Abstract
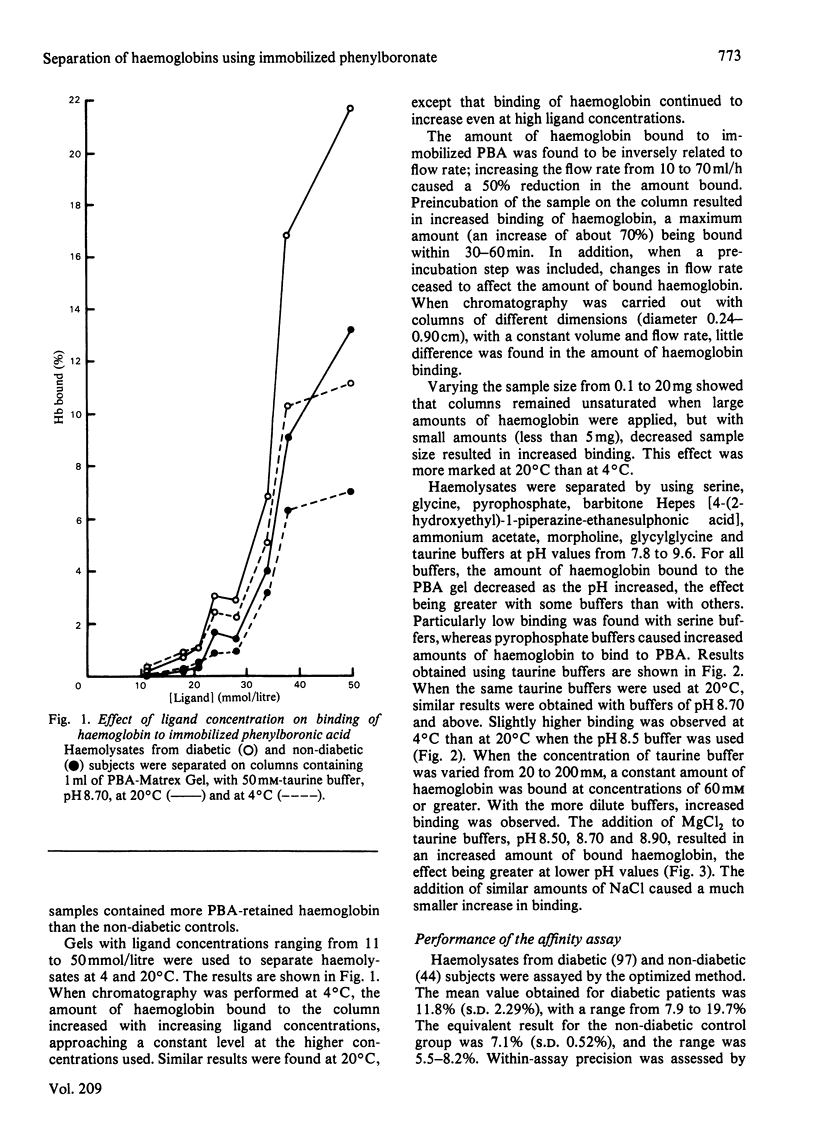
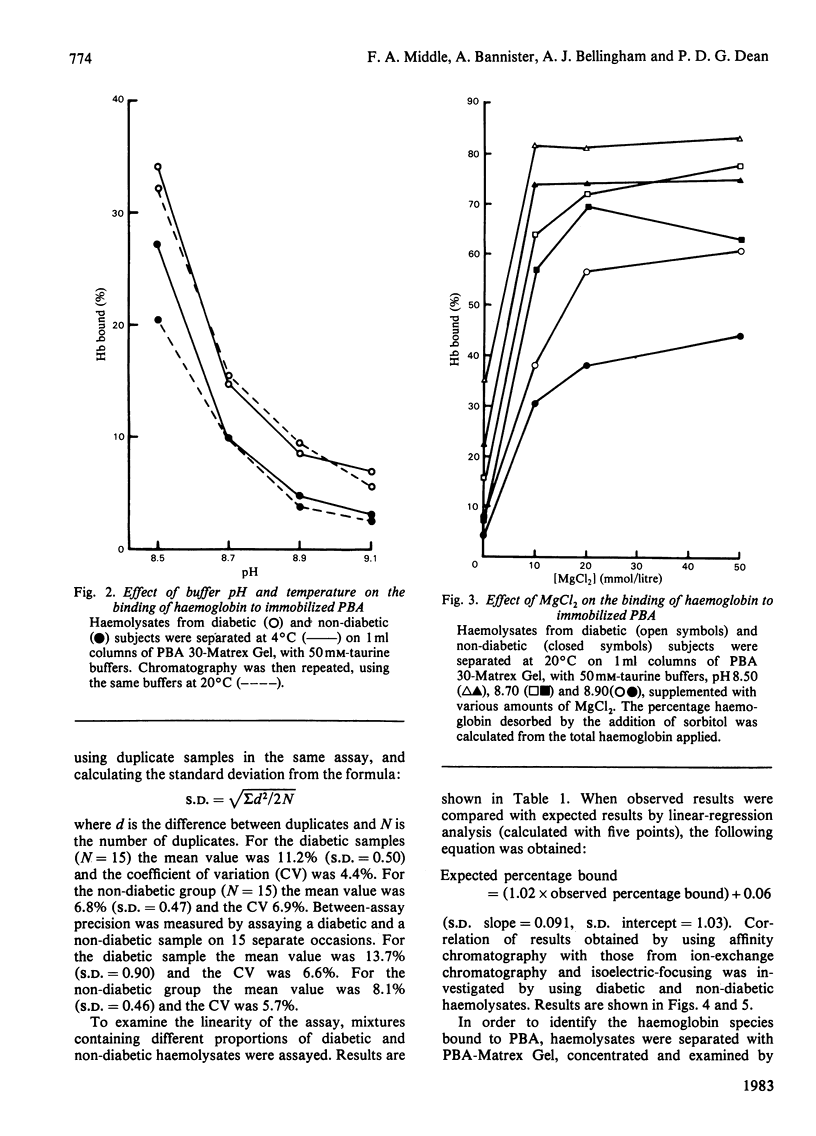
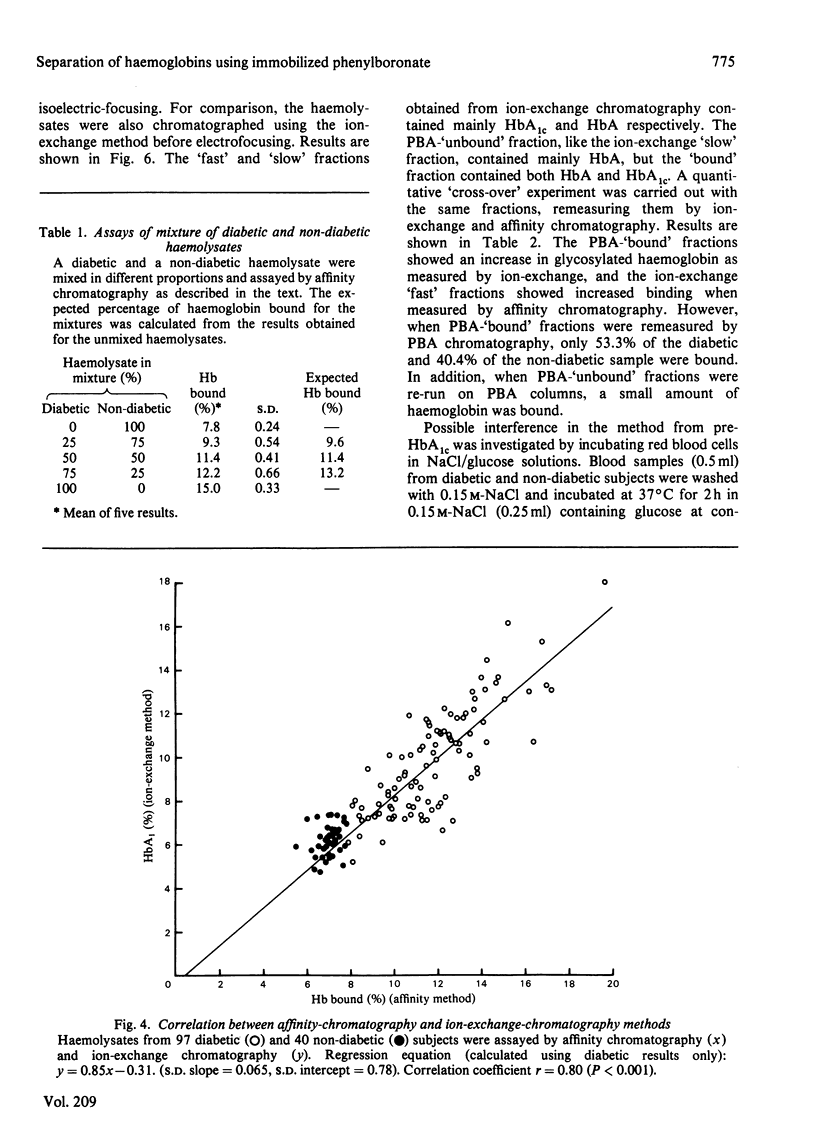
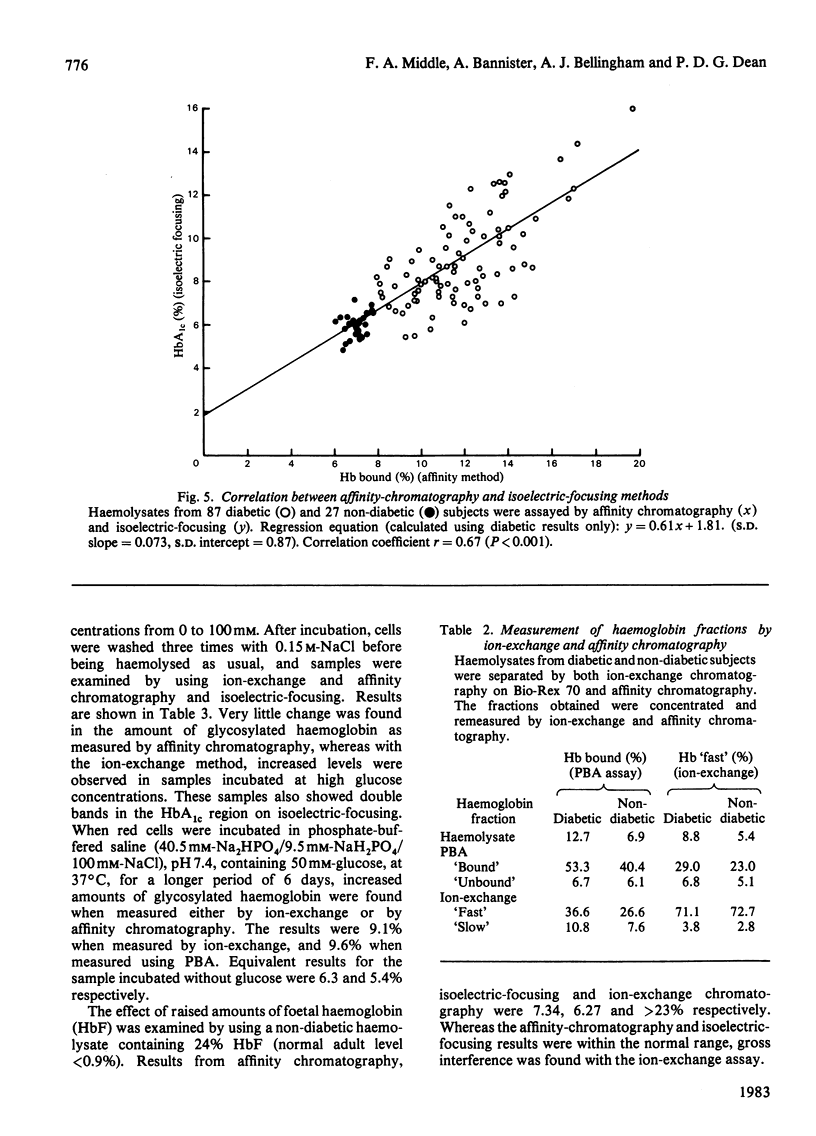
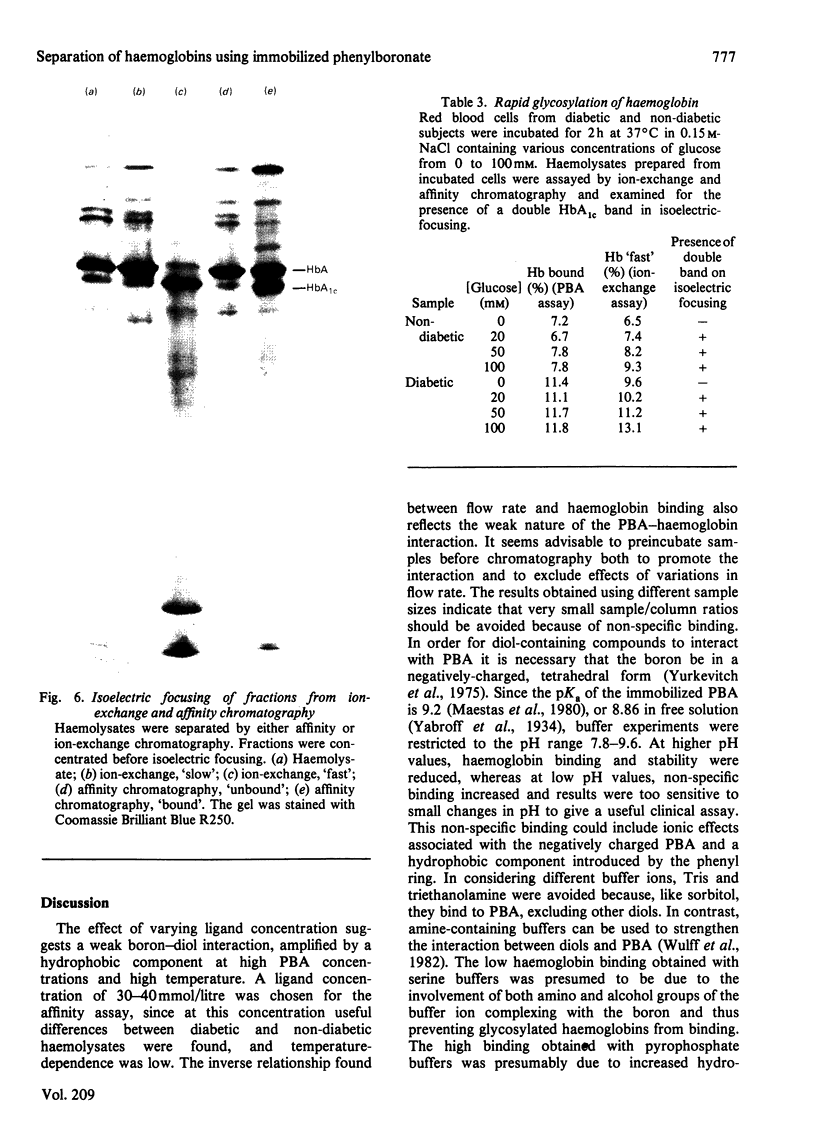
Haemoglobins from diabetic and non-diabetic individuals have been separated by affinity chromatography using immobilized phenylboronate, which interacts specifically with diol-containing compounds such as glycosylated haemoglobin. The effects of ligand concentration, flow rate, column geometry, preincubation of sample, buffer composition and temperature have been investigated. Significant correlation was found between results from affinity-chromatography and ion-exchange and isoelectric-focusing methods. Isoelectric-focusing of the haemoglobin fractions obtained from affinity chromatography indicate that, in addition to haemoglobin A1c, some haemoglobin A is also bound to immobilized phenylboronic acid. Assays of haemolysates obtained from red blood cells incubated in glucose solutions suggest that unstable pre-(haemoglobin A1c) does not interfere. The assay is not affected by the presence of haemoglobin F.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunn H. F. Evaluation of glycosylated hemoglobin diabetic patients. Diabetes. 1981 Jul;30(7):613–617. doi: 10.2337/diab.30.7.613. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Shapiro R., McManus M., Garrick L., McDonald M. J., Gallop P. M., Gabbay K. H. Structural heterogeneity of human hemoglobin A due to nonenzymatic glycosylation. J Biol Chem. 1979 May 25;254(10):3892–3898. [PubMed] [Google Scholar]
- Compagnucci P., Cartechini M. G., Bolli G., de Feo P., Santeusanio F., Brunetti P. The importance of determining irreversibly glycosylated hemoglobin in diabetics. Diabetes. 1981 Jul;30(7):607–612. doi: 10.2337/diab.30.7.607. [DOI] [PubMed] [Google Scholar]
- Dolhofer R., Wieland O. H. Increased glycosylation of serum albumin in diabetes mellitus. Diabetes. 1980 Jun;29(6):417–422. doi: 10.2337/diab.29.6.417. [DOI] [PubMed] [Google Scholar]
- Fischer R. W., Winterhalter K. H. The carbohydrate moiety in hemoglobin A1C is present in the ring form. FEBS Lett. 1981 Nov 30;135(1):145–147. doi: 10.1016/0014-5793(81)80963-5. [DOI] [PubMed] [Google Scholar]
- Flückiger R., Winterhalter K. H. In vitro synthesis of hemoglobin AIc. FEBS Lett. 1976 Dec 1;71(2):356–360. doi: 10.1016/0014-5793(76)80969-6. [DOI] [PubMed] [Google Scholar]
- Gabbay K. H., Sosenko J. M., Banuchi G. A., Mininsohn M. J., Flückiger R. Glycosylated hemoglobins: increased glycosylation of hemoglobin A in diabetic patients. Diabetes. 1979 Apr;28(4):337–340. doi: 10.2337/diab.28.4.337. [DOI] [PubMed] [Google Scholar]
- Higgins P. J., Bunn H. F. Kinetic analysis of the nonenzymatic glycosylation of hemoglobin. J Biol Chem. 1981 May 25;256(10):5204–5208. [PubMed] [Google Scholar]
- McCutchan T. F., Gilham P. T., Söll D. An improved method for the purification of tRNA by chromatography on dihydroxyboryl substituted cellulose. Nucleic Acids Res. 1975 Jun;2(6):853–864. doi: 10.1093/nar/2.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald M. J., Shapiro R., Bleichman M., Solway J., Bunn H. F. Glycosylated minor components of human adult hemoglobin. Purification, identification, and partial structural analysis. J Biol Chem. 1978 Apr 10;253(7):2327–2332. [PubMed] [Google Scholar]
- Rosenberg M., Wiebers J. L., Gilham P. T. Studies on the interactions of nucleotides, polynucleotides, and nucleic acids with dihydroxyboryl-substituted celluloses. Biochemistry. 1972 Sep 12;11(19):3623–3628. doi: 10.1021/bi00769a020. [DOI] [PubMed] [Google Scholar]
- Schellekens A. P., Sanders G. T., Thornton W., van Groenestein T. Sources of variation in the column-chromatographic determination of glycohemoglobin (HbA1). Clin Chem. 1981 Jan;27(1):94–99. [PubMed] [Google Scholar]
- Shapiro R., McManus M. J., Zalut C., Bunn H. F. Sites of nonenzymatic glycosylation of human hemoglobin A. J Biol Chem. 1980 Apr 10;255(7):3120–3127. [PubMed] [Google Scholar]
- Svendsen P. A., Christiansen J. S., Søegaard U., Welinder B. S., Nerup J. Rapid changes in chromatographically determined haemoglobin A1c induced by short-term changes in glucose concentration. Diabetologia. 1980 Aug;19(2):130–136. doi: 10.1007/BF00421859. [DOI] [PubMed] [Google Scholar]
- Trivelli L. A., Ranney H. M., Lai H. T. Hemoglobin components in patients with diabetes mellitus. N Engl J Med. 1971 Feb 18;284(7):353–357. doi: 10.1056/NEJM197102182840703. [DOI] [PubMed] [Google Scholar]
- Weith H. L., Wiebers J. L., Gilham P. T. Synthesis of cellulose derivatives containing the dihydroxyboryl group and a study of their capacity to form specific complexes with sugars and nucleic acid components. Biochemistry. 1970 Oct 27;9(22):4396–4401. doi: 10.1021/bi00824a021. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Johnstone A. P., Bouriotis V., Dean P. D. Affinity chromatography of membrane proteins on dihydroxyboryl-matrix gel. Biochem Soc Trans. 1981 Feb;9(1):137–139. doi: 10.1042/bst0090137. [DOI] [PubMed] [Google Scholar]
- Williams G. T., Johnstone A. P., Dean P. D. Fractionation of membrane proteins on phenylboronic acid-agarose. Biochem J. 1982 Jul 1;205(1):167–171. doi: 10.1042/bj2050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. K., McLennan S., Church D. B., Turtle J. R. The measurement of glycosylated hemoglobin in man and animals by aminophenylboronic acid affinity chromatography. Diabetes. 1982 Aug;31(8 Pt 1):701–705. doi: 10.2337/diab.31.8.701. [DOI] [PubMed] [Google Scholar]