme in being more susceptible to proteolysis by trypsin.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bickerstaff G. F., Paterson C., Price N. C. The refolding of denatured rabbit muscle creatine kinase. Biochim Biophys Acta. 1980 Feb 27;621(2):305–314. doi: 10.1016/0005-2795(80)90182-8. [DOI] [PubMed] [Google Scholar]
- Bickerstaff G. F., Price N. C. Properties of matrix-bound dimer and monomer derivatives of immobilized creatine kinase from rabbit skeletal muscle. Biochem J. 1978 Jul 1;173(1):85–93. doi: 10.1042/bj1730085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas J. M., Hubbard D. R., Anderson S. Subunit structure and hybrid formation of bovine pyruvate kinases. Biochemistry. 1977 Jan 25;16(2):191–197. doi: 10.1021/bi00621a005. [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Yang J. T., Chau K. H. Determination of the helix and beta form of proteins in aqueous solution by circular dichroism. Biochemistry. 1974 Jul 30;13(16):3350–3359. doi: 10.1021/bi00713a027. [DOI] [PubMed] [Google Scholar]
- Cottam G. L., Hollenberg P. F., Coon M. J. Subunit structure of rabbit muscle pyruvate kinase. J Biol Chem. 1969 Mar 25;244(6):1481–1486. [PubMed] [Google Scholar]
- Doster W., Hess B. Reversible solvent denaturation of rabbit muscle pyruvate kinase. Biochemistry. 1981 Feb 17;20(4):772–780. doi: 10.1021/bi00507a019. [DOI] [PubMed] [Google Scholar]
- Hermann R., Jaenicke R., Rudolph R. Analysis of the reconstitution of oligomeric enzymes by cross-linking with glutaraldehyde: kinetics of reassociation of lactic dehydrogenase. Biochemistry. 1981 Sep 1;20(18):5195–5201. doi: 10.1021/bi00521a015. [DOI] [PubMed] [Google Scholar]
- Johnson G. S., Kayne M. S., Deal W. C., Jr Metabolic control and structure of glycolytic enzymes. 8. Reversal of the dissociation of rabbit muscle pyruvate kinase into unfolded subunits. Biochemistry. 1969 Jun;8(6):2455–2462. doi: 10.1021/bi00834a030. [DOI] [PubMed] [Google Scholar]
- Kayne F. J., Suelter C. H. The temperature-dependent conformational transitions of pyruvate kinase. Biochemistry. 1968 May;7(5):1678–1684. doi: 10.1021/bi00845a009. [DOI] [PubMed] [Google Scholar]
- Kayne F. J. Thallium (I) activation of pyruvate kinase. Arch Biochem Biophys. 1971 Mar;143(1):232–239. doi: 10.1016/0003-9861(71)90204-9. [DOI] [PubMed] [Google Scholar]
- Porter D. H., Cardenas J. M. Analysis of the renaturation kinetics of bovine muscle pyruvate kinase. Biochemistry. 1980 Jul 22;19(15):3447–3452. doi: 10.1021/bi00556a007. [DOI] [PubMed] [Google Scholar]
- Price N. C., Jaenicke R. The quaternary structure of phosphoglycerate mutase from yeast: evidence against dissociation of the tetrameric enzyme at low concentrations. FEBS Lett. 1982 Jul 5;143(2):283–286. doi: 10.1016/0014-5793(82)80117-8. [DOI] [PubMed] [Google Scholar]
- Price N. C., Stevens E. The refolding of denatured rabbit muscle creatine kinase. Search for intermediates in the refolding process and effect of modification at the reactive thiol group on refolding. Biochem J. 1982 Jan 1;201(1):171–177. doi: 10.1042/bj2010171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph R., Heider I., Westhof E., Jaenicke R. Mechanism of refolding and reactivation of lactic dehydrogenase from pig heart after dissociation in various solvent media. Biochemistry. 1977 Jul 26;16(15):3384–3390. doi: 10.1021/bi00634a015. [DOI] [PubMed] [Google Scholar]
- Rudolph R., Zettlmeissl G., Jaenicke R. Reconstitution of lactic dehydrogenase. Noncovalent aggregation vs. reactivation. 2. Reactivation of irreversibly denatured aggregates. Biochemistry. 1979 Dec 11;18(25):5572–5575. doi: 10.1021/bi00592a008. [DOI] [PubMed] [Google Scholar]
- Stammers D. K., Muirhead H. Three-dimensional structure of cat muscle pyruvate kinase at 6 Angstrom resolution. J Mol Biol. 1975 Jun 25;95(2):213–225. doi: 10.1016/0022-2836(75)90391-5. [DOI] [PubMed] [Google Scholar]
- Stuart D. I., Levine M., Muirhead H., Stammers D. K. Crystal structure of cat muscle pyruvate kinase at a resolution of 2.6 A. J Mol Biol. 1979 Oct 15;134(1):109–142. doi: 10.1016/0022-2836(79)90416-9. [DOI] [PubMed] [Google Scholar]
- Sugimoto E., Sasaki R., Chiba H. Reversible urea denaturation of crystalline yeast phosphoglyceric acid mutase. Arch Biochem Biophys. 1966 Feb;113(2):444–450. doi: 10.1016/0003-9861(66)90212-8. [DOI] [PubMed] [Google Scholar]
- Teipel J. W., Koshland D. E., Jr Kinetic aspects of conformational changes in proteins. I. Rate of regain of enzyme activity from denatured proteins. Biochemistry. 1971 Mar 2;10(5):792–798. doi: 10.1021/bi00781a011. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Zettlmeissl G., Rudolph R., Jaenicke R. Reconstitution of lactic dehydrogenase. Noncovalent aggregation vs. reactivation. 1. Physical properties and kinetics of aggregation. Biochemistry. 1979 Dec 11;18(25):5567–5571. doi: 10.1021/bi00592a007. [DOI] [PubMed] [Google Scholar]


